Abstract
Background
Antibody-mediated rejection (AMR) has been associated with poor outcome after heart transplantation. The diagnosis of AMR usually includes endomyocardial biopsy findings of endothelial cell swelling, intravascular macrophages, C4d+ staining, and associated left ventricular dysfunction. The significance of AMR findings in biopsy specimens of asymptomatic heart transplant patients (normal cardiac function and no symptoms of heart failure) is unclear.
Methods
Between July 1997 and September 2001, AMR was found in the biopsy specimens of 43 patients. Patients were divided into 2 groups: asymptomatic AMR (AsAMR, n = 21) and treated AMR (TxAMR with associated left ventricular dysfunction, n = 22). For comparison, a control group of 86 contemporaneous patients, without AMR, was matched for age, gender, and time from transplant. Outcomes included 5-year actuarial survival and development of cardiac allograft vasculopathy (CAV). Patients were considered to have AMR if they had ≥ 1 endomyocardial biopsy specimen positive for AMR.
Results
The 5-year actuarial survival for the AsAMR (86%), TxAMR (68%), and control groups (79%) was not significantly different (p = 0.41). Five-year freedom from CAV (≥ 30% stenosis in any vessel) was AsAMR, 52%; TxAMR, 68%; and control, 79%. Individually, freedom from CAV was significantly lower in the AsAMR group compared with the control group (p = 0.02). There was no significant difference between AsAMR vs TxAMR and TxAMR vs control for CAV.
Conclusions
Despite comparable 5-year survival with controls after heart transplantation, AsAMR rejection is associated with a greater risk of CAV. Trials to treat AsAMR to alter outcome are warranted.
Although advances in heart transplant immunosuppression flourished in the 1990s, which lowered the incidence of acute cellular rejection, the incidence of antibody-mediated rejection (AMR) remained relatively unaffected.1,2 The problem of AMR remains unsolved because standardized schemes for diagnosis and treatment remain contentious, and current immunosuppressive regimens are largely intended to interfere in T-cell signaling pathways.3 Accordingly, AMR manifests in approximately 10% to 20% of heart transplant patients and has been associated with poor outcome, including greater development of CAV, increased incidence for hemodynamic compromise, rejection, and higher incidence of mortality.4–7
The definition and diagnosis of AMR has developed significantly since Herskowitz et al8 initially described it in 1987 as a type of rejection characterized by arteriolar vasculitis and poor outcome in heart transplant recipients. Hammond et al9 were the first to recognize the importance of AMR, providing the initial immunohistochemical evidence that AMR involved antibody deposition with subsequent complement activation.9 Still, some critics have doubted the existence of “antibody-mediated” or “vascular” rejection throughout the years, debating that some of the effects may be due to nonimmunologic factors such as ischemic injury or reperfusion.
At present, AMR has become a better defined entity, reviewed by the International Society for Heart and Lung Transplantation (ISHLT) Immunopathology Task Force and identified by a typical histopathologic blueprint of capillary endothelial changes, macrophages and neutrophils infiltration, interstitial edema, and linear accumulations of immunoglobulins and complement, especially complement component C4d.10
Numerous studies have examined AMR in the heart transplant population. AMR was found to commonly occur early after transplantation.4 Risk factors associated with the development of AMR include female gender, elevated pre-transplant panel-reactive antibodies (PRAs), positive donor-specific crossmatch, prior sensitization to OKT3, cytomegalovirus (CMV) seropositivity, prior implantation of ventricular assist device, and/or retransplantation.4,10–13 In clinical practice, AMR is generally treated in patients with clinical symptoms of heart failure and evidence for left ventricular dysfunction (which may be without symptoms) in the absence of cellular infiltrates on their endomyocardial biopsy specimen.
On the other hand, AMR has been noted histologically in patients with normal cardiac function and no symptoms of heart failure (asymptomatic) and, in general, has not been treated. At present, however, there is nothing in the AMR literature clarifying the significance of AMR findings in endomyocardial biopsy specimens of asymptomatic patients. Previous AMR studies, like that of Kfoury et al,5 Michaels et al,4 and Casarez et al,14 usually merged asymptomatic AMR patients and treated AMR patients into one group. In contrast, the literature in cellular rejection has already analyzed the significance of asymptomatic cellular rejection in context with symptomatic cellular rejection.15
The following study is an assessment of the significance of untreated asymptomatic AMR. The purpose of this study was to analyze the clinical consequence of this asymptomatic AMR by investigating 5-year outcomes of adult heart transplant patients with asymptomatic and treated AMR.
METHODS
Patients
Between July 1, 1997, and September 30, 2000, we retrospectively reviewed all adult patients who received a heart transplant at our center for findings of AMR on biopsy specimens. We found 43 patients who had at least 1 biopsy specimen revealing AMR findings without concurrent acute cellular rejection (defined as ISHLT biopsy grade ≥ 3A). The 43 patients with AMR were then divided into 2 groups: untreated asymptomatic AMR (AsAMR, n = 21) with preserved left ventricular function, and treated AMR (TxAMR, n = 22) with left ventricular dysfunction, defined as an echocardiographic left ventricular ejection fraction (LVEF) of ≤ 40%. For comparison, 86 contemporaneous patients were selected as age- and gender-matched controls in a ratio of 2:1 control patients per AMR patient. Time from transplant to AMR of each AMR patient was matched to time from transplant of the 2 corresponding control patients.
Tissue Analysis and Diagnosis of AMR
Right ventricular endomyocardial biopsy specimens were obtained at routine intervals by percutaneous transcatheter techniques. The cardiac tissue was placed immediately into Bayley’s fixative and embedded in paraffin. Paraffin blocks were serially cut into 3-μm-thick sections. For each biopsy specimen, 3 slides with multiple sections were stained with hematoxylin and eosin to be read by a cardiac pathologist using ISHLT standards.
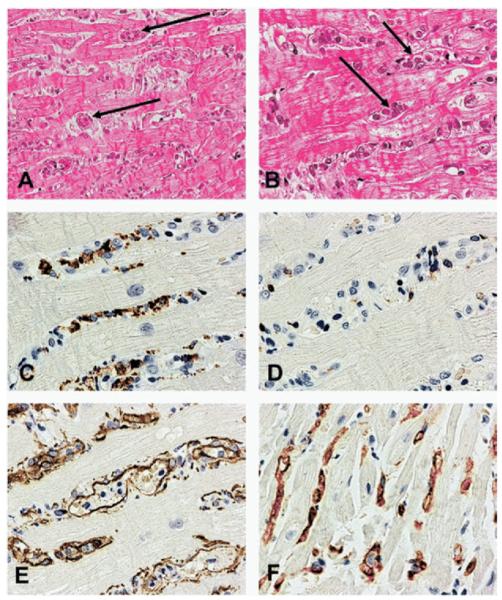
AMR was diagnosed when tissue from endomyocardial biopsies showed histologic evidence of capillary endothelial cell swelling, interstitial hemorrhage, interstitial edema, and neutrophil infiltration. Additionally, AMR was diagnosed when immunoperoxidase staining of paraffin-embedded tissue showed the presence of CD68-positive macrophages within capillary cells (anti-factor VIII-related antigen and/or anti-CD34) and C4d complement coating the walls of the myocardial capillaries16 (Figure 1). AMR was graded according to severity based on the degree of cell swelling, interstitial hemorrhage, and interstitial edema.
Figure 1.
Endomyocardial biopsy findings in antibody-mediated rejection. (A) Prominent intravascular cells are present in the myocardium in the absence of lymphoid cellular infiltrate (hematoxylin and eosin [H&E]; original magnification ×200). (B) Magnification of panel A at ×400 (H&E). (C) Prominence of macrophages amongst the cells with a CD68 immunoperoxidase stain (original magnification ×400). (D) Presence of only rare T lymphocytes with CD3 immunoperoxidase stain (original magnification ×400); (E) Highlighting of endothelial cells and confirmation of the macrophages’ intravascular location with the CD34 immunoperoxidase stain (original magnification ×400). (F) Deposition of C4d complement component in capillaries with the C4d immunoperoxidase stain (original magnification ×400). From: Fishbein and Kobashigawa: Current Opin. Cardiol 2004;19:166. Reprinted with permission of Wolters Kluwer.
Pre-transplant PRA and Donor-Specific Antibody Screening
When patients were listed for a transplant, pre-transplant human leukocyte antigen (HLA) antibody identification and specificity were performed using the National Institutes of Health cytotoxicity assay. A T-lymphocyte panel with 120 reference cells and a B-lymphocyte panel with 60 reference cells were used to measure antibody reactivity as a percentage of the panel reactive to the patient’s serum (PRA). Samples with PRA > 10% were retested with dithiothreitol to remove immunoglobulin M antibody. Prospective donor-specific crossmatching was performed on sensitized pre-heart transplant patients with elevated PRA > 10%. Sera collected at the time of transplantation were crossmatched directly with the donor’s T lymphocytes using complement-dependent cytotoxicity and the flow cytometry crossmatch techniques.
Coronary Angiography
All patients underwent annual coronary angiography after heart transplantation. Cardiac allograft vasculopathy (CAV) was defined as an angiographic coronary lesion with ≥ 30% luminal stenosis. Two physicians reviewed each angiogram.
Immunosuppression
During the study period, the routine immunosuppression at our institution consisted of cyclosporine, corticosteroids, and azathioprine or mycophenolate mofetil (routine use after April 1998). Tacrolimus replaced cyclosporine in January 2000. No patient received cytolytic induction, and steroids were weaned off for most patients with little or no previous rejection after 6 months post-transplant. Although asymptomatic AMR was not treated, symptomatic AMR was treated with intravenous gammaglobulin in association with high-dose corticosteroids. Some AMR patients were also switched from azathioprine to mycophenolate mofetil per treatment protocol.
Statistical Methods
Non-continuous variables were analyzed with the chi-square test, as appropriate, and continuous variables were analyzed by the 2-tailed t-test. The outcome end-points were analyzed according to the Kaplan-Meier method, and the results were compared with the log-rank test. A value of p < 0.05 was considered statistically significant for all tests.
RESULTS
Patient Demographics
Between July 1, 1997, and September 30, 2001, 21 patients had asymptomatic AMR (AsAMR group) and were untreated, 22 patients had treated AMR (TxAMR group), and 86 contemporaneous patients were selected as age- and gender-matched controls in a ratio of 2:1 control subjects for every AMR patient. Time from transplant to AMR of each AMR patient was similar to time from transplant of the two corresponding control patients.
Ischemic time, donor gender, donor age, and cytomegalovirus positivity were similar among the 3 groups (Table 1). Baseline immunosuppression was also similar for the 3 groups. Cyclosporine was given (instead of tacrolimus) for 72% of patients in the AsAMR group, 80% in the TxAMR group, and 77% in the control group (p = 0.66). Correspondingly, mycophenolate mofetil was given as the anti-proliferative agent for 65% of patients in the AsAMR group, 58% in the TxAMR group, and 49% in the control group (p = 0.39).
Table 1.
Patient Demographics and Baseline Characteristicsa
| Variable | AsAMR | TxAMR | Control |
|---|---|---|---|
| No. | 21 | 22 | 86 |
| Female, No. (%) | 9 (43) | 9 (41) | 34 (40) |
| Recipient age at Tx, mean ± SD, years |
54 ± 13 | 50 ± 14 | 52 ± 14 |
| Ischemic time, mean ± SD, min | 218 ± 77 | 204 ± 56 | 220 ± 84 |
| Donor female, No. (%) | 10 (48) | 5 (22) | 34 (40) |
| Donor, mean age ± SD, years | 38 ± 12 | 33 ± 13 | 31 ± 12 |
| CMV mismatch D+/R−, No. (%) | 3 (18) | 0 | 14 (16) |
AsAMR, asymptomatic antibody-mediated rejection; CMV, cytomegalovirus; SD, standard deviation; TxAMR, treated antibody-mediated rejection.
No significant differences in any baseline characteristics.
Hemodynamic Dysfunction in AMR Patients
Hemodynamic dysfunction, consisting of shock, hypotension, reduced cardiac index (≤ 2.0 liters/min/m2), and/or a rise in capillary wedge (> 20 mm Hg) or pulmonary artery systolic pressure (> 50 mm Hg) necessitating inotropic support was observed in 9 of 22 (41%) of the TxAMR patients at the time of their AMR diagnosis. By definition, none of the AsAMR patients had concurrent hemodynamic dysfunction at the time of their diagnosis. The remaining 13 patients in the TxAMR group had no hemodynamic dysfunction but had an echocardiographic LVEF of ≤ 40%. The mean LVEF in TxAMR patients at the time of the first AMR diagnosis was 39.4%, which was significantly different than that of the AsAMR group (55.5%, p < 0.001).
Outcomes of Patients With AMR
Actuarial survival at 5 years was comparable among the AsAMR (85.7%), TxAMR (68.2%), and control groups (79.1%; Figure 2). Freedom from CAV at 5 years was significantly lower in the AsAMR group (52.4%) compared with the control group (79.1%, p = 0.02; Figure 3). The TxAMR group had numerically less freedom from CAV than control patients (68.2% vs 79.1%, p = 0.27). There was no significant difference in freedom from CAV between the AsAMR and TxAMR groups (52.4% vs 68.2%, p = 0.36).
Figure 2.
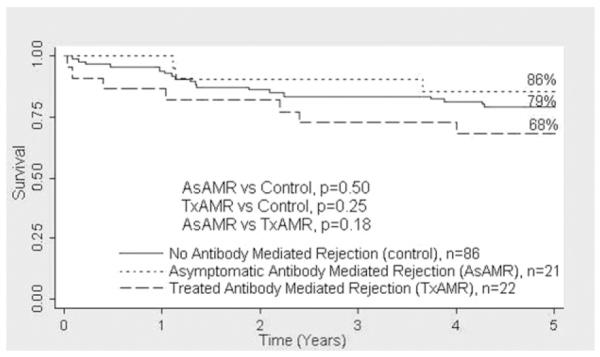
There was no significant difference in 5-year actuarial survival among the patients with asymptomatic antibody-mediated rejection (AsAMR), treated antibody-mediated rejection (TxAMR), and control patients.
Figure 3.
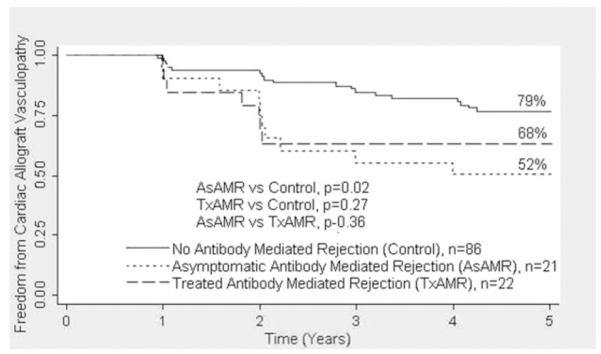
The 5-year freedom from cardiac allograft vasculopathy was lower in the treated antibody-mediated rejection (TxAMR) and asymptomatic antibody-mediated rejection (AsAMR) groups compared with the control group. Cardiac allograft vasculopathy is defined as ≥ 30% stenosis in any vessel on an angiogram.
The mean time to the first diagnosis of AMR from endomyocardial biopsy was 157.1 ± 193.8 days for the AsAMR group and 189.2 ± 259.8 days for the TxAMR group. Ten AsAMR patients (48%) were first diagnosed with AMR within 1 month after transplant, 8 (38%) were diagnosed between 1 month and 1 year after transplant, and 3 (14%) were diagnosed at more than 1 year after transplant. Similarly, 11 (50%) TxAMR patients were first diagnosed with AMR within 1 month after transplant, 5 (23%) were diagnosed between 1 month and 1 year after transplant, and 6 (27%) were diagnosed at more than 1 year after transplant. There is no difference in the distribution of time to the first diagnosis of AMR for the 2 groups (p = 0.65). Moreover, after the first diagnosis of AMR, 8 AsAMR patients (38%) and 9 TxAMR patients (41%) continued to have at least 1 repeat episode of AMR (p = 0.90). Four patients (19%) in the AsAMR group and 5 patients (23%) in the TxAMR group ultimately had ≥ 3 episodes of AMR. These patients with ≥ 3 episodes of AMR were not at greater risk for lower 5-year actuarial survival or freedom from CAV in the AsAMR or TxAMR groups, but the numbers are small.
In the AsAMR group, 7 of 21 patients had combined mild cellular rejection (ISHLT 1A, 1B)/AMR and the remaining 14 had AMR alone. In the TxAMR group, 5 of 22 patients had combined mild cellular rejection/AMR and the remaining 17 had AMR alone. There was no difference between the combined mild cellular rejection/AMR and AMR-alone patients on 5-year actuarial survival or freedom from CAV in the AsAMR and TxAMR groups (data not shown). There were no ISHLT grade 2 findings in either AMR group.
Pre-transplant PRA and Donor-Specific Crossmatching
Data on pre-transplant PRA and T-cell donor-specific crossmatches was available for all the patients in the AsAMR group, 20 patients (91%) in the TxAMR group, and 77 patients (90%) in the control group. Positive T-cell PRAs, defined as ≥ 10% reactivity with the cellular reference panel, were found in 1 AsAMR patient (4.8%), 6 TxAMR patients (30%), and 6 control patients (7.8%; p = 0.011 for TxAMR vs controls). Also, positive B-cell PRAs, defined as ≥ 10% reactivity with the cellular reference panel, were found in 3 AsAMR patients (14%), 4 TxAMR patients (20%), and 11 control patients (14%; p = 0.81; Table 2).
Table 2.
Patient Pre-transplant Panel-Reactive Antibody Results
| Variable | AsAMR | TxAMR | Control |
|---|---|---|---|
| No. | 21 | 20 | 77 |
| PRA, No. (%) | |||
| T cells (≥ 10%) | 1 (4.8) | 6 (30)a | 6 (7.8) |
| B cells (≥ 10%) | 3 (14) | 4 (20) | 11 (14) |
AsAMR, asymptomatic antibody-mediated rejection; PRA, panel-reactive antibody; TxAMR, treated antibody-mediated rejection.
P = 0.011 for TxAMR vs control.
One AsAMR patient (4.8%), 3 TxAMR patients (15%), and 3 control patients (3.9%) had retrospective positive T-cell flow cytometry crossmatches (p = 0.17). Unfortunately, routine post-transplant antibody levels were not obtained.
DISCUSSION
Controversy over the management of first-year untreated AsAMR had been unresolved because of the lack of data on the significance of AsAMR on patient outcome. Our data suggest that patients with AsAMR can be identified with existing pathologic criteria and are at greater risk for developing CAV.
We found that AsAMR rejection patients developed significantly less freedom from CAV (defined as ≥ 30% stenosis in any vessel) at 5 years compared with control patients. By 5 years after transplant, only 52% of patients in the AsAMR group compared with 79% of the control patients were free from CAV. AMR, in general, has been associated with greater development of CAV.4,17 However, no study to date has isolated AsAMR patients from those treated. Hammond et al9 found a significant difference in the time to the development of CAV between patients who had cellular rejection alone, mixed cellular and AMR, and AMR alone. Patients with mixed rejection had an intermediate time to the development of CAV between that of patients with cellular and AMR alone. Similarly, Michaels et al25 found CAV to be 10% greater at 1 year and 36% greater at 3 years in patients with AMR compared with age- and gendermatched controls who did not have AMR.
Our study yielded no difference in the frequency of CAV between AsAMR and TxAMR (p = 0.36) patients. This comes as no surprise given that the combination of alloantibody presence, fibrin deposition, and endothelial cell activation sufficiently promote the release of growth factors from platelets, monocytes, and endothelial cells. These growth factors likely lead to progressive vascular injury by stimulating migration, proliferation, and functional modification of medial smooth muscle cells.18,19 In addition, a small study involving 17 heart transplant patients has shown that asymptomatic C4d has a positive predictive value of 60% and a negative predictive value of 92% for CAV by intravascular ultrasound at 1 year.20
Correspondingly, after prospectively examining endomyocardial biopsy specimens reporting greater than 2+C4d deposition, Rodriguez et al21 found that CAV developed in 4 of the 15 heart transplant patients who had C4d and/or C3d deposition without allograft dysfunction and in 4 of 5 patients with allograft dysfunction. It may appear that not all asymptomatic patients with C4d deposition have clinical sequelae,16 but the relatively small number of patients in this study is inadequate to form any conclusions. Moreover, though immunofluorescence for C4d was considered a standard for diagnosing AMR, the presence of clinically significant AMR in the absence of C4d is now widely accepted.22–24
Patients in the AsAMR and TxAMR groups appear to have comparable actuarial survival at 5 years after heart transplantation compared with controls (AsAMR, 86%; TxAMR, 68%; control, 79%; p = 0.41). This is different than many published results from other centers that show AMR is associated with increased mortality.7,9,17,22 In a more recent article from the Utah heart transplant program, Kfoury et al5 defined patterns of rejection (AMR and cellular rejection) from a review of biopsy diagnoses taken in the first 6 to 12 weeks after transplant. They report that cardiovascular deaths were significantly increased in patients with ≥ 3 episodes of AMR. By contrast, cellular rejection episodes did not increase the risk of cardiovascular death. The cardiovascular deaths included acute myocardial infarction, arrhythmic death, sudden death, and deaths related to cardiac allograft vasculopathy.
The presence of pre-transplant donor-specific anti-HLA antibodies is reported to be associated with AMR and the presence of C4d, in heart biopsies after transplant.25 The current study did not find that an elevated pre-transplant PRA (AsAMR, 4.8%; control, 7.8%, p = 0.59) and/or a retrospective positive donor specific crossmatch result (AsAMR, 4.8%; control, 3.9%, p = 0.86) was predictive of AsAMR compared with the control. However, an elevated pre-transplant PRA was associated with treated AMR after transplant (AsAMR, 4.8%; TxAMR, 30%; control, 7.8%; p = .011). Likewise, a retrospective positive donor specific crossmatch trended toward a prediction of AMR as a whole (p = .08). These latter results are consistent with the literature.4,9,15,17,26
Nonetheless, despite a lack of clinical symptoms and an affect on mortality, AsAMR is still a marker for poor outcomes after heart transplantation. After the first diagnosis of AMR, 38% of AsAMR patients and 41% of TxAMR had at least 1 repeat episode of AMR (p = 0.90). Moreover, 4 patients (19%) in the AsAMR group and 5 patients (23%) in the TxAMR group had ≥ 3 episodes of AMR. A recent study has found that the incremental risk of cardiovascular mortality was 8% for each episode of AMR.5 In light of this and of the increased risk of CAV that it carries, AsAMR may be of long-term clinical consequence.
The limitations of this study are the retrospective format and the small sample size. A larger number of AMR patients may be needed to demonstrate a difference in survival, as there appeared to be a trend towards lower survival in the TxAMR group compared with the control group in our study. Blood for circulating antibodies was not routinely drawn after transplant. The detection of donor-specific antibodies at the time of AsAMR would have been of great interest to assess whether the presence of these antibodies also affects outcome.
We conclude that despite comparable 5-year survival with control patients after heart transplantation, AsAMR rejection is associated with a greater risk of CAV. Whether AsAMR should be treated is unknown. Some parties have advocated early detection, followed by early increase of immunosuppression to improve outcome in this patient population.4,17 Plasmapheresis, intravenous immunoglobulin, and more recently, rituximab, based on studies of these therapies in sensitized pre-heart transplant patients, can also be considered for treatment of AsAMR patients.22–29 As an alternative to early treatment, however, some have proposed a strategy of close observation and follow-up for evidence of graft dysfunction.30 Prospective, randomized studies to treat AsAMR will be needed to demonstrate any benefit of treatment to improve outcome.
Acknowledgments
This work was supported in part by the National Institute of Allergy and Infectious Diseases Grant R01 A1 42819 and the National Heart, Lung and Blood Institute Grant R01 HL 090995 to E.F.R.
REFERENCES
- 1.Eisen H, Ross H. Optimizing the immunosuppressive regimen in heart transplantation. J Heart Lung Transplant. 2004;23:S207–13. doi: 10.1016/j.healun.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Cogert GA, Subherwal S, Wu G, et al. Incidence of non-cellular (humoral) rejection unchanged in the 1990 decade despite a decrease in cellular rejection. J Heart Lung Transplant. 2003;22:S119–42. [Google Scholar]
- 3.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807–17. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 4.Michaels PJ, Espejo ML, Kobashigawa JA, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 5.Kfoury AG, Stehlik J, Renlund DG, et al. Impact of repetitive episodes of antibody-mediated or cellular rejection on cardiovascular mortality in cardiac transplant recipients: defining rejection patterns. J Heart Lung Transplant. 2006;25:1277–82. doi: 10.1016/j.healun.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Tambur AR, Pamboukian SV, Constanzao MR, et al. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–25. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DO, Yowell RL, Kfoury AG, et al. Allograft coronary artery disease clinical correlations with circulating anti-HLA antibodies and the immunohistophathologic pattern of vascular rejection. J Heart Lung Transplant. 2000;19:518–21. doi: 10.1016/s1053-2498(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 8.Herskowitz A, Soule LM, Ueda K, et al. Arteriolar vasculitis on endomyocardial biopsy: A histologic predictor of poor outcome in cyclosporine-treated heart transplant recipients. J Heart Transplant. 1987;6:127. [PubMed] [Google Scholar]
- 9.Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation (pathologic observations and clinical implications) J Heart Transplant. 1989;8:430–43. [PubMed] [Google Scholar]
- 10.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Leech SH, Rubin S, Eisen HJ, et al. Cardiac transplantation across a positive prospective lymphocyte cross-match in sensitized recipients. Clin Transplant. 2003;17(suppl 9):17–26. doi: 10.1034/j.1399-0012.17.s9.3.x. [DOI] [PubMed] [Google Scholar]
- 12.Hammond EH, Wittwer CT, Greenwood J, et al. Relationship of OKT3 sensitization and vascular rejection in cardiac transplant patients receiving OKT3 rejection prophylaxis. Transplantation. 1990;50:776. doi: 10.1097/00007890-199011000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Toyoda M, Petrosian A, Jordan SC. Immunological characterization of anti-endothelial cell antibodies induced by cytomegalovirus infection. Transplantation. 1999;68:1311. doi: 10.1097/00007890-199911150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Casarez TW, Perens G, Williams RJ, et al. Humoral rejection in pediatric orthotopic heart transplantation. J Heart Lung Transplant. 2007;26:114–9. doi: 10.1016/j.healun.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Kirklin JK. Is biopsy-proven cellular rejection an important clinical consideration in heart transplantation? Curr Opin Cardiol. 2005;20:127–31. doi: 10.1097/01.hco.0000153950.49813.d2. [DOI] [PubMed] [Google Scholar]
- 16.Fedson SE, Daniel SS, Husain AN. Immunohistochemistry staining of C4d to diagnose antibody-mediated rejection in cardiac transplantation. J Heart Lung Transplant. 2008;27:372–9. doi: 10.1016/j.healun.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Hammond EH, Yowell RL, Price GD, et al. Vascular rejection and its relationship to allograft coronary artery disease. J Heart Lung Transplant. 1992;11:S111–9. [PubMed] [Google Scholar]
- 18.Forrester A. Vascular rejection in cardiac transplantation: a morphological study of 25 human cardiac allografts. APMIS. 1992;100:367–76. [PubMed] [Google Scholar]
- 19.Miller LW. The diagnosis, incidence, and management of vascular rejection in heart transplantation. J Heart Lung Transplant. 1993;12(suppl):S111–2. [PubMed] [Google Scholar]
- 20.Poelzl G, Ullrich R, Huber A, et al. Capillary deposition of the complement fragment fragment C4d in cardiac allograft biopsies is associated with allograft vasculopathy. Transplant Int. 2005;18:313–7. doi: 10.1111/j.1432-2277.2004.00037.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez ER, Skojec DV, Tan CD, et al. Antibody-mediated rejection in human cardiac allografts: Evaluation of immunoglobulins and complement activation products C4d and C3d as markers. Am J Transplant. 2005;5:2778–85. doi: 10.1111/j.1600-6143.2005.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crespo-Leiro MG, Viega-Barreiro A, Domenech N, et al. Humoral heart rejection (severe allograft dysfunction with no signs of cellular rejection or ischemia): incidence, management, and the value of C4d for diagnosis. Am J Transplant. 2005;5:2560–4. doi: 10.1111/j.1600-6143.2005.01039.x. [DOI] [PubMed] [Google Scholar]
- 23.Behr TM, Feucht HE, Richter K, et al. Detection of humoral rejection in human cardiac allografts by assessing the capillary deposition of complement fragment C4d in endomyocardial biopsies. J Heart Lung Transplant. 1999;18:904–12. doi: 10.1016/s1053-2498(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 24.Chantranuwat C, Qiao JH, Kobashigawa J, et al. Immunoperoxidase staining for C4d on paraffinembedded tissue in cardiac allograft endomyocardial biopsies. Appl Immunohistochem Mol Morphol. 2004;12:166–71. doi: 10.1097/00129039-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Michaels PJ, Fishbein MC, Colvin RB. Humoral rejection of human organ transplants. Springer Semin Immunopathol. 2003;25:119–40. doi: 10.1007/s00281-003-0139-x. [DOI] [PubMed] [Google Scholar]
- 26.Greger B, Brossmann T, Gartner H, et al. Positive post-operative donor-specific crossmatch correlates with B-cell infiltration and poor graft prognosis. Transplant Proc. 1990;22:1900–2. [PubMed] [Google Scholar]
- 27.Pisani BA, Mullen GM, Malinowska K, et al. Plasmapheresis with intravenous immunoglobulin G is effective in patients with elevated panel reactive antibody prior to cardiac transplantation. J Heart Lung Transplant. 1999;18:701–6. doi: 10.1016/s1053-2498(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 28.Leech SH, Lopez-Cepero M, LeFor WM, et al. Management of the sensitized cardiac recipient: the use of plasmapheresis and intravenous immunoglobulin. Clin Transplant. 2006;20:476–84. doi: 10.1111/j.1399-0012.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 29.Vieira CA, Agarwal A, Book BK, et al. Rituximab for reduction of anti-HLA antibodies in patients awaiting renal transplantation: safety, pharmacodynamics, and pharmacokinetics. Transplantation. 2004;77:542–8. doi: 10.1097/01.tp.0000112934.12622.2b. [DOI] [PubMed] [Google Scholar]
- 30.Uber WE, Self SE, Van Bakel AB, et al. Acute antibody-mediated rejection following heart transplantation. Am J Transplant. 2007;7:2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]