Abstract
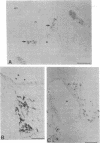
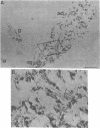
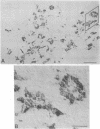
We sought to determine, in rat embryo, when and at what site in their migration cells derived from the neural crest differentiate into sympathetic neuroblasts. This has been accomplished by immunocytochemical detection, within the cells, of the enzymes catalyzing catecholamine biosynthesis—tyrosine hydroxylase [TH; tyrosine 3-monooxygenase, L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2] dopamine-β-hydroxylase [DBH; 3,4-dihydroxyphenylethylamine,ascorbate:oxygen oxidoreductase (β-hydroxylating), EC 1.14.17.1)]—and, as a marker of prospective adrenal medullary cells, the enzyme phenylethanolamine N-methyltransferase (PNMT; S-adenosyl-L-methionine:phenylethanolamine N-methyltransferase, EC 2.1.1.28). TH and DBH, not detected in the neural crest, appear almost simultaneously in cells of the thoracic sympathetic ganglia in 11-day-old embryos, and in abdominal and lumbar ganglia 1-2 days later, thereby exhibiting a characteristic rostral-caudal gradient of differentiation. Cells stained for TH and DBH are seen in the gut wall from day 11 to day 14, but not thereafter. Cells stained for TH and DBH appear in the adrenal anlage at day 15. However, PNMT is not detected in the adrenal until day 17 of development, and is present only in the sympathoblasts in contact with the adrenal cortex. Treatment of pregnant rats with dexamethasone failed to accelerate the appearance of PNMT in the embryo or to initiate its expression in cells of other sympathetic organs. We conclude that neural crest cells express a noradrenergic phenotype only after leaving the neural crest and that these cells are labile with respect to their neurotransmitter and are capable of transformation in response to environmental stimuli.
Keywords: immunocytochemistry, tyrosine 3-monooxygenase (tyrosine hydroxylase), dopamine-β-hydroxylase, phenylethanolamine N-methyltransferase
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjerre B. The production of catecholamine-containing cells in vitro by young chick embryos studies by the histochemical fluorescence method. J Anat. 1973 May;115(Pt 1):119–131. [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Cegrell L., Falck B., Ritzén M., Rosengren E. Dopamine-containing cells in sympathetic ganglia. Acta Physiol Scand. 1970 Mar;78(3):334–338. doi: 10.1111/j.1748-1716.1970.tb04668.x. [DOI] [PubMed] [Google Scholar]
- Bunge R., Johnson M., Ross C. D. Nature and nurture in development of the autonomic neuron. Science. 1978 Mar 31;199(4336):1409–1416. doi: 10.1126/science.24273. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. M. Factors directing the expression of sympathetic nerve traits in cells of neural crest origin. J Exp Zool. 1972 Feb;179(2):167–182. doi: 10.1002/jez.1401790204. [DOI] [PubMed] [Google Scholar]
- Cohen A. M. Independent expression of the adrenergic phenotype by neural crest cells in vitro. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2899–2903. doi: 10.1073/pnas.74.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Champlain J., Malmfors T., Olson L., Sachs C. Ontogenesis of peripheral adrenergic neurons in the rat: pre- and postnatal observations. Acta Physiol Scand. 1970 Oct;80(2):276–288. doi: 10.1111/j.1748-1716.1970.tb04791.x. [DOI] [PubMed] [Google Scholar]
- ENEMAR A., FALCK B. OBSERVATIONS ON THE APPEARANCE OF NOREPINEPHRINE IN THE SYMPATHETIC NERVOUS SYSTEM OF THE CHICK EMBRYO. Dev Biol. 1965 Apr;11:268–283. doi: 10.1016/0012-1606(65)90060-6. [DOI] [PubMed] [Google Scholar]
- Eränkö L., Eränkö O. Effect of hydrocortisone on histochemically demonstrable catecholamines in the sympathetic ganglia and extra-adrenal chromaffin tissue of the rat. Acta Physiol Scand. 1972 Jan;84(1):125–133. doi: 10.1111/j.1748-1716.1972.tb05161.x. [DOI] [PubMed] [Google Scholar]
- Gianutsos G., Moore K. E. Effects of pre- or postnatal dexamethasone, adrenocorticotrophic hormone and environmental stress on phenylethanolamine N-methyltransferase activity and catecholamines in sympathetic ganglia of neonatal rats. J Neurochem. 1977 May;28(5):935–940. doi: 10.1111/j.1471-4159.1977.tb10653.x. [DOI] [PubMed] [Google Scholar]
- Greenberg J. H., Schrier B. K. Development of choline acetyltransferase activity in chick cranial neural crest cells in culture. Dev Biol. 1977 Nov;61(1):86–93. doi: 10.1016/0012-1606(77)90344-x. [DOI] [PubMed] [Google Scholar]
- Hess A. A simple procedure for distinguishing dopamine from noradrenaline in peripheral nervous structures in the fluorescence microscope. J Histochem Cytochem. 1978 Feb;26(2):141–144. doi: 10.1177/26.2.624834. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Shideman F. E. Appearance and concentrations of catecholamines and their biosynthesis in the embryonic and developing chick. J Pharmacol Exp Ther. 1968 Jan;159(1):38–48. [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Goldstein M. Isolation and characterization of multiple forms of phenylethanolamine N-methyltransferase. Mol Pharmacol. 1973 Jan;9(1):117–129. [PubMed] [Google Scholar]
- Kanerva L., Hervonen A., Hervonen H. Morphological characteristics of the ontogenesis of the mammalian peripheral adrenergic nervous system with special remarks on the human fetus. Med Biol. 1974 Jun;52(3):144–153. [PubMed] [Google Scholar]
- Le Douarin N. M., Renaud D., Teillet M. A., Le Douarin G. H. Cholinergic differentiation of presumptive adrenergic neuroblasts in interspecific chimeras after heterotopic transplantations. Proc Natl Acad Sci U S A. 1975 Feb;72(2):728–732. doi: 10.1073/pnas.72.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Margolis F. L., Roffi J., Jost A. Norepinephrine methylation in fetal rat adrenals. Science. 1966 Oct 14;154(3746):275–276. doi: 10.1126/science.154.3746.275. [DOI] [PubMed] [Google Scholar]
- Maxwell G. D. Cell cycle changes during neural crest cell differentiation in vitro. Dev Biol. 1976 Mar;49(1):66–79. doi: 10.1016/0012-1606(76)90258-x. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc Natl Acad Sci U S A. 1967 Jul;58(1):344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norr S. C. In vitro analysis of sympathetic neuron differentiation from chick neural crest cells. Dev Biol. 1973 Sep;34(1):16–38. doi: 10.1016/0012-1606(73)90336-9. [DOI] [PubMed] [Google Scholar]
- Papka R. E. Ultrastructural and fluorescence histochemical studies of developing sympathetic ganglia in the rabbit. Am J Anat. 1972 Jul;134(3):337–364. doi: 10.1002/aja.1001340306. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Chun L. L. The induction of acetylcholine synthesis in primary cultures of dissociated rat sympathetic neurons. I. Effects of conditioned medium. Dev Biol. 1977 Apr;56(2):263–280. doi: 10.1016/0012-1606(77)90269-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H., Reichardt L. F., Chun L. L. Biochemical studies on the development of primary sympathetic neurons in cell culture. Cold Spring Harb Symp Quant Biol. 1976;40:389–397. doi: 10.1101/sqb.1976.040.01.037. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Joh T. H., Reis D. J. Monoamine-synthesizing enzymes in central dopaminergic, noradrenergic and serotonergic neurons. Immunocytochemical localization by light and electron microscopy. J Histochem Cytochem. 1976 Jul;24(7):792–306. doi: 10.1177/24.7.8567. [DOI] [PubMed] [Google Scholar]
- Ross D., Johnson M., Bunge R. Development of cholinergic characteristics in adrenergic neurones is age dependent. Nature. 1977 Jun 9;267(5611):536–539. doi: 10.1038/267536a0. [DOI] [PubMed] [Google Scholar]
- Ross R. A., Joh T. H., Reis D. J. Reversible changes in the accumulation and activities of tyrosine hydroxylase and dopamine-beta-hydroxylase in neurons of nucleus locus coeruleus during the retrograde reaction. Brain Res. 1975 Jul 4;92(1):57–72. doi: 10.1016/0006-8993(75)90527-2. [DOI] [PubMed] [Google Scholar]
- Smith J., Cochard P., Le Douarin N. M. Development of choline acetyltransferase and cholinesterase activities in enteric ganglia derives from presumptive adrenergic and cholinergic levels of the neural crest. Cell Differ. 1977 Oct;6(3-4):199–216. doi: 10.1016/0045-6039(77)90016-1. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- WESTON J. A. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev Biol. 1963 Jun;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]
- Weston J. A. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]
- Wurtman R. J., Pohorecky L. A., Baliga B. S. Adrenocortical control of the biosynthesis of epinephrine and proteins in the adrenal medulla. Pharmacol Rev. 1972 Jun;24(2):411–426. [PubMed] [Google Scholar]