Abstract
Drotrecogin alpha activated (DAA), trade name Xigris, is a recombinant human protein C that has been the subject of controversy since 2001 when it became the first biologic agent approved for the treatment of severe sepsis and septic shock. The PROWESS trial showed a 6.1% absolute reduction in 28-day mortality although these findings were not replicated in later trials, ultimately leading to the withdrawal of DAA in 2011. Observational trials, however, have consistently shown a mortality benefit with the use of DAA, leading to the questions, did DAA truly fail and if so, why? While these questions may never be definitively answered based on available evidence, several factors may explain the conflicting results.. In clinical practice, DAA may have been preferentially given to subjects more likely to survive. Contemporary treatments including early antibiotic administration and volume resuscitation may have mitigated the inflammatory processes leading to disordered coagulation and microvascular thrombosis and thus reduced or abolished the therapeutic opportunity for DAA. Later randomized clinical trials of DAA focused on the clinical phenotype of refractory shock largely due to a strong efficacy signal in this subset from PROWESS; however, this clinical phenotype may not be tightly linked, at least after contemporary early resuscitation strategies, to the mechanistic phenotype of dysregulated coagulation that may have been a better target for DAA. Future trials of biologic therapies in severe sepsis and septic shock should use a combination of clinical phenotype and biomarkers to identify responsive populations that may benefit from such therapies.
Keywords: Sepsis, septic shock, Systemic inflammatory response syndrome, Drotrecogin alfa activated, Protein C
Sepsis is a clinical syndrome of systemic inflammation caused by infection, and can range in severity depending on the presence of shock and organ failure.(1) It remains a worldwide problem with over 750,000 cases of sepsis reported annually in the United States,(2) and accounts for over 25% of intensive care unit admissions in Europe.(3) Historically, mortality rates for patients with severe sepsis or septic shock have been as high as 60%.(4) While contemporary treatments have improved mortality rates,(5, 6) a substantial number of patients with sepsis still die.
In 2001, enthusiasm for the use of biologic therapy for sepsis peaked with the approval of drotrecogin alpha activated (DAA), trade name Xigris, a recombinant human activated protein C (rhAPC). These regulatory decisions were based on the results of the PROWESS trial which demonstrated that treatment with DAA led to a 6.1% absolute risk reduction in 28-day mortality in patients with severe sepsis or septic shock as compared to placebo.(7) rhAPCs appear to modify disease course through modulation of dysregulated coagulation and subsequent microvascular thrombosis as well as additional anti-inflammatory effects.(8–10) DAA was quickly adopted into clinical practice, although debate regarding its efficacy began almost immediately due to several issues. First, the PROWESS trial was terminated early as it met a priori stopping criteria for efficacy. Second, a study amendment was made midway through the trial. The amendment included changes in the inclusion and exclusion criteria for study enrollment, a change in the placebo used (saline vs. albumin), and changes in the formulation of the study drug. While there was no benefit observed for DAA prior to the study amendment, after the study amendment the results favored the use of DAA.(11)
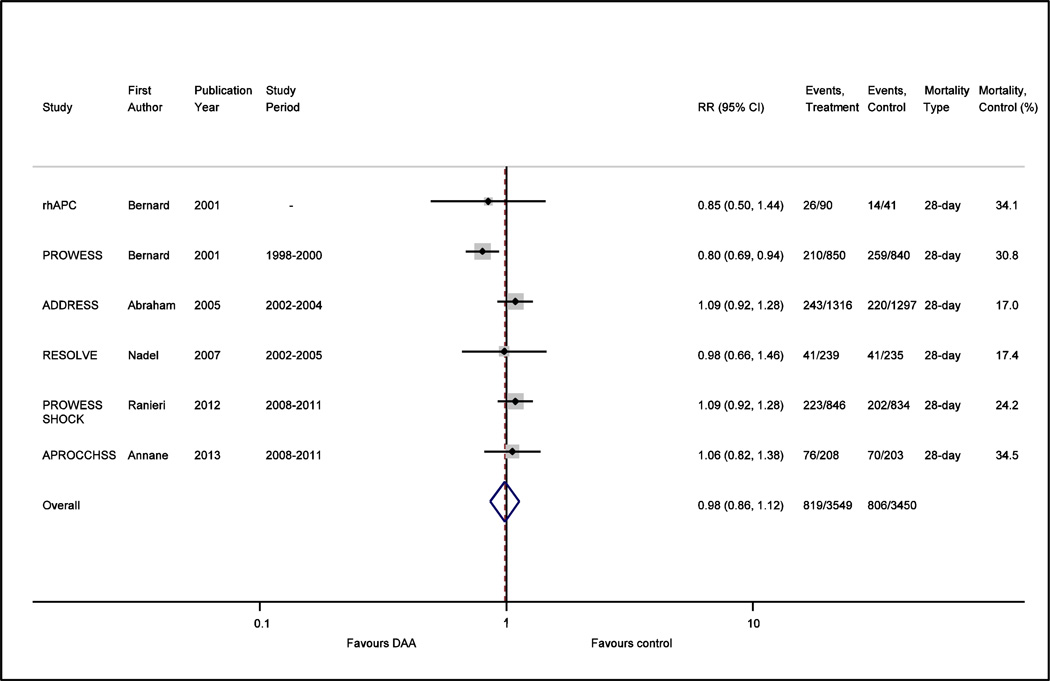
Due to lingering questions regarding the efficacy of DAA, especially in subgroups that were less acutely ill, the ADDRESS trial was performed focusing on adults with a lower risk of death from severe sepsis (APACHE II scores <25 or single organ failure) and showed no benefit.(12) Subsequent trials including a pediatric trial(13) and a trial using an extended infusion of DAA(14) also showed no benefit. Given the rising controversy, the PROWESS-SHOCK trial(15) was designed to provide a definitive answer in the era of modern critical care by randomizing adults with persistent septic shock after protocol-specified early volume resuscitation to either DAA or placebo. On October 2011, Eli Lilly withdrew DAA from the market(16) based on the preliminary results from PROWESS-SHOCK that showed no effect on 28 day all-cause mortality. At the time, there was an ongoing multicenter study performed in French intensive care units(17) assessing the benefit of DAA with and without low dose corticosteroids in patients with vasopressor dependent septic shock (APROCCHSS). APROCCHSS was prematurely terminated with the withdrawal of DAA from the market although it too showed no mortality benefit when data from already enrolled patients was analyzed (see Figure 1).
Figure 1.
Forest plot comparing the effect of DAA vs. placebo on risk ratio (RR) for 28-day all-cause mortality in all placebo controlled randomized clinical trials of DAA for severe sepsis and septic shock.
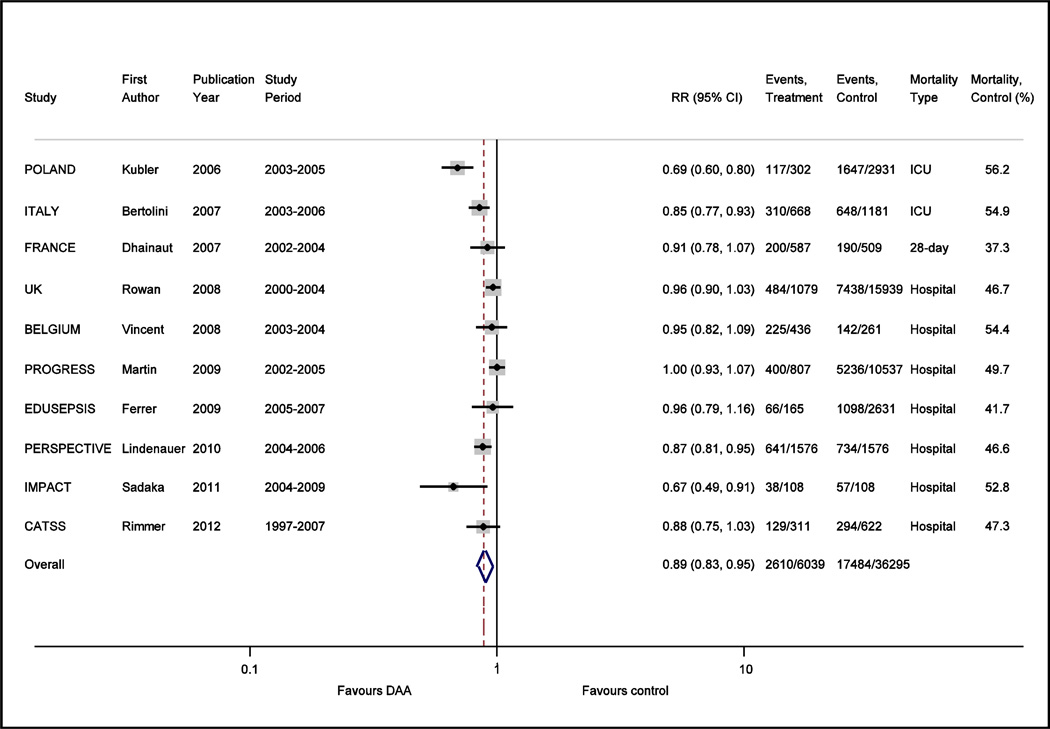
The controversy continued, however, with the publication of a number of observational trials based on large numbers of patients which argued that “real life” use of DAA consistently shows a mortality benefit (see Figure 2).(18–26) This was further reinforced by a meta-analysis (27) that focused on observational trials and found that in these patients, hospital mortality was reduced by 18% with DAA use; this effect estimate was similar to that observed for PROWESS. Metaregression suggested that increased mortality in the control arm and more severe disease as defined by the APACHE II score were associated with DAA benefit; this is in contrast to a meta-analysis of randomized placebo-controlled trials that showed no mortality benefit to the use of DAA.(28) Thus the question remains; is DAA beneficial in patients with severe sepsis and septic shock? If so, why did PROWESS demonstrate a mortality benefit while later randomized placebo controlled trials did not, and likewise, why do observational trials consistently identify a mortality benefit when randomized trials do not?
Figure 2.
Forest plot comparing the unadjusted effect of DAA vs. control on risk ratio (RR) for mortality (ICU, hospital, or 28-day as specified) in all observational trials which included a comparison arm for DAA in studies on severe sepsis and septic shock.
From a strictly epidemiologic perspective, when observational trials disagree with randomized clinical trials, two major reasons are cited. First, only randomized clinical trials are able to account for unknown risk factors which can be controlled for by randomization alone. Even when observational studies are of high quality and known confounders are carefully controlled for using multiple methods with consistent results, this remains an issue. In critical care, such a factor can be confounding by indication, where patients thought to be more likely to benefit from an intervention are also more likely to receive it. In this setting it may be impossible to detect indication bias, even after the propensity to use the intervention is adjusted for. Thus observational studies may not be able to determine whether the association found with treatment is due to the effect of treatment, or due to underlying and often unmeasured patient characteristics that led clinicians to use the intervention.
Studies in the use of pulmonary artery catheters (PACs) serve as an enlightening example. In the 1990s, PACs were in widespread use in critically ill patients despite a lack of randomized clinical trials demonstrating efficacy due to the belief that they were essential to the care of critically ill patients. Connors et al performed a study evaluating the use of pulmonary artery catheters using high quality data gathered from the observational SUPPORT trial. (29) Both propensity score based matching and multivariate regression demonstrated an association between PAC use and increased risk of death. This finding spurred five large multicenter randomized controlled trials to determine whether PACs were associated with increased mortality; all randomized trials found no evidence of harm.(30–34) It is only in retrospect that we can conclude that the findings of Connors et al were biased, likely because of confounding by indication. Physician judgment that led to the placement of a PAC to guide therapy was not well captured in standard variables collected for SUPPORT, and sicker patients were more likely to receive PACs. This propensity was not captured even by traditional measures of disease severity such as APACHE II scores, underscoring the difficulty observational trials or databases have in capturing this element. The “missed” effect size can be quite large. As an example, a recent observational study of patients with H1N1 who were mechanically ventilated found that prone positioning was associated with a 4.07 increased odds of hospital mortality even after adjusting for severity of illness.(35) Yet, a subsequent randomized controlled trial found that early prone positioning in ARDS led to a 16.8% absolute reduction in 28-day mortality and 17.4% absolute reduction in 90-day mortality, again demonstrating the difficulty in disentangling the effect of treatment from underlying patient characteristics in observational trials.
In the case of DAA, if unmeasured confounding accounts for the divergent results seen between randomized clinical trials and observational trials, then the subjects who received DAA in observational studies would be more likely to survive than those who did not. DAA has been estimated to add up to $16,000 to overall costs per patient treated,(36) and one could argue that in clinical practice, given the known high cost of treatment, physicians may have consciously or subconsciously selected subjects more likely to survive in order to justify the use of an expensive intervention. One international multi-center observational study described patients who received DAA in clinical practice as being younger with fewer co-morbidities(22) suggesting that such a selection process did indeed occur with real life use of DAA.
However, unmeasured confounding would not explain why early randomized clinical trials with DAA showed a benefit while later ones did not. A second factor cited in discrepant results between randomized trials vs. observational trials relates to the limited generalizability of study populations enrolled in clinical trials. It is apparent that the mortality rate of the control arms in clinical trials is substantially lower than that reported in observational trials (see Figure 1 vs. Figure 2); on average 23.4% vs. 48.2%. Although heterogeneity exists in the reporting of type of mortality (ICU vs. hospital vs. 28 day mortality) in observational trials, it would be difficult to imagine that this heterogeneity in reporting can account for a doubling of the mortality rate seen in observational trials as compared to clinical trials. One potential explanation for the large differences in the baseline mortality may be due to changes in contemporary care of sepsis. In 2001, the same year that PROWESS was published, Rivers et al demonstrated that with early goal directed therapy, hospital mortality decreased from 46.5 to 30.5% in patients with severe sepsis or septic shock.(37) Relevant to the question of DAA, subjects receiving early goal directed therapy had a lower prothrombin time, d-dimer, and concentration of fibrin splitproducts in the six to 72 hour interval following the early resuscitation intervention. This raises the question of whether favorable modulation of the coagulation system with early resuscitation may have reduced or eliminated the potential benefit of DAA. Through the efforts of the surviving sepsis campaign, early identification of severe sepsis, adherence to early volume resuscitation, and early appropriate antibiotic therapy has been slowly adopted over the years.(5, 6) An observational study recently demonstrated that the odds ratio of death decreased by 4% a year from 1997 to 2007; this was accompanied by a decrease in the time to administration of appropriate antibiotics.(26) This study also found that while DAA was associated with a 6.1% absolute reduction in 30-day mortality in patients with septic shock, the mortality benefit was confined to the subgroup who received delayed antibiotics. No effect of DAA was seen in subjects who received antibiotics within 6 hours of shock onset when DAA treated patients were compared to propensity matched controls.
Further supporting the idea that early fluid resuscitation and appropriate antibiotic administration may have modulated the potential benefit of DAA comes from looking at these measures in PROWESS-SHOCK and APROCCHSS. These are the only two clinical trials in DAA which reported the timeliness and appropriateness of antibiotic administration and volume resuscitation. An important inclusion criterion of PROWESS-SHOCK was the requirement for at least 30 ml/kg of intravenous fluids early in the course. Further, patients in PROWESS-SHOCK were treated with antibiotics a median of 2.5 hours prior to shock onset, subsequently determined to have received initial appropriate antibiotics 84% of the time, and source control was judged to be adequate in 90% of subjects who needed it. These measures may have accounted for the lower than expected mortality rate of the control arm in PROWESS-SHOCK as compared to PROWESS (24.2% vs. 30.3%). Although the mortality of the control arm in APROCCHSS approached PROWESS at 34.5%, like PROWESS-SHOCK an inclusion criterion was the presence of vasopressor dependent shock despite adequate volume resuscitation; subjects received on average 1,626 ml of crystalloid and or 909 ml of colloid and median time from onset of infection to initial antibiotics was reported as 0 (mean 1±7) hours. Therefore both PROWESS-SHOCK and APROCCHSS was performed in subjects who received early goal directed therapy and antibiotics. Early goal directed therapy in these clinical trials, which likely was not or was only variably implemented in patient care in the observational trials (based on the included study periods, Figure 2), may have reduced or possibly eliminated the potential benefit of DAA.
An additional factor that may explain the striking difference in mortality rates between the randomized and observational trials in DAA may simply relate to the population studied. Was there in fact a responsive subset receiving DAA in clinical practice that was systematically excluded from clinical trials? While all clinical trials with DAA excluded subjects with significant liver disease, dialysis dependent renal failure, recent use of high dose aspirin, glycoprotein IIb/IIIa antagonists, warfarin or non-prophylactic doses of heparin, these were relative contraindications for use in clinical practice and left to the discretion of the treating physician. Off-label use of DAA has been reported to be over 10% in one observational study(19) although it most frequently pertained to delayed initiation of DAA. Use of DAA in those with coagulopathy has been associated with increased mortality in a Veterans Administration study (38) although the sample size of this study was small. Patients with a significant coagulopathy were, however, excluded from PROWESS as well, and yet PROWESS showed a mortality benefit with the use of DAA.
It is also important to note that both PROWESS-SHOCK and APROCCHSS both focused on vasopressor dependent shock, in part due to the observation that this subgroup appeared to be particularly responsive to DAA in PROWESS.(7) While this clinical phenotype of persistent septic shock was thought to be the “optimal” clinical phenotype in which to test the efficacy of DAA 12 years ago, it is not clear that patients with persistent septic shock, especially after early resuscitation, uniformly experience dysregulation of coagulation or inflammation. Consider that only 40% of patients in PROWESS-SHOCK had protein C activity less than 40% even though the median norepinephrine dose was 21–24 mcg/min approximately 17 hours after shock onset and after nearly 4 liters of fluid was administered. While it remains unclear whether low protein C activity is a good biomarker to identify patients likely to benefit from DAA (as a predefined subgroup analysis in PROWESS-SHOCK did not show evidence of benefit in those with protein C deficiency), this example shows the disconnect between Protein C activity as a potential biomarker and the septic shock phenotype targeted in PROWESS-SHOCK.(39)
It is possible then that the wrong subgroup of patients was selected for later definitive trials to assess the efficacy of DAA. In retrospect, the regulatory requirement to study lower disease severity subjects in ADDRESS, a subgroup with a very tenuous link to DAA responsiveness, was probably a mistake. A confirmatory trial, perhaps combined with a biomarker discovery aim, would have been a more sensible approach. Potential biomarkers for DAA responsiveness that could have been tested were elevated plasma levels of D-dimer, IL-6,(7) or microvascular alterations visualized by orthogonal polarized spectroscopy (OPS).(40) The development and regulatory pathway tilted toward testing in subpopulations defined by clinical scores (APACHE and sequential organ failure scores) or use of catecholamines for shock. As noted above, these phenotypes are not necessarily linked to a pathophysiologic process or processes that would be expected to predict DAA responsiveness. However, this path of development was probably necessary to define the indicated population given the regulatory climate of the time. Future drug development for patients with severe sepsis should be more strongly tied to subpopulations with the specific pathophysiologic alterations targeted by the new therapies. Such targeting will almost certainly require biomarkers.
In conclusion, we return to the past. It has long been recognized that sepsis clinical phenotypes, including “systemic inflammatory response syndrome” have substantial limitations and are not strongly linked to underlying pathophysiologic pathways or alterations. Twenty six years ago, after yet another failed sepsis trial, Roger Bone referred to the troubled 17th century baroque innkeeper Don Quixote, who returned to his senses and his home after attacking windmills masquerading in his mind as giants.(41) Dr. Bone lamented, “In sepsis, we have had a similar quixotic adventure with a simplistic and elementary understanding of the pathogenesis of sepsis. We tilted at windmills by trying to block "evil humors" with magic potions…. We are now searching for the imaginary "magic bullet" without even a semblance of a homogeneous patient population under the categoric definition "sepsis syndrome.” I hope we will return home to our senses as did Don Quixote.” We hope so too.
Acknowledgments
Taylor Thompson has been a board member for Astra Zeneca; a consultant for Sirius Genetics, Immunetrics, and Lilly; and has received grant funding from NHLBI.
Abbreviations
- DAA
Drotrecogin alpha activated
- OPS
orthogonal polarized spectroscopy
- PAC
pulmonary artery catheter
- rhAPC
Recombinant human activated protein C
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Peggy S. Lai declares no conflict of interest.
Contributor Information
Peggy S. Lai, Pulmonary and Critical Care Unit, Department of Medicine, Massachusetts General Hospital, Boston, MA USA.
B. Taylor Thompson, Pulmonary and Critical Care Unit, Department of Medicine, Massachusetts General Hospital, Boston, MA USA.
REFERENCES
- 1.Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. Journal of global health. 2012 Jun;2(1):10404. doi: 10.7189/jogh.02.010404. PubMed PMID: 23198133. Pubmed Central PMCID: 3484761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical care medicine. 2001 Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. PubMed PMID: 11445675. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006 Feb;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. PubMed PMID: 16424713. Epub 2006/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 4.Russell JA. Management of sepsis. N Engl J Med. 2006 Oct 19;355(16):1699–1713. doi: 10.1056/NEJMra043632. PubMed PMID: 17050894. Epub 2006/10/20. eng. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008 May 21;299(19):2294–2303. doi: 10.1001/jama.299.19.2294. PubMed PMID: 18492971. Epub 2008/05/22. eng. [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Critical care medicine. 2010 Feb;38(2):367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. PubMed PMID: 20035219. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. New England Journal of Medicine. 2001 Apr 08;344(10):699–709. doi: 10.1056/NEJM200103083441001. English. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, van der Poll T. Recombinant human activated protein C: current insights into its mechanism of action. Critical care. 2007;11(Suppl 5):S3. doi: 10.1186/cc6154. PubMed PMID: 18269690. Pubmed Central PMCID: 2230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002 May;30(5 Suppl):S288–S293. doi: 10.1097/00003246-200205001-00019. PubMed PMID: 12004250. Epub 2002/05/11. eng. [DOI] [PubMed] [Google Scholar]
- 10.Schouten M, van 't Veer C, Roelofs JJ, Gerlitz B, Grinnell BW, Levi M, et al. Recombinant activated protein C attenuates coagulopathy and inflammation when administered early in murine pneumococcal pneumonia. Thromb Haemost. 2011 Dec;106(6):1189–1196. doi: 10.1160/TH11-06-0438. PubMed PMID: 21901240. Epub 2011/09/09. eng. [DOI] [PubMed] [Google Scholar]
- 11.Committee A-IA. FDA briefing document: drotrecogin alfa (activated)[recombinant human activated protein C (rhAPC)] Xigris, BLA# 125029/0. Rockville, Md: Food and Drug Administration; 2001. Sep, p. 12. [Google Scholar]
- 12.Abraham E, Laterre P-F, Garg R, Levy H, Talwar D, Trzaskoma BL, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. The New England journal of medicine. 2005 Sep 29;353(13):1332–1341. doi: 10.1056/NEJMoa050935. English. [DOI] [PubMed] [Google Scholar]
- 13.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Apr 10;369(9564):836–843. doi: 10.1016/S0140-6736(07)60411-5. English. [DOI] [PubMed] [Google Scholar]
- 14.Dhainaut JF, Antonelli M, Wright P, Desachy A, Reignier J, Lavoue S, et al. Extended drotrecogin alfa (activated) treatment in patients with prolonged septic shock. Intensive Care Medicine. 2009 Apr 05;35(7):1187–1195. doi: 10.1007/s00134-009-1436-1. English. [DOI] [PubMed] [Google Scholar]
- 15.Ranieri VM, Thompson BT, Barie PS, Dhainaut J-F, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. The New England journal of medicine. 2012 May 22;366(21):1–10. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed May 9, 2012];Lilly Announces Withdrawal of Xigris® Following Recent Clinical Trial. http://newsroomlillycom/releasedetailcfm?ReleaseID=617602.
- 17.Annane D, Timsit JF, Megarbane B, Martin C, Misset B, Mourvillier B, et al. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2013 May 15;187(10):1091–1097. doi: 10.1164/rccm.201211-2020OC. PMID: 23525934. [DOI] [PubMed] [Google Scholar]
- 18.Kubler A, Mayzner-Zawadzka E, Durek G, Gaszynski W, Karpel E, Mikaszewska-Sokolewicz M, et al. Results of severe sepsis treatment program using recombinant human activated protein C in Poland. Medical science monitor : international medical journal of experimental and clinical research. 2006 Mar;12(3):CR107–CR112. PubMed PMID: 16501420. Epub 2006/02/28. eng. [PubMed] [Google Scholar]
- 19.Bertolini G, Rossi C, Anghileri A, Livigni S, Addis A, Poole D. Use of Drotrecogin alfa (activated) in Italian intensive care units: the results of a nationwide survey. Intensive Care Med. 2007 Mar;33(3):426–434. doi: 10.1007/s00134-007-0554-x. PubMed PMID: 17325836. [DOI] [PubMed] [Google Scholar]
- 20.Rowan KM, Welch CA, North E, Harrison DA. Drotrecogin alfa (activated): real-life use and outcomes for the UK. Critical care. 2008;12(2):R58. doi: 10.1186/cc6879. PubMed PMID: 18430215. Pubmed Central PMCID: PMC2447613. Epub 2008/04/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent JL, Laterre PF, Decruyenaere J, Spapen H, Raemaekers J, Damas F, et al. A registry of patients treated with drotrecogin alfa (activated) in Belgian intensive care units--an observational study. Acta clinica Belgica. 2008 Jan-Feb;63(1):25–30. doi: 10.1179/acb.2008.004. PubMed PMID: 18386762. Epub 2008/04/05. eng. [DOI] [PubMed] [Google Scholar]
- 22.Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, et al. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Critical care. 2009;13(3):R103. doi: 10.1186/cc7936. PubMed PMID: 19566927. Pubmed Central PMCID: 2717475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrer R, Artigas A, Suarez D, Palencia E, Levy MM, Arenzana A, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009 Nov 1;180(9):861–866. doi: 10.1164/rccm.200812-1912OC. PubMed PMID: 19696442. Epub 2009/08/22. eng. [DOI] [PubMed] [Google Scholar]
- 24.Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS. Activated protein C and hospital mortality in septic shock: a propensity-matched analysis. Critical care medicine. 2010 Apr;38(4):1101–1107. doi: 10.1097/CCM.0b013e3181d423b7. PubMed PMID: 20154607. Epub 2010/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 25.Sadaka F, O'Brien J, Migneron M, Stortz J, Vanston A, Taylor RW. Activated protein C in septic shock: a propensity-matched analysis. Critical care. 2011;15(2):R89. doi: 10.1186/cc10089. PubMed PMID: 21385410. Pubmed Central PMCID: PMC3219349. Epub 2011/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rimmer E, Kumar A, Doucette S, Marshall J, Dial S, Gurka D, et al. Activated protein C and septic shock: a propensity-matched cohort study*. Critical care medicine. 2012 Nov;40(11):2974–2981. doi: 10.1097/CCM.0b013e31825fd6d9. PubMed PMID: 22932397. Epub 2012/08/31. eng. [DOI] [PubMed] [Google Scholar]
- 27.Kalil AC, LaRosa SP. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012 Sep;12(9):678–686. doi: 10.1016/S1473-3099(12)70157-3. PubMed PMID: 22809883. [DOI] [PubMed] [Google Scholar]
- 28.Lai PS, Matteau A, Iddriss A, Hawes JC, Ranieri V, Thompson BT. An updated meta-analysis to understand the variable efficacy of drotrecogin alfa (activated) in severe sepsis and septic shock. Minerva anestesiologica. 2013 Jan;79(1):33–43. PubMed PMID: 23174922. [PMC free article] [PubMed] [Google Scholar]
- 29.Connors AF, Jr, Speroff T, Dawson NV, Thomas C, Harrell FE, Jr, Wagner D, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA : the journal of the American Medical Association. 1996 Sep 18;276(11):889–897. doi: 10.1001/jama.276.11.889. PubMed PMID: 8782638. [DOI] [PubMed] [Google Scholar]
- 30.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. The New England journal of medicine. 2006 May 25;354(21):2213–2224. doi: 10.1056/NEJMoa061895. PubMed PMID: 16714768. [DOI] [PubMed] [Google Scholar]
- 31.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2003 Nov 26;290(20):2713–2720. doi: 10.1001/jama.290.20.2713. PubMed PMID: 14645314. [DOI] [PubMed] [Google Scholar]
- 32.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005 Aug 6–12;366(9484):472–477. doi: 10.1016/S0140-6736(05)67061-4. PubMed PMID: 16084255. [DOI] [PubMed] [Google Scholar]
- 33.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005 Oct 5;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. PubMed PMID: 16204662. [DOI] [PubMed] [Google Scholar]
- 34.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003 Jan 2;348(1):5–14. doi: 10.1056/NEJMoa021108. PubMed PMID: 12510037. [DOI] [PubMed] [Google Scholar]
- 35.Estenssoro E, Rios FG, Apezteguia C, Reina R, Neira J, Ceraso DH, et al. Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010 Jul 1;182(1):41–48. doi: 10.1164/201001-0037OC. PubMed PMID: 20203241. [DOI] [PubMed] [Google Scholar]
- 36.Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al. Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris) for the treatment of severe sepsis in adults: a systematic review and economic evaluation. Health technology assessment. 2005 Mar;9(11):1–126. iii–iv. doi: 10.3310/hta9110. PubMed PMID: 15774234. [DOI] [PubMed] [Google Scholar]
- 37.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine. 2001 Nov 08;345(19):1368–1377. doi: 10.1056/NEJMoa010307. English. [DOI] [PubMed] [Google Scholar]
- 38.Gentry CA, Gross KB, Sud B, Drevets DA. Adverse outcomes associated with the use of drotrecogin alfa (activated) in patients with severe sepsis and baseline bleeding precautions. Crit Care Med. 2009 Jan;37(1):19–25. doi: 10.1097/CCM.0b013e318192843b. PubMed PMID: 19050637. [DOI] [PubMed] [Google Scholar]
- 39.Shorr AF, Bernard GR, Dhainaut JF, Russell JR, Macias WL, Nelson DR, et al. Protein C concentrations in severe sepsis: an early directional change in plasma levels predicts outcome. Critical care. 2006;10(3):R92. doi: 10.1186/cc4946. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013 Mar;41(3):791–799. doi: 10.1097/CCM.0b013e3182742e8b. PubMed PMID: 23318492. [DOI] [PubMed] [Google Scholar]
- 41.Bone RC. Sepsis clinical trials. Don Quixote revisited. Chest. 1995 Feb;107(2):298–299. doi: 10.1378/chest.107.2.298. PubMed PMID: 7842747. [DOI] [PubMed] [Google Scholar]