Abstract
In skeletal muscles, motor units comprise a motoneuron and the group of muscle fibers innervated by it, which are usually classified based on myosin heavy chain isoform expression. Motor units displaying diverse contractile and fatigue properties are important in determining the range of motor behaviors that can be accomplished by a muscle. Muscle fiber atrophy and weakness may disproportionately affect specific fiber types across a variety of diseases or clinical conditions, thus impacting neuromotor control. In this regard, fiber atrophy that affects a specific fiber type will alter the relative contribution of different motor units to overall muscle structure and function. For example, in various diseases there is fairly selective atrophy of type IIx and/or IIb fibers comprising the strongest yet most fatigable motor units. As a result, there is muscle weakness (i.e., reductions in force per cross-sectional area) associated with an apparent improvement in resistance to fatiguing contractions. This review will examine neuromotor control of respiratory muscles such as the diaphragm muscle and the impact of muscle fiber atrophy on motor performance.
1. Introduction
The final common effector of skeletal muscle neuromotor control is the motor unit consisting of a motoneuron and the group of muscle fibers it innervates (Liddell and Sherrington, 1925). With recruitment of additional motor units force increases (Fournier and Sieck, 1988; Sieck, 1988b). In addition, for those units recruited, force increases with increasing discharge frequency (Iscoe et al., 1976). The muscle fibers comprising motor units express uniform contractile proteins that underlie distinction of different fiber types (Enad et al., 1989; Fournier and Sieck, 1988; Hamm et al., 1988; Nemeth et al., 1986; Sieck et al., 1989a; Sieck et al., 1996). Specifically, the expression of different isoforms of myosin heavy chain (MyHC) corresponds with the classification of different muscle fiber types (Fig. 1) (Butler et al., 1999; Sieck, 1991; Sieck, 1994; Su et al., 1997). The contractile and fatigue properties of motor units vary widely depending on their fiber type composition (Burke et al., 1973; Fournier and Sieck, 1988). The overall diversity of motor unit contractile and fatigue properties determines the range of function of a skeletal muscle.
Figure 1.
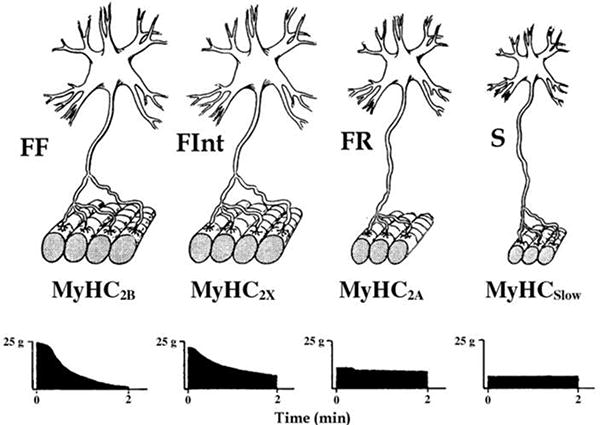
Motor units display differences in their contractile and fatigue properties that underlie the most common classification scheme: type S, FR, FInt and FF. Importantly, muscle fibers in a motor unit share a homogeneous fiber type composition as determined by myosin heavy chain (MyHC) isoform expression (MyHCSlow, MyHC2A, MyHC2X and/or MyHC2B for type S, FR, FInt and FF units, respectively). Reprinted with permission from Mantilla and Sieck (2013).
The diaphragm muscle (DIAm) is the major muscle for inspiration in mammals, and in accomplishing this ventilatory behavior it is active ∼30-40% of the time (duty cycle) each day throughout life (Hensbergen and Kernell, 1997). However, ventilatory behaviors can be accomplished by activating only ∼10-25% of the total force-generating capacity of the DIAm (Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1991; Sieck and Fournier, 1989). This level of force generation by the DIAm is accomplished by the recruitment of only fatigue resistant slow- and fast-twitch motor units. From a design standpoint it would be inefficient to repeatedly recruit more fatigable fast-twitch motor units to accomplish sustained ventilatory behaviors.
It is important to recognize that the DIAm is also activated during non-ventilatory motor behaviors such as coughing, sneezing, sighing, vomiting, defecation, vocalization and maintenance of posture (Butler et al., 2001; Mantilla et al., 2011; Mantilla et al., 2010; Milano et al., 1992). Forces generated by the DIAm are much greater during expulsive, airway-protective (clearance) behaviors (Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1991; Sieck and Fournier, 1989). For instance, near maximal co-activation of the DIAm and abdominal muscles during coughing and sneezing is necessary to generate the large inspiratory effort and high intra-abdominal pressure that precedes diaphragm elevation and increased intra-thoracic pressure to “clear” the airway (Milano et al., 1992; Rybak et al., 2008; Shannon et al., 1998). Indeed, these non-ventilatory motor behaviors of the DIAm require activation of all motor unit types, but particularly more fatigable fast-twitch motor units.
In a variety of diseases or treatment conditions, there is muscle fiber wasting that affects the contractile and fatigue properties of the DIAm. Specific fiber type atrophy is often noted that disproportionately affects the relative contribution of different motor unit types and possibly the ability to accomplish certain motor behaviors. This review will discuss various disease conditions where DIAm fiber atrophy occurs and the impact on performance of ventilatory versus non-ventilatory motor behaviors. Diseases that affect survival of respiratory motor neurons (e.g., amyotrophic lateral sclerosis) will affect neuromotor control directly as a result of motor neuron loss. Furthermore, if motor neuron loss is specific to certain motor unit types (Pun et al., 2006), perhaps reflecting specific vulnerability of certain motor neuron populations, then functional impairments will display the confounding contribution of motor unit types to muscle performance, as discussed herein.
2. Classification of Muscle Fiber and Motor Unit Types
In the DIAm, four different types of motor units are classified based on the contractile and fatigue properties of their muscle fibers (Fig. 1) (Burke, 1981; Fournier and Sieck, 1988; Kernell, 2006; Sieck et al., 1989a). Based on their twitch contraction times, motor units are classified as slow-twitch (type S) or fast-twitch (type F). Motor units are also classified based on their fatigue resistance. All type S motor units are fatigue resistant, while fatigue resistance of type F units varies considerably. These contractile and fatigue properties of motor units correspond with the MyHC isoform composition of muscle fibers. Type S motor units comprise type I fibers that express MyHCSlow. These type I fibers have smaller cross-sectional areas (Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 1989b; Zhan et al., 1997), higher mitochondrial volume densities and higher capacities for oxidative phosphorylation (Enad et al., 1989; Sieck et al., 1996). Single fiber studies show that type I muscle fibers have slower maximum shortening velocities, consistent with slower cross-bridge cycling kinetics (Sieck and Prakash, 1997). Type I fibers also have lower specific force (force per cross-sectional area) (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999).
Type F motor units that are fatigue-resistant are classified as type FR and comprise type IIa fibers that express MyHC2A. Type IIa fibers have smaller cross-sectional areas (Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 1989b; Zhan et al., 1997), higher mitochondrial volume densities and higher oxidative capacities compared to other type II fibers (Enad et al., 1989; Sieck et al., 1996). Type IIa muscle fibers have faster maximum shortening velocities, consistent with faster cross-bridge cycling kinetics (Sieck and Prakash, 1997). The specific force of type IIa fibers is comparable to type I fibers (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999).
More fatigable type F motor units are classified as either fast-twitch fatigue intermediate (FInt) or fast-twitch fatigable (FF), although a continuum of fatigue resistance exists across these units. Type FInt and FF units comprise type IIx and IIb fibers that commonly co-express MyHC2X and MyHC2B isoforms, albeit in varying proportions (Sieck et al., 1996). Cross-sectional areas of type IIx and IIb DIAm fibers are usually larger than type I and IIa fibers (Greising et al., 2013a; Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 2012; Sieck et al., 1989b; Zhan et al., 1997). Mitochondrial volume densities and oxidative capacities of type IIx and IIb fibers are lower than type I and IIa fibers (Enad et al., 1989; Sieck et al., 1996). Type IIx and IIb fibers also have the fastest maximum shortening velocities and generate the greatest specific forces of all DIAm fiber types (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999).
Differences also exist in the force-Ca2+ relationships of type I, IIa, IIx and IIb fibers that underlie differences in their force-frequency responses (Geiger et al., 2000; Geiger et al., 1999). At a given myoplasmic Ca2+ concentration, the force generated by type I DIAm fibers is greater than type II fibers, reflecting increased Ca2+ sensitivity, most likely due to the expression of a slow troponin C isoform. Correspondingly, the force-frequency response curve of type S motor units is shifted leftward compared to type F motor units. No differences in Ca2+ sensitivity exist across type F units. In agreement, motor units with lower recruitment thresholds (most likely type S units) have slower initial and peak discharge rates than type F motor units with higher recruitment thresholds.
The innervation ratio (i.e., the number of muscle fibers innervated by a motoneuron) of type FInt and FF motor units is greater than that of type S and FR units (Sieck, 1988b). Thus, as a result of larger fiber cross-sectional areas, greater innervation ratios and greater specific forces, the overall force contributed by type FInt and FF DIAm motor units is substantially greater than that contributed by type S and FR units (Fig. 2) (Fournier and Sieck, 1988; Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck and Fournier, 1989).
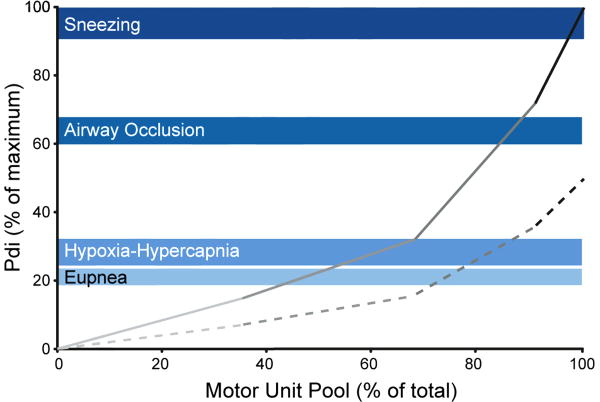
Figure 2.
Motor unit recruitment order across a range of ventilatory and non-ventilatory behaviors. In this model, recruitment of type S motor units is followed by fast-twitch units according to fatigue resistance (type FR before FInt and FF units). Transdiaphragmatic pressures (Pdi) generated during eupnea, hypoxia–hypercapnia, airway occlusion and sneezing (shown in shades of blue with the shaded area representing mean ± SE) were used as a surrogate of diaphragm muscle (DIAm) force and are expressed as percent of maximum Pdi obtained by bilateral phrenic nerve stimulation. For the diaphragm muscle (DIAm), most ventilatory behaviors (eupnea and hypoxia-hypercapnia) can be accomplished by recruitment of only fatigue-resistant type S and FR motor units, when motor units are maximally activated (solid line) and when activated submaximally (at 50% of maximal motor unit force; dashed line). Reprinted with permission from Mantilla and Sieck (2011).
3. Motor Unit Recruitment Order
Based on recordings from ventral root filaments, it was shown that motor units that are recruited first display smaller amplitudes and slower conduction velocities than units recruited later (Henneman, 1957; Henneman and Olson, 1965; Henneman et al., 1965; McPhedran et al., 1965). Gasser had previously demonstrated a relationship between action potential amplitude, conduction velocity and axon diameter that reflects motoneuron size (Gasser and Grundfest, 1939). Consequently, Henneman formulated the “size principle”, proposing that motor units are recruited in an orderly fashion according to intrinsic electrophysiological properties related to motoneuron size. Subsequently, the size principle was tested by demonstrating that the order of motor unit recruitment was consistently related to axonal conduction velocity (Henneman and Olson, 1965; Henneman et al., 1965; McPhedran et al., 1965). Those motor units recruited first (i.e., with lower threshold) displayed slower axonal conduction velocities compared to higher threshold motor units that were recruited later.
The size principle has been validated in a variety of muscles (Gordon et al., 2004; Mendell, 2005), including the DIAm (Dick et al., 1987). It has also been shown that the order of motor unit recruitment matches the mechanical and fatigue properties of motor units such that type S units are recruited first, followed by type FR, FInt and FF units (Burke et al., 1973; Mendell, 2005; Sypert and Munson, 1981). In addition, the force contributed by a motor unit is a strong predictor of its recruitment order (Zajac and Faden, 1985), such that those units generating the least amount of force (e.g., type S and FR) are recruited before those units generating greater forces (e.g., type FInt and FF). During inspiratory efforts, DIAm motor units with slower conduction velocities are preferentially recruited (Jodkowski et al., 1987, 1988; Seven et al., 2013).
Sieck and Fournier (1989) developed a model of motor unit recruitment in the cat DIAm based on a progressive, orderly recruitment of type S, FR, FInt and FF units and their relative force contributions. DIAm forces were estimated based on measurements of transdiaphragmatic pressure (Pdi) across a range of ventilatory and non-ventilatory motor behaviors as well as bilateral phrenic nerve stimulation. In this model, the Pdi generated during quiet breathing (eupnea) was accomplished by recruitment of only type S and FR motor units. When breathing was stimulated by 10% O2 and 5% CO2 (hypoxic-hypercapnic ventilatory stimulus), Pdi increased to ∼40% of the maximum Pdi generated by bilateral phrenic nerve stimulation (Pdimax). This level of force was accomplished by the maximal recruitment of all type S and FR units. The Pdi generated during non-ventilatory behaviors was also assessed to estimate motor unit recruitment. During tracheal occlusion, Pdi increased to ∼60% of Pdimax and it was necessary to recruit all type S, FR and a portion of type FInt units to accomplish this force level. During coughing, Pdi approximated Pdimax necessitating full recruitment of all motor unit types. This model has been subsequently validated in hamsters and rats (Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1995). In these models, the mechanical properties of DIAm motor units were not directly measured but were approximated based on the following measurements: 1) specific force developed by type-identified muscle fibers (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999; Sieck, 1988b; Sieck and Fournier, 1989; Walmsley et al., 1978), 2) cross-sectional areas of type-identified muscle fibers (Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 1989b; Zhan et al., 1997), and 3) proportion of different fiber types (Enad et al., 1989; Fournier and Sieck, 1988; Sieck, 1988b; Sieck et al., 1989a; Sieck et al., 1996). Similar to the cat, ventilatory behaviors such as resting breathing and breathing in response to 10% O2 and 5% CO2 can be accomplished by recruitment of only type S and FR motor units. In agreement, a recent study in mice showed that Pdi generated during eupnea and hypoxia-hypercapnia was ∼10-12% of Pdimax (Greising et al., 2013b). More forceful, ventilatory and non-ventilatory behaviors, such as efforts against an occluded airway and sneezing induced by intranasal capsaicin, would require progressive recruitment of type FInt and FF units.
Across all species near maximal Pdi was generated during expulsive airway clearance behaviors (e.g., sneezing or coughing) (Mantilla et al., 2010; Sieck, 1991; Sieck, 1994; Sieck and Fournier, 1989) that require coordinated activation of the DIAm during an initial inspiratory phase followed by activation of abdominal muscles to generate the necessary intra-thoracic pressures to clear the airway. In the cough reflex, high levels of intra-abdominal and intra-thoracic pressures are generated during the subsequent compressive and expulsive phases (Milano et al., 1992; Rybak et al., 2008; Shannon et al., 1998). The recruitment of highly fatigable motor units restricts the performance of these expulsive behaviors to short-durations and relatively low incidence to avoid fatigue. Conditions that selectively affect type IIx and/or IIb fibers and the forces contributed by the corresponding type FInt and FF motor units will compromise the ability to perform behaviors important to airway clearance.
4. Diseases and Conditions Resulting in Selective DIAm Fiber Atrophy
4.1. Chronic Obstructive Pulmonary Disease (COPD)
In a hamster model of COPD using elastase treatment to induce emphysematous changes in the lung, there was a 30% increase in the cross-sectional area of type II DIAm fibers (Lewis et al., 1992b). Unfortunately, these investigators did not distinguish between type IIa, IIx or IIb fibers, but they did note that oxidative capacity (succinate dehydrogenase activity) increased across all fiber types. In addition, with an increase in functional residual capacity of the lung, the DIAm flattened and shortened. However, optimal DIAm fiber length also shortened as a result of a decrease in the number of sarcomeres in series; thereby preserving the ability to generate force (Mantilla and Sieck, 2013). Yet, the DIAm in COPD animals generated ∼25% lower maximum specific force at optimal sarcomere length (Lewis et al., 1992b). In a similar mouse model of emphysema, in which lung changes were induced by elastase treatment, a similar shortening of DIAm length was noted, but without a concomitant reduction in maximum specific force (Luthje et al., 2009).
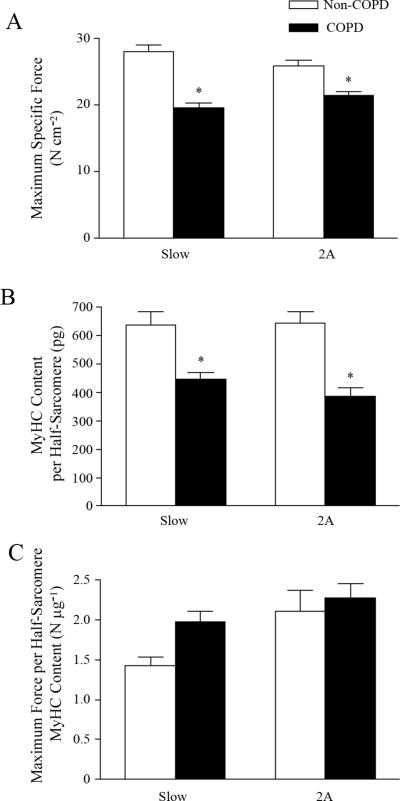
Several studies have examined DIAm biopsies obtained from moderate to severe COPD patients and compared DIAm fiber properties to those obtained from biopsies from age-matched controls (Decramer and Stas, 1992; Levine et al., 2003; Nguyen et al.,2005; Nguyen et al., 2000; Ottenheijm et al., 2006; Ottenheijm et al.,2005). Most striking is the apparent increase in the proportion of type I fibers in the COPD DIAm compared to controls, while the proportion of type IIx fibers decreases (Levine et al., 2012; Levine et al.,2002; Levine et al., 2003; Ottenheijm et al., 2006; Ottenheijm et al.,2005). However, clear distinction of DIAm fiber type in COPD patients is obscured due to the co-expression of MyHC isoforms (Nguyen et al., 2005; Nguyen et al., 2000; Stubbings et al., 2008). In COPD DIAm, fiber CSA is slightly increased across all fibers, but MyHC content per half-sarcomere volume is reduced (Levine et al., 2003; Ottenheijm et al., 2005). This decrease in MyHC content per half sarcomere likely accounts for the decrease in maximum specific force observed across all DIAm fibers from COPD patients (Levine et al., 2012; Levine et al., 2003; Ottenheijm et al., 2005). Indeed, normalizing force for MyHC content per half-sarcomere mitigated the differences in maximum specific force between COPD and control DIAm (Fig. 3) (Ottenheijm et al., 2005). Furthermore, the sensitivity of force generation to myoplasmic Ca2+ was reduced across all DIAm fibers in COPD patients compared to controls (Ottenheijm et al., 2005). This reduction in Ca2+ sensitivity of force generation in the COPD DIAm would affect the force-frequency response of motor units. In fact, changes in Ca2+ sensitivity may account for the higher discharge frequencies of DIAm motor units observed during eupnea in patients with severe COPD compared to controls (De Troyer et al., 1997; Mantilla and Sieck, 2013). Finally, cross-bridge cycling rate was slower in all DIAm fibers in COPD patients (Ottenheijm et al., 2005).
Figure 3.
A. Maximum specific force (force normalized per cross-sectional area) in single type-identified DIAm fibers expressing MyHC isoforms slow and 2A from patients with (black bars) and without (white bars) chronic obstructive pulmonary disease (COPD). Specific force is reduced across DIAm fiber types in COPD patients. B. In single DIAm fibers, MyHC content per half-sarcomere was determined from the volume of a half-sarcomere and electrophoretically-determined MyHC concentration. MyHC content per half-sarcomere is reduced across DIAm fiber types in COPD patients. C. Maximum specific force normalized by the MyHC content per half-sarcomere no longer differs across fiber types in the DIAm of patients with and without COPD. Data are mean ± SE; *p < 0.05, different from non-COPD group. Data from Ottenheijm et al. (2005).
4.2. Chronic Corticosteroid Treatment
A number of animal studies report that chronic treatment with systemic corticosteroids induces selective atrophy of type IIx and IIb DIAm fibers (Dekhuijzen et al., 1993; Fournier et al., 2003; Koerts-de Lang et al., 2000; Koerts-De Lang et al., 1998; Pellegrino et al., 2004; Sieck et al., 1999; Verheul et al., 2004). Systemic corticosteroid treatment may also result in weight loss and thus studies commonly compare corticosteroid effects with those induced by undernutrition (Dekhuijzen, 1995; Fournier et al., 2003; Koerts-de Lang et al., 2000; Koerts-De Lang et al., 1998; Lewis et al., 1986; Sieck et al., 1989b). Regardless of whether corticosteroid effects are compared to weight-matched animals as a result of pair-feeding (undernutrition) or younger age (van Balkom et al., 1997; Verheul et al., 2004), corticosteroid treatment substantially reduces DIAm fiber cross-sectional area in a fiber type selective manner – type IIx and/or IIb DIAm fibers are disproportionately affected. These morphological changes are also associated with mechanical alterations in DIAm fibers including a reduction of specific force and a slowing of cross-bridge cycling kinetics evident by a marked slowing of maximum shortening velocity and power output (van Balkom et al., 1997). Generally, type I and IIa DIAm fibers have slower maximum shortening velocities compared to type IIx and/or IIb fibers, consistent with their slower cross-bridge cycling kinetics and lower ATP hydrolysis rates (actomyosin ATPase activity) (Han et al., 2003; Sieck and Prakash, 1997; Sieck et al., 2003; Sieck et al., 1995). In agreement with a reduced contribution of more fatigable type IIx and IIb fibers to total DIAm fiber cross-sectional area, fatigue resistance generally increases after chronic corticosteroid treatment (Moore et al., 1989; van Balkom et al., 1997; Wilcox et al., 1989). Specific force either is unchanged (Dekhuijzen, 1995; Dekhuijzen et al., 1993; Lewis et al., 1992a) or reduced (van Balkom et al., 1997) after chronic corticosteroid treatment, possibly reflecting differences in the type, dose and duration of corticosteroid administration (Dekhuijzen et al., 1993; van Balkom et al., 1997).
4.3. Inactivity Induced by Cervical Spinal Cord Injury
Following a unilateral C2 spinal cord hemisection, descending excitatory premotor input to phrenic motoneurons is interrupted and DIAm motor units are inactivated (Goshgarian, 2003; Mantilla and Sieck, 2009). Unlike other models of DIAm paralysis (e.g., unilateral phrenicotomy), inactivity of DIAm motor units does not result in overt DIAm fiber atrophy following 2 weeks in rats (Miyata et al., 1995; Prakash et al., 1999; Prakash et al., 1995; Zhan et al., 1997). Furthermore, specific force is maintained for up to 2 weeks after C2 hemisection (Miyata et al., 1995). However, longer-term inactivity of DIAm motor units imposed by 6 weeks of C2 hemisection results in only modest atrophy (∼20%) of type IIx and/or IIb fibers (Mantilla et al., 2013). In addition, by 6 weeks after C2 hemisection, specific force is reduced to a greater extent (∼40% compared to uninjured control animals). Based on studies using single, type-identified rat DIAm fibers, which demonstrate fiber type-dependent differences in specific force with type I and IIa fibers exerting ∼50% of the force that type IIx and IIb fibers exert (Geiger et al., 2000), the anticipated reduction in total DIAm force would only be ∼14% of control after 6 weeks of C2 hemisection, with minimal (∼3%) changes in specific force. Clearly, when phrenic motoneurons are injured and lost (e.g., by contusion at the midcervical level), DIAm atrophy is evident (Nicaise et al., 2012a; Nicaise et al., 2012b). However, whether this atrophy is fiber type selective was not explored.
4.4. Mechanical Ventilation
In several studies involving rodents, controlled mechanical ventilation results in both rapid and dramatic reductions in DIAm specific force and fiber atrophy across all fiber types (Hudson et al., 2012; Levine et al., 2008; Mrozek et al., 2012; Powers et al., 2009; Powers et al., 2002; Sassoon et al., 2002; Whidden et al., 2010). Generalized DIAm fiber atrophy was reported following 12-18 hours of controlled mechanical ventilation, with reductions in DIAm fiber CSA of 15-45% in type I and IIa fibers and 20-30% in type IIx and/or IIb fibers (Hudson et al., 2012; McClung et al., 2007; Powers et al., 2002; Shanely et al., 2002; Whidden et al., 2010). In agreement, brain dead patients subjected to mechanical ventilation for periods between 18-69 hours also exhibited greater than 50% atrophy across all types of DIAm fibers, when compared to patients who were mechanically ventilated for 2-3 hours while undergoing lung surgery (Levine et al., 2008). Interestingly, DIAm specific force was reduced to a similar extent (∼20%) despite substantial differences in fiber atrophy following 12 vs. 18 hours of controlled mechanical ventilation (Hudson et al., 2012; McClung et al., 2007; Powers et al., 2002; Shanely et al., 2002; Whidden et al., 2010). Longer periods of controlled mechanical ventilation (24 to 72 hours) result in further reductions in DIAm force in both rats and rabbits (∼50%) (Le Bourdelles et al., 1994; Powers et al., 2002; Sassoon et al., 2002). Clearly, loss of DIAm force and fiber atrophy do not display a straightforward relationship (Sieck and Mantilla, 2008), and taken together, the results of these studies indicate that force loss following controlled mechanical ventilation may reflect different underlying mechanisms, distinct from those dependent exclusively on muscle inactivity. For example, it is difficult to attribute the effects of controlled mechanical ventilation to inactivity per se, since complete removal of activity and the influence of innervation (by phrenicotomy) results in DIAm fiber atrophy only after 7 days and even then only type IIx and/or IIb fibers are affected (Aravamudan et al., 2006; Lewis et al., 1996; Zhan et al., 1995). Moreover, with assist-mode ventilation where a small degree of neural activation triggers the mechanical ventilator, DIAm fiber atrophy is largely mitigated (Sassoon et al., 2002; Sassoon et al., 2004). Although studies examining the effects of controlled mechanical ventilation on DIAm structure and function have attempted to exclude the potential confounding influence of inflammation and sepsis, it is during such conditions that such rapid catabolic effects are observed (Ermilov et al., 2010; Supinski et al., 1999; Supinski and Callahan, 2006).
It is worth noting that recent studies suggest that corticosteroid treatment (usually high-dose, short duration) may mitigate the deleterious effects of controlled mechanical ventilation on DIAm structural and functional properties (Maes et al., 2010; Maes et al., 2008; Sassoon et al., 2011). The mechanisms underlying such an effect remain unclear but may include effects on lung injury (and the resultant systemic inflammatory response) or nutritional status in addition to direct effects on the DIAm.
5. Changing Motor Unit Contributions: Impact on Ventilatory and Non-Ventilatory Behaviors
Many diseases are associated with a selective atrophy of type IIx and/or IIb fibers. During ventilatory behaviors of the DIAm, only type S and FR motor units are recruited, consistent with the sustained nature of these motor tasks (e.g., a duty cycle of 30-40%) (Mantilla et al., 2010; Sieck, 1991; Sieck, 1994; Sieck and Fournier, 1989). However, a significant portion of the maximum force generating capacity of the DIAm is not necessary for ventilatory behaviors but is required to accomplish expulsive airway clearance behaviors. Thus, in diseases that selectively atrophy type IIx and/or IIb fibers and thereby impact the relative contribution of more fatigable fast-twitch motor units, the ability to perform these non-ventilatory behaviors may become impaired. This may account for the higher incidence of airway tract infections and pneumonias associated with diseases such as COPD or conditions such as spinal cord injury or even old age. As diseases progress and muscle fibers comprising more fatigue-resistant motor units display atrophy, the ability of the DIAm to generate forces required for normal ventilation may become impaired thereby affecting the ability of these patients to sustain ventilation during exercise and other activities requiring elevated efforts. Thus, there is a normal progression of the severity of these diseases with mortality being related to the inability to sustain ventilation during basal conditions.
5.1 Emphasis on DIAm Fatigue and Endurance is Misplaced
Many studies have emphasized the importance of DIAm fatigue and/or endurance of respiratory muscles in various diseases and have suggested therapeutic approaches to train the DIAm to ameliorate the ventilatory impairments associated with severe disease (e.g., respiratory training in COPD). First of all, it is important to distinguish between muscle fatigue and endurance. They are not the same but reflect different underlying mechanisms that affect motor performance. Fatigue is an intrinsic property of muscle fibers that relates to inability to sustain maximum force. At submaximal activation, muscle fibers may become less sensitive to Ca2+ activation; however, to be unambiguous this is referred to as a reduction in Ca2+ sensitivity of force generation and may not reflect any change in fatigability. With both fatigue and Ca2+ sensitivity there is a fiber type dependence. For example, in the DIAm type I and IIa fibers are fatigue-resistant (Enad et al., 1989; Fournier and Sieck, 1988; Sieck, 1988a; Sieck et al., 1989a; Sieck et al., 1996); i.e., display minimal decrements in maximum force with repetitive stimulation. Type I fibers display greater Ca2+ sensitivity compared to all type II fibers, which appear to have similar Ca2+ sensitivities (Geiger et al., 1999). The underlying mechanism may relate to phosphorylation of regulatory proteins such as troponin I or troponin T on the thin filament. In contrast, endurance relates to the time during which a certain level of force can be sustained. Determination of endurance does not typically require maximum force generation by a muscle, and thus the level of motor unit activation and the relative contributions of motor unit types are poorly controlled. As such, endurance is largely a descriptive measure of the ability of a muscle to sustain a motor task and it is difficult to ascribe unique underlying physiological mechanisms to endurance. Endurance may reflect varying neural and muscle mechanisms. Muscle fiber fatigue may contribute (peripheral fatigue), but impaired neural activation of muscle fibers (central or peripheral fatigue) may also contribute; thus, interpretation of changes in endurance is ambiguous and often complicated.
5.2. Therapeutic Implications
Many therapeutic interventions are targeted towards improving respiratory muscle fatigue resistance and endurance; e.g., respiratory muscle training. Unfortunately, the literature does not consistently address and recognize the important distinctions between muscle fatigue and endurance, as these measures are often confused. For example, with type IIx and IIb fiber atrophy, the relative contribution of these more fatigable muscle fibers is reduced with an apparent improvement in overall muscle fatigue resistance, yet the ability of the DIAm to perform essential non-ventilatory motor tasks is greatly impaired. Paradoxically, the ability of the DIAm to sustain ventilatory tasks (i.e., motor tasks) may be unaffected or impaired. Thus, therapies targeted at improving fatigue resistance (e.g., respiratory muscle training) may be off mark as diseases progress and may actually be counterproductive.
6. Concluding Remarks and Recommendations
It is obvious that in studying changes in DIAm structure and function under various disease and clinical conditions it is important to distinguish impact on different muscle fiber and motor unit types and how their relative contribution to force generation might be affected. Impact of disease progression on the DIAm appears to follow a selective impairment of more fatigable but less frequently activated motor units that are not required for sustained ventilatory behaviors and may thus be more expendable with respect to vital functions. However, these selective effects come at a cost, because of the resulting inability to generate the higher DIAm forces that are required for expulsive airway clearance behaviors, which likely results in an increased risk for airway infections. The emphasis on improving respiratory muscle fatigue resistance and endurance is misplaced since respiratory muscle weakness may be the culprit in the progression of disease. Improving muscle strength may have far greater beneficial effects. Future studies should directly address how atrophy and reduced specific forces of more fatigable, fast-twitch motor units (comprising type IIx and/or IIb fibers) can be ameliorated and/or reversed. Importantly, potential beneficial effects of any therapy may only be evident if a range of ventilatory and non-ventilatory behaviors is assessed. Forces generated during coughing, sneezing or even deep sighs will likely provide necessary insight into the therapeutic benefit of rehabilitative interventions.
Highlights.
Motor units display diverse contractile and fatigue properties
Muscle fiber atrophy and weakness may disproportionately affect specific fiber types
Various diseases result in selective atrophy of muscle fibers in the most fatigable motor units
Impact of muscle fiber atrophy on motor performance depends on affected motor unit types
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. 2006;100:1617–1622. doi: 10.1152/japplphysiol.01277.2005. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology and functional organization. In: Peachey LD, editor. Handbook of Physiology The Nervous System Motor Control. Am Physiol Soc; Bethesda, MD: 1981. pp. 345–422. [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol (London) 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol. 1999;518(Pt 3):907–920. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge frequencies of single motor units in human diaphragm and parasternal muscles in lying and standing. J Appl Physiol. 2001;90:147–154. doi: 10.1152/jappl.2001.90.1.147. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- Decramer M, Stas KJ. Corticosteroid-induced myopathy involving respiratory muscles in patients with chronic obstructive pulmonary disease or asthma. Am Rev Respir Dis. 1992;146:800–802. doi: 10.1164/ajrccm/146.3.800. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PNR. Corticosteroid treatment and nutritional deprivation cause a different pattern of atrophy in rat diaphragm. J Appl Physiol. 1995;78:629–637. doi: 10.1152/jappl.1995.78.2.629. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PNR, Gayan-Ramirez G, de Block V, Dom R, Decramer M. Triamcinolone and prednisolone affect contractile properties and histopathology of rat diaphragm differently. J Clin Invest. 1993;92:1534–1542. doi: 10.1172/JCI116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J Appl Physiol. 1989;67:620–627. doi: 10.1152/jappl.1989.67.2.620. [DOI] [PubMed] [Google Scholar]
- Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low dose inhaled CO. Am J Physiol Regul Integr Comp Physiol. 2010;298:R784–789. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Huang ZS, Li H, Da X, Cercek B, Lewis MI. Insulin-like growth factor I prevents corticosteroid-induced diaphragm muscle atrophy in emphysematous hamsters. Am J Physiol Regul Integr Comp Physiol. 2003;285:R34–43. doi: 10.1152/ajpregu.00177.2002. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol. 1988;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Gasser HS, Grundfest H. Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. Am J Physiol. 1939;127:393–414. [Google Scholar]
- Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol. 2002;92:1506–1514. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J Appl Physiol. 2001;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J Appl Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can J Physiol Pharmacol. 2004;82:645–661. doi: 10.1139/y04-081. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Plasticity in Respiratory Motor Control: Invited Review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013a doi: 10.1016/j.exger.2013.06.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. 2013b;188:56–59. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TM, Nemeth PM, Solanki L, Gordon DA, Reinking RM, Stuart DG. Association between biochemical and physiological properties in single motor units. Muscle Nerve. 1988;11:245–254. doi: 10.1002/mus.880110309. [DOI] [PubMed] [Google Scholar]
- Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–2196. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Olson CB. Relations between structure and function in the design of skeletal muscles. J Neurophysiol. 1965;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat's ankle muscles. Exp Brain Res. 1997;115:325–332. doi: 10.1007/pl00005701. [DOI] [PubMed] [Google Scholar]
- Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med. 2012;40:1254–1260. doi: 10.1097/CCM.0b013e31823c8cc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol Neurobiol. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Kernell D. The motoneurone and its muscle fibres. Oxford University Press Inc; New York: 2006. [Google Scholar]
- Koerts-de Lang E, Schols AM, Rooyackers OE, Gayan-Ramirez G, Decramer M, Wouters EF. Different effects of corticosteroid-induced muscle wasting compared with undernutrition on rat diaphragm energy metabolism. Eur J Appl Physiol. 2000;82:493–498. doi: 10.1007/s004210000231. [DOI] [PubMed] [Google Scholar]
- Koerts-De Lang E, Schols AM, Wouters EF, Gayan-Ramirez G, Decramer M. Contractile properties and histochemical characteristics of the rat diaphragm after prolonged triamcinolone treatment and nutritional deprivation. J Muscle Res Cell Motil. 1998;19:549–555. doi: 10.1023/a:1005364627467. [DOI] [PubMed] [Google Scholar]
- Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med. 1994;149:1539–1544. doi: 10.1164/ajrccm.149.6.8004310. [DOI] [PubMed] [Google Scholar]
- Levine S, Bashir MH, Clanton TL, Powers SK, Singhal S. COPD elicits remodeling of the diaphragm and vastus lateralis muscles in humans. J Appl Physiol. 2013;114:1235–1245. doi: 10.1152/japplphysiol.01121.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Gregory C, Nguyen T, Shrager J, Kaiser L, Rubinstein N, Dudley G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol. 2002;92:1205–1213. doi: 10.1152/japplphysiol.00116.2001. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, Rome LC, Dudley GA, Sieck GC, Shrager JB. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168:706–713. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. New Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- Lewis MI, LoRusso TJ, Zhan WZ, Sieck GC. Interactive effects of denervation and malnutrition on diaphragm structure and function. J Appl Physiol. 1996;81:2165–2172. doi: 10.1152/jappl.1996.81.5.2165. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. J Appl Physiol. 1992a;72:293–301. doi: 10.1152/jappl.1992.72.1.293. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC. Effect of acute nutritional deprivation on diaphragm structure and function. J Appl Physiol. 1990;68:1938–1944. doi: 10.1152/jappl.1990.68.5.1938. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC, Fournier M, Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol. 1986;60:596–603. doi: 10.1152/jappl.1986.60.2.596. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Zhan WZ, Sieck GC. Adaptations of the diaphragm in emphysema. J Appl Physiol. 1992b;72:934–943. doi: 10.1152/jappl.1992.72.3.934. [DOI] [PubMed] [Google Scholar]
- Liddell EGT, Sherrington CS. Recruitment and some other factors of reflex inhibition. Proc Roy Soc Lond (Biol) 1925;97:488–518. [Google Scholar]
- Luthje L, Raupach T, Michels H, Unsold B, Hasenfuss G, Kogler H, Andreas S. Exercise intolerance and systemic manifestations of pulmonary emphysema in a mouse model. Respir Res. 2009;10:7. doi: 10.1186/1465-9921-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes K, Agten A, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. Corticosteroid effects on ventilator-induced diaphragm dysfunction in anesthetized rats depend on the dose administered. Respir Res. 2010;11:178. doi: 10.1186/1465-9921-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes K, Testelmans D, Cadot P, Deruisseau K, Powers SK, Decramer M, Gayan-Ramirez G. Effects of acute administration of corticosteroids during mechanical ventilation on rat diaphragm. Am J Respir Crit Care Med. 2008;178:1219–1226. doi: 10.1164/rccm.200702-296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Greising SM, Zhan WZ, Seven YB, Sieck GC. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013;114:380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. 2011;179:57–63. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromotor Control in Chronic Obstructive Pulmonary Disease. J Appl Physiol. 2013;114:1246–1252. doi: 10.1152/japplphysiol.01212.2012. [DOI] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhedran AM, Wuerker RB, Henneman E. Properties of motor units in a homogeneous red muscle (soleus) of the cat. J Neurophysiol. 1965;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J Neurophysiol. 2005;93:3024–3026. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- Milano S, Grelot L, Bianchi AL, Iscoe S. Discharge patterns of phrenic motoneurons during fictive coughing and vomiting in decerebrate cats. J Appl Physiol. 1992;73:1626–1636. doi: 10.1152/jappl.1992.73.4.1626. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Moore BJ, Miller MJ, Feldman HA, Reid MJ. Diaphragm atrophy and weakness in cortisone-treated rats. J Appl Physiol. 1989;67:2420–2426. doi: 10.1152/jappl.1989.67.6.2420. [DOI] [PubMed] [Google Scholar]
- Mrozek S, Jung B, Petrof BJ, Pauly M, Roberge S, Lacampagne A, Cassan C, Thireau J, Molinari N, Futier E, Scheuermann V, Constantin JM, Matecki S, Jaber S. Rapid onset of specific diaphragm weakness in a healthy murine model of ventilator-induced diaphragmatic dysfunction. Anesthesiol. 2012;117:560–567. doi: 10.1097/ALN.0b013e318261e7f8. [DOI] [PubMed] [Google Scholar]
- Nemeth PM, Solanki L, Gordon DA, Hamm TM, Reinking RM, Stuart DG. Uniformity of metabolic enzymes within individual motor units. J Neurosci. 1986;6:892–898. doi: 10.1523/JNEUROSCI.06-03-00892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Rubinstein NA, Vijayasarathy C, Rome LC, Kaiser LR, Shrager JB, Levine S. Effect of chronic obstructive pulmonary disease on calcium pump ATPase expression in human diaphragm. J Appl Physiol. 2005;98:2004–2010. doi: 10.1152/japplphysiol.00767.2004. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Shrager J, Kaiser L, Mei L, Daood M, Watchko J, Rubinstein N, Levine S. Developmental myosin heavy chains in the adult human diaphragm: coexpression patterns and effect of COPD. J Appl Physiol. 2000;88:1446–1456. doi: 10.1152/jappl.2000.88.4.1446. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Hala TJ, Frank DM, Parker JL, Authelet M, Leroy K, Brion JP, Wright MC, Lepore AC. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol. 2012a;235:539–552. doi: 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Nicaise C, Putatunda R, Hala TJ, Regan KA, Frank DM, Brion JP, Leroy K, Pochet R, Wright MC, Lepore AC. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J Neurotrauma. 2012b;29:2748–2760. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, Labeit S, Granzier HL, Dekhuijzen PN. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MA, D'Antona G, Bortolotto S, Boschi F, Pastoris O, Bottinelli R, Polla B, Reggiani C. Clenbuterol antagonizes glucocorticoid-induced atrophy and fibre type transformation in mice. Exp Physiol. 2004;89:89–100. doi: 10.1113/expphysiol.2003.002609. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37:S347–353. doi: 10.1097/CCM.0b013e3181b6e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol. 2002;92:1851–1858. doi: 10.1152/japplphysiol.00881.2001. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Zhan WZ, Miyata H, Sieck GC. Adaptations of diaphragm neuromuscular junction following inactivity. Acta Anat. 1995;154:147–161. doi: 10.1159/000147762. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Rybak IA, O'Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, Shannon R, Dick TE, Dunin-Barkowski WL, Orem JM, Solomon IC, Morris KF, Lindsey BG. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100:1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol. 2002;92:2585–2595. doi: 10.1152/japplphysiol.01213.2001. [DOI] [PubMed] [Google Scholar]
- Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- Sassoon CS, Zhu E, Fang L, Ramar K, Jiao GY, Caiozzo VJ. Interactive effects of corticosteroid and mechanical ventilation on diaphragm muscle function. Muscle Nerve. 2011;43:103–111. doi: 10.1002/mus.21821. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol. 2013;185:400–409. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med. 2002;166:1369–1374. doi: 10.1164/rccm.200202-088OC. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84:2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: Structural and functional organization. In: Belman MJ, editor. Clinics in Chest Medicine, Respiratory Muscles: Function in Health and Disease. W. B. Saunders Company; Philadelphia, PA: 1988a. pp. 195–210. [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med. 1988b;9:195–210. [PubMed] [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- Sieck GC. Organization and recruitment of diaphragm motor units. In: Roussos C, editor. The Thorax. Second. Marcel Dekker; New York, NY: 1995. pp. 783–820. [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett. 1989a;97:29–34. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol. 1996;80:2179–2189. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol. 1989b;66:2196–2205. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. New Eng J Med. 2008;358:1392–1394. doi: 10.1056/NEJMe0801226. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Cross bridge kinetics in respiratory muscles. Eur Respir J. 1997;10:2147–2158. doi: 10.1183/09031936.97.10092147. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS, Han YS, Fang YH, Geiger PC, Zhan WZ. Changes in actomyosin ATP consumption rate in rat diaphragm muscle fibers during postnatal development. J Appl Physiol. 2003;94:1896–1902. doi: 10.1152/japplphysiol.00617.2002. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Van Balkom RH, Prakash YS, Zhan WZ, Dekhuijzen PN. Corticosteroid effects on diaphragm neuromuscular junctions. J Appl Physiol. 1999;86:114–122. doi: 10.1152/jappl.1999.86.1.114. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF. SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol. 1995;79:1629–1639. doi: 10.1152/jappl.1995.79.5.1629. [DOI] [PubMed] [Google Scholar]
- Stubbings AK, Moore AJ, Dusmet M, Goldstraw P, West TG, Polkey MI, Ferenczi MA. Physiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary disease. J Physiol. 2008;586:2637–2650. doi: 10.1113/jphysiol.2007.149799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CK, Mellen NM, Feldman JL. Intrinsic and extrinsic factors affecting phrenic motoneuronal excitability in neonatal rats. Brain Res. 1997;774:62–68. doi: 10.1016/s0006-8993(97)81688-5. [DOI] [PubMed] [Google Scholar]
- Supinski G, Stofan D, Callahan LA, Nethery D, Nosek TM, DiMarco A. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol. 1999;87:783–791. doi: 10.1152/jappl.1999.87.2.783. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Caspase activation contributes to endotoxin-induced diaphragm weakness. J Appl Physiol. 2006;100:1770–1777. doi: 10.1152/japplphysiol.01288.2005. [DOI] [PubMed] [Google Scholar]
- Sypert GW, Munson JB. Basis of segmental motor control: Motoneuron size or motor unit type? Neurosurg. 1981;8:608–621. doi: 10.1227/00006123-198105000-00020. [DOI] [PubMed] [Google Scholar]
- van Balkom RHH, Zhan WZ, Prakash YS, Dekhuijzen PNR, Sieck GC. Corticosteroid effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol. 1997;83:1062–1067. doi: 10.1152/jappl.1997.83.4.1062. [DOI] [PubMed] [Google Scholar]
- Verheul AJ, Mantilla CB, Zhan WZ, Bernal M, Dekhuijzen PN, Sieck GC. Influence of corticosteroids on myonuclear domain size in the rat diaphragm muscle. J Appl Physiol. 2004;97:1715–1722. doi: 10.1152/japplphysiol.00625.2003. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108:1376–1382. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox PG, Hards JM, Bockhold K, Bressler B, Pardy RL. Pathologic changes and contractile properties of the diaphragm in corticosteroid myopathy in hamsters: comparison to peripheral muscle. Am J Respir Cell Mol Biol. 1989;1:191–199. doi: 10.1165/ajrcmb/1.3.191. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Faden JS. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J Neurophysiol. 1985;53(5):1303–1322. doi: 10.1152/jn.1985.53.5.1303. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Farkas GA, Schroeder MA, Gosselin LE, Sieck GC. Regional adaptations of rabbit diaphragm muscle fibers to unilateral denervation. J Appl Physiol. 1995;79:941–950. doi: 10.1152/jappl.1995.79.3.941. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]