Abstract
In recent years, it has become clear that both AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)- and NMDA (N-methyl-D-aspartate)-type glutamate receptors, and many of their interacting partners, are palmitoylated proteins. Interfering with palmitoylation dramatically affects receptor trafficking and distribution and, in turn, can profoundly alter synaptic transmission. Increased knowledge of synaptic palmitoylation not only will aid our understanding of physiological neuronal regulation, but also may provide insights into, and even novel treatments for, neuropathological conditions. In the present paper, we review recent advances regarding the regulation of ionotropic glutamate receptor trafficking and function by palmitoylation.
Keywords: α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR), glutamate receptor, N-methyl-D-aspartate receptor (NMDAR), palmitoylation, PDZ domain, synapse
Introduction
Glutamate receptors, the major excitatory neurotransmitter receptors in the mammalian brain, must be targeted to precise subcellular locations. In mature neurons, glutamate receptors are concentrated on dendritic spines. These tiny structures are the sites of synaptic connections, via which neurons communicate with one another. For normal higher brain function, neurons face the challenge of not only targeting glutamate receptors correctly to spines and synapses, but also regulating receptor number and function in response to experience. Considerable evidence suggests that experience-dependent changes in glutamate receptor number and function are critical for higher forms of behaviour such as learning and memory, and are impaired in disease states.
Post-translational synaptic regulation: phosphorylation compared with palmitoylation
Changes in glutamate receptor number and/or function must be rapid, and thus often involve post-translational modification of existing receptors [1–4]. It is therefore unsurprising that protein phosphorylation, the best-known post-translational regulatory mechanism, is heavily linked to the regulation of receptor trafficking and function. Indeed, both serine/threonine and tyrosine phosphorylation influence distribution and channel properties of different glutamate receptor subtypes [1,4–6]. Glutamate-receptor-binding proteins are also key targets for phosphorylation-dependent regulation [7–9].
Despite its clear importance in receptor regulation, phos-phorylation is a charge-based regulatory mechanism, whereas receptors are predominantly found in a highly hydrophobic lipid-rich environment. Phosphorylation therefore appears to be imperfectly suited to regulate the countless budding and fusion events between trafficking vesicles and the plasma membrane that receptors undergo. In contrast, these events would appear to be perfect candidates for regulation by S-palmitoylation, the reversible addition of the fatty acid palmitate to cysteine residues in target proteins [10–12]. Palmitoylation can direct transmembrane proteins to specific microdomains within the plasma membrane and can also target otherwise soluble proteins to either the plasma membrane or to specific vesicles [12]. It is now clear that the major ionotropic glutamate receptors and several of their binding partners are also targets for regulation by palmitoylation [13–16]. Moreover, research in this field has revealed several new roles for palmitoylation at the cell biological level. It is now apparent that palmitoylation is frequently not simply a constitutive targeting motif; instead, cycles of palmitate addition and removal are highly dynamic, on both receptors and their interacting proteins. Moreover, palmitoylation levels can be regulated by changes in neuronal activity or other stimuli [17]. Finally, recent evidence suggests that this multilayered regulation is facilitated by the targeting of specific DHHC (Asp-His-His-Cys) family PAT (palmitoyl acyltransferase) enzymes to different subcellular locations in neurons [13,15,18]. In the present paper, we focus on the palmitoylation-dependent regulation of AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) and NMDA (N-methyl-D-aspartate) subtype glutamate receptors (AM-PARs and NMDARs respectively) and their binding partners, which is emerging as a key synaptic regulatory mechanism.
Regulation of AMPA-type glutamate receptors by palmitoylation
AMPARs mediate the majority of fast excitatory transmission at central synapses. Neurons expend huge amounts of energy to dynamically traffic AMPARs, which are constantly inserted, internalized and recycled, even at synapses that maintain a consistent stable synaptic strength [2]. Changing the balance of AMPAR trafficking into and out of synapses is a major way by which synaptic strength can be altered and probably underlies several forms of synaptic plasticity and behaviour [1,19,20]. The short cytoplasmic tails of AMPARs are critical for regulation of their trafficking. AMPAR tails contain multiple phosphorylation sites, plus binding motifs for several interactors. In addition, all four AMPAR subunits (GluA1–GluA4) also have a cysteine residue close to the beginning of their cytoplasmic tail, which is palmitoylated [13]. All four mammalian AMPAR subtypes were found to be palmitoylated in cultured neurons and endogenously in forebrain [17]. Palmitoylation of the AMPAR GluA1 subunit juxtamembrane cysteine residue is reported to influence binding of GluA1 to the protein 4.1N and to regulate GluA1 insertion into the plasma membrane [21] (Figure 1).
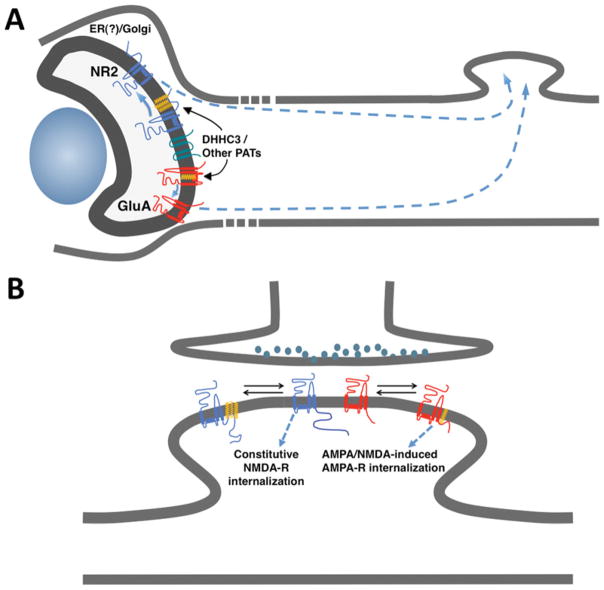
Figure 1. Two distinct roles for palmitoylation in trafficking and targeting of AMPA- and NMDA-type glutamate receptors.
(A) In neurons transfected with DHHC3 (orange), both AMPARs (red) and NMDARs (blue) are palmitoylated, causing their retention in the Golgi. Only depalmitoylated receptors can traffic forward to the plasma membrane. AMPAR retention requires a site near the AMPAR channel pore, whereas NMDAR retention requires a cluster of cysteine residues (cysteine cluster II) in the NR2 C-terminus. (B) Additional sites on both AMPARs and NMDARs are also palmitoylated. These sites do not affect initial targeting to the plasma membrane. However, a C-terminal juxtamembrane site regulates activity-induced internalization of AMPARs, whereas a distinct cluster of cysteines residues (cysteine cluster I) regulates constitutive internalization of NMDARs.
The C-terminal juxtamembrane cysteine residue is the major palmitoylation site on AMPARs expressed alone [13]. However, AMPARs co-expressed with the broad-specificity DHHC family PAT DHHC3/GODZ are also palmitoylated at a second site, close to the channel-forming region of the receptor subunit [13,22]. GODZ is exclusively localized to the Golgi apparatus [22] and AMPARs palmitoylated by co-transfected GODZ are trapped intracellularly [13] (Figure 1). This palmitoylation event may act as a ‘quality-control’ step, perhaps preventing forward trafficking of AMPARs that are incompletely folded and/or have not yet undergone a full complement of other modifications, e.g. glycosylation. However, more recent studies suggest that, particularly for the GluA2 subunit, endogenous palmitoylation occurs within the ER (endoplasmic reticulum) and is actually increased by agents that disrupt Golgi membrane integrity [23]. As GODZ has only been demonstrated to palmitoylate AMPARs when overexpressed, it is possible that other PATs perform this role endogenously in neurons. More work will be necessary to determine where in neurons AMPAR channel pore palmitoylation occurs and how it is regulated.
Although cell biological effects of AMPAR palmitoylation have been described in cultured neurons, how palmitoylation affects AMPAR trafficking and plasticity in vivo is largely unexplored. However, in a recent impressive series of experiments, AMPAR subunits were reported to be palmitoylated in the nucleus accumbens, a brain region central to the effects of drugs of abuse [24]. Palmitoylation of the GluA1 and GluA3 subunits was markedly, but transiently, increased following intraperitoneal injection of cocaine. The broad-spectrum palmitoylation inhibitor 2-Br (2-bromopalmitate) blocked both cocaine-induced palmitoylation and intracellular redistribution of GluA1 and GluA3. When combined with earlier reports of glutamate-dependent AMPAR depalmitoylation in cortical neurons [13], this suggests that AMPAR palmitoylation occurs in multiple brain regions and can be dynamically regulated by extracellular signals.
Regulation of NMDA-type glutamate receptors by palmitoylation
In contrast with AMPARs, which are constantly trafficked into and out of synapses, synaptic NMDARs have been historically considered to be more stable, especially in mature neurons. However, it is now clear that NMDARs can also be trafficked rapidly, both constitutively and in response to changes in neuronal activity [3]. Palmitoylation is now emerging as an additional regulator of NMDAR trafficking.
NMDARs are heteromeric combinations of an obligatory NR1 subunit plus different NR2 subunits, with NR2A and NR2B the major NR2 subunit types in forebrain [3,4]. Both NR2A and NR2B contain long intracellular tails, both of which are palmitoylated [14,17]. In this case, the ‘two-site’ arrangement of palmitoylation sites seen in AMPARs is replaced by a ‘two-cluster’ arrangement, with palmitoylation occurring on two separate groups of cysteine residues in the long NR2 intracellular C-terminal tails [14]. In transfected non-neuronal cells, it appears that no single site in either cluster is dominant, such that all must be mutated for palmitoylation to be eliminated. The consequences of palmitoylation of each cluster differ markedly. Cluster II palmitoylation causes both NR2A- and NR2B-containing receptors to accumulate in the Golgi apparatus, again possibly serving as a quality-control step in receptor maturation, as postulated for the channel pore AMPAR palmitoylation site (Figure 1A). In contrast, NR2A/NR2B palmitoylation on cysteine cluster I is linked to increased levels of phosphorylation by Src-family tyrosine kinases, which in turn decreases rates of receptor internalization [14] (Figure 1B). As with AMPAR palmitoyla-tion, NMDAR palmitoylation is regulated by neuronal activity, but little is known about the PATs responsible, and which site(s) are most sensitive to activity changes.
Regulation of glutamate-receptor-binding proteins by palmitoylation
Direct palmitoylation of glutamate receptors themselves is an important regulatory mechanism. However, receptor-interacting proteins, particularly those of the PDZ domain family [25], guide receptors to specific subcellular locations and play important roles in receptor regulation. Many PDZ domain proteins are themselves palmitoylated [10], and this is emerging as another locus for the control of receptor localization and trafficking.
Several years before reports of NMDAR palmitoylation, it was known that the major NR2-interacting protein PSD (postsynaptic density)-95 is palmitoylated and that this modification is critical for PSD-95 targeting to synapses [26,27]. At first glance, these findings would suggest that PSD-95 palmitoylation should influence NMDAR synaptic trafficking, clustering and/or function. Indeed, the PSD-95 palmitoylation state does alter NMDAR desensitization, a property that in turn may affect NMDAR activation during prolonged or repeated exposure to glutamate [28]. However, completely removing palmitate from PSD-95 with the inhibitor 2-Br does not affect NMDAR clustering, at least over short time courses (8 h) [26].
However, over the same periods during which NMDARs are minimally affected, blocking PSD-95 palmitoylation with 2-Br dramatically affects AMPAR synaptic clustering. Thus synaptic AMPARs are rapidly dispersed by 2-Br treatment, or by overexpression of a non-palmitoylatable PSD-95 [26,29]. Links between palmitoylated PSD-95 and AMPAR regulation are strengthened further when the influence of neuronal activity is considered. In neurons treated with glutamate, PSD-95 is depalmitoylated and dispersed and AMPARs internalize. Overexpression of a constitutively membrane-bound (prenylated) form of PSD-95 blocks both PSD-95 cluster dispersal and AMPAR internalization [26]. The underlying molecular basis for these dramatic effects of PSD-95 palmitoylation on AMPARs has not been fully elucidated. However, evidence points to a key role for a pool of PSD-95 complexed with AMPAR auxiliary subunits of the TARP (transmembrane AMPA receptor regulatory protein) family (Figure 2). In particular, wild-type PSD-95 overexpression increases AMPAR-mediated transmission, an effect likely to be mediated by direct TARP binding, but this effect is not seen with non-palmitoylatable C3S/C5S PSD-95 [30]. Intriguingly, the major hippocampal TARP CACNG8 (calcium channel, voltage-dependent, γ subunit 8) is also likely to be palmitoylated [17], although the role of this modification is as yet unknown.
Figure 2. Possible mechanism for the selective loss of synaptic AMPARs and PSD-95 caused by 2-Br.
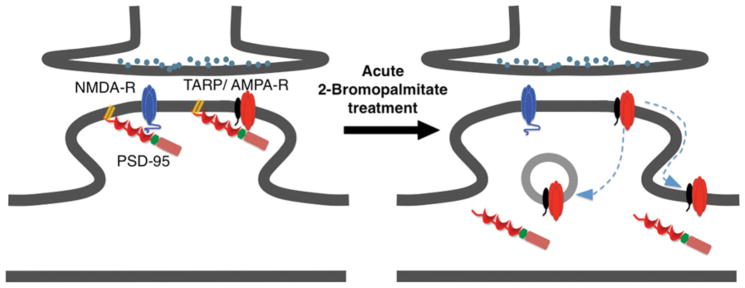
Under basal conditions, PSD-95 is palmitoylated at synapses and probably forms distinct complexes with both NMDARs and AMPARs. NMDARs bind PSD-95 directly, whereas AMPARs bind PSD-95 via TARPs. Acute (6–8 h) 2-Br treatment depalmitoylates and declusters PSD-95. This leads to diffusion of synaptic AMPARs to extra-synaptic and/or internal sites. However, despite the loss of synaptic PSD-95, synaptic NMDARs are minimally affected, perhaps because they are stabilized by other protein–protein interactions.
Enzymatic control of PSD-95 palmitoylation is also multilayered. Originally, several DHHC family PATs (DHHC2, DHHC3, DHHC7 and DHHC15) capable of palmitoylating PSD-95 were identified [31]. Of these, DHHC2 and DHHC3 are the major PSD-95 PATs expressed in hippocampal neurons, where they play different roles [18]. The Golgi-localized DHHC3 appears to regulate constitutive PSD-95 palmitoylation, whereas the synaptodendritic DHHC2 regulates activity-dependent changes in PSD-95 palmitoylation at and near synapses. Synaptodendritic PSD-95 palmitoylation is functionally important because both DHHC2 and palmitoylated PSD-95 are required for long-term activity-dependent increases in surface AMPAR expression [18].
Other palmitoylated MAGUKs (membrane-associated guanylate kinases)
PSD-95 belongs to a family of MAGUKs, each containing three PDZ domains, an SH3 (Src homology 3) domain and a region homologous with yeast guanylate kinases [25]. Of the three other mammalian MAGUKs, PSD-93 (also known as Chapsyn), SAP (synapse-associated protein) 97 and SAP102, only PSD-93 has been demonstrated to be palmitoylated [32]. Indeed, two PSD-93 isoforms, each with a different pattern of N-terminal cysteine residues, can be palmitoylated. However, the role of PSD-93 palmitoylation is less clear than that of PSD-95. PSD-93 palmitoylation can drive clustering of ion channels in transfected non-neuronal cells [32], but appears to be dispensable for synaptic targeting of either PSD-93 splice form in neurons [33]. The precise role of neuronal PSD-93 palmitoylation, and the PAT(s) responsible, therefore remains to be determined. It is noteworthy that the MAGUK SAP102 is not palmitoylated, despite possessing multiple N-terminal cysteine residues that score highly in palmitoylation site prediction programs (e.g. [34]). Instead, the cysteine-containing N-terminal domain of SAP102 is dedicated to binding Zn2+ ions [32]. This finding demonstrates the need to confirm bioinformatically predicted palmitoylation sites experimentally.
Palmitoylation of GRIP (glutamate-receptor-interacting protein) 1, GRIP2 and direct AMPAR interactors
Whereas NMDARs predominantly bind MAGUK family PDZ domain proteins, AMPARs have a distinct set of PDZ domain interactors, which include the multi-PDZ domain GRIP1 and GRIP2 (also known as ABP for AMPAR-binding protein) [35,36]. Both GRIPs directly bind and regulate GluA2-containing AMPARs.
Each GRIP exists in multiple splice forms, and the splice forms GRIP1b and GRIP2b (the latter is also known as pABP-L) contain a unique N-terminal cysteine residue that is palmitoylated [37]. Similar to the role of PSD-95 palmitoylation, overexpressed palmitoylated GRIP2b is targeted to dendritic spines [16,38]. GRIP2b overexpression dramatically increases spine number and size, even causing enlarged presynaptic puncta of synaptophysin that contact the GRIP2b-expressing neuron [38]. However, whether endogenously palmitoylated GRIP2b plays a similar role is unclear.
In contrast with the synaptic targeting of palmitoylated PSD-95 and GRIP2b, palmitoylation of GRIP1b has a different function. In this case, palmitoylation directs GRIP1b to a specific type of dendritic vesicle: recycling endo-somes [15]. GRIP1 plays a specific role in activity-dependent GluA2 recycling [39] and mimicking palmitoylation of GRIP1b with a constitutive lipid-attachment motif enhances activity-dependent GluA2 trafficking back to the plasma membrane [15] (Figure 3). The palmitoylation-mimicking form of GRIP1b also interacts more strongly with kinesin motor proteins. This suggests that palmitoylated GRIP1b may serve to ‘bridge’ receptor-containing vesicles with the motor proteins that move these vesicles within dendrites [15].
Figure 3. Unique substrate recognition by DHHC5/DHHC8 PATs allows palmitoylated GRIP1b to regulate vesicular AMPAR trafficking.
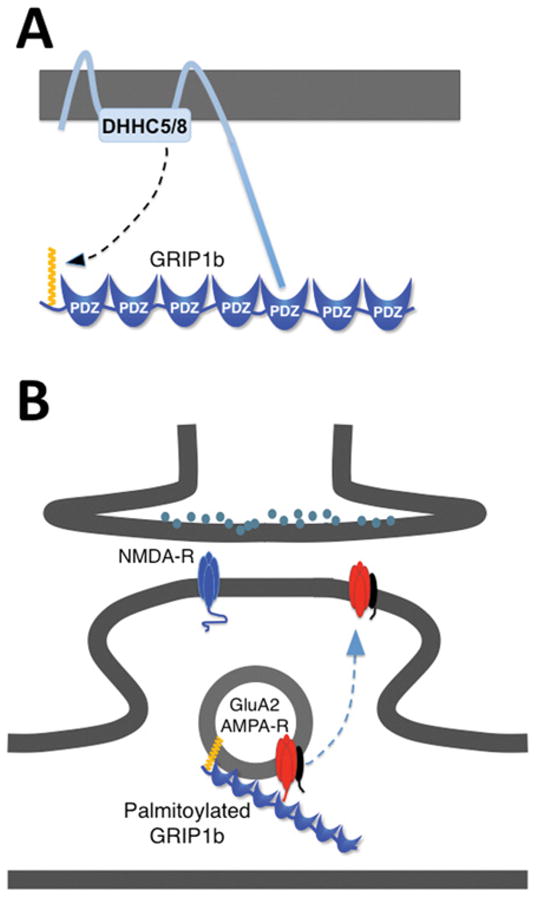
(A) The C-terminal PDZ ligands of DHHC5 and DHHC8 bind GRIP1b. This binding is necessary for the recognition of GRIP1b as a substrate for palmitoylation. (B) Palmitoylated GRIP1b is targeted to recycling endosomes, where it accelerates activity-dependent recycling of GluA2-containing AMPARs back to the plasma membrane.
Why should palmitoylated GRIP1b and GRIP2b localize to such distinct places in neurons and play such different roles? At least two plausible explanations exist for this. First, the GRIP2b N-terminus surrounding the palmitoylated cysteine residue contains a string of basic residues, whereas GRIP1b lacks this polybasic motif. Polybasic motifs co-operate with lipid modifications to direct plasma membrane/spine targeting of other proteins [40,41], whereas a single lipid modification can result in intracellular or vesicular targeting [42]. Secondly, the kinesin-binding region of GRIP1b is poorly conserved in GRIP2, suggesting that GRIP2 is unlikely to regulate trafficking of vesicular cargo. Thus differential lipid modification, often in combination with neighbouring amino acids, can allow otherwise similar proteins to play very different roles in neurons. This increases the molecular toolkit available to a neuron to regulate different aspects of receptor trafficking and localization.
How is palmitoylation of GRIP1b and GRIP2b regulated? The PATs responsible for GRIP1b palmitoylation were identified recently as the related enzymes DHHC5 and DHHC8 [15], with DHHC5 being the dominant GRIP1b PAT. Consistent with this observation, DHHC5 and palmitoylated GRIP1b are similarly localized predominantly in dendritic shafts, and only rarely at synapses.
Interestingly, both DHHC5 and DHHC8 terminate in identical PDZ ligands that directly bind the PDZ domains of GRIP1b. This direct binding is necessary for DHHC5/DHHC8 to palmitoylate GRIP1b in transfected heterologous cells, and for DHHC5 to target GRIP1b to dendritic vesicles in neurons [15] (Figure 3). PDZ ligand-dependent recognition of specific substrates has been reported previously for protein kinases [43,44], and its use by DHHC5/8 may be an example of convergent evolution.
The PAT(s) responsible for GRIP2b palmitoylation is less clear. Although co-transfected DHHC5 and DHHC8 also bind the PDZ domains of GRIP2 in heterologous cells, shRNA (short hairpin RNA)-mediated DHHC5 knockdown in neurons only mildly reduces GRIP2b palmitoylation (G.M. Thomas and R.L. Huganir, unpublished work). This is consistent with the localization of DHHC5 in dendritic shafts, compared with the spine localization of GRIP2b. It is possible that GRIP2b palmitoylation is mediated by DHHC8, which is reported to have a more synaptic localization than DHHC5 [15,45]. However, other PATs may contribute to GRIP2b regulation.
Consistent with their roles in synaptodendritic palmitoyla-tion, it is also of note that loss of either DHHC5 or DHHC8 leads to behavioural impairments in mice [45,46]. Even more intriguingly, both of these PATs are linked to neurodevelopmental and/or neuropsychiatric conditions in human genetic studies [15,45]. Whether known or additional DHHC5/DHHC8 substrates underlie these links is unclear. PSD-95 palmitoylation is reported to be reduced in DHHC8-knockout mice [47]. However, in hippocampal neurons, DHHC2 appears to be the dominant PSD-95 PAT, raising the possibility that DHHC8-dependent effects may be indirect consequences of impaired neurodevelopment.
Palmitoylation of other glutamate-receptor-regulatory proteins
In the present paper, we have focused on the palmitoylation of glutamate receptors and their direct interactors, but many other synaptic proteins are now known to be palmitoylated. Currently, the effects of palmitoylation of the majority of these proteins remain uninvestigated, but will probably turn out to regulate diverse aspects of synaptic function. For example, palmitoylation of AKAP (A-kinase-anchoring protein) 79/150, a scaffold protein that binds both protein phosphatases and kinases, and regulates both NMDARs and AMPARs, was reported to be palmitoylated [17,48]. Similar to GRIP1b, palmitoylation targets AKAP79 to recycling endosomes. Unexpectedly, a non-palmitoylatable form of AKAP79 markedly increases the number of glutamatergic synapses, suggesting that palmitoylation normally restricts AKAP79-dependent signals to appropriate locations [48]. Thus palmitoylation, normally considered a ‘gain-of-function’ modification, can also serve as a tether or ‘brake’ to restrict inappropriate signaling. Additional investigation may thus shed new light on to new roles for palmitoylation at the cellular level.
Current knowledge, outstanding questions
The last few years have seen considerable steps forward in our understanding of the extent and roles of synaptic palmitoylation. Several advances aided this progress, including the discovery of the DHHC family PATs, refinements of non-radioactive methods to track palmitoylation [49] and the use of shRNA knockdown/rescue approaches to probe the roles of specific palmitoylation events [48]. This last approach is particularly well suited to investigating palmitoylation in cultured neurons. Despite this progress, current knowledge is clearly far from complete. So what major challenges lie ahead?
First, the specific roles and substrates of many neuronal PATs remain unclear. This knowledge is likely to increase as individual PATs and their binding partners are studied. These advances will require some effort, but would appear achievable with current methods. However, additional progress may require technical challenges to be overcome. For example, studies with transfected non-palmitoylatable mutants strongly suggest that AMPAR and NMDAR subunits are palmitoylated at multiple sites [13,14], yet current methods cannot monitor individual palmitoylation sites on endogenous proteins. This situation is similar to studies of protein phosphorylation some years ago, an issue that was largely solved by the development of phospho-specific antibodies. Palmitoylation state-specific antibodies that recognize individual sites could be similarly invaluable. Moreover, if suitable for immunocytochemistry, such antibodies would help to address where specific palmitoylation events occur in neurons, another pressing question. Successfully generated palmitoylation state-specific antibodies were reported recently in abstract form [50], and their full description in the published literature is eagerly awaited.
Despite these challenges, recent progress has highlighted the importance of palmitoylation in neuronal regulation. Hundreds of synaptic palmitoylated proteins have already been identified proteomically, and this number will probably rise as lower-abundance proteins are studied. The importance of palmitoylation for normal neuronal function is underscored by the dramatic phenotypes frequently observed when specific palmitoylation events are either mimicked or prevented, or when individual PATs are mutated or knocked out. One largely unexplored area, however, is how mimicking or preventing specific palmitoylation events could affect outcomes in neuropathological conditions. As mentioned above, DHHC family PATs and several palmitoylated proteins are already linked to both neurodevelopmental and neuropsychiatric disorders. As PATs and thioesterases are, at least a priori, ‘druggable’, they represent a novel group of enzymes that could be targeted to ameliorate a wide variety of disease conditions. This exciting possibility gives us even more reason to look forward to the next years of progress in the field of neuronal palmitoylation.
Acknowledgments
We thank Dr Cindy Benedict-Alderfer for comments on the paper.
Funding
Work in the laboratory of G.M.T. is funded by a Basic Research Grant and Seed Funding from the Shriners Hospitals for Children. Work in the laboratory of R.L.H. is funded by the Howard Hughes Medical Institute and by grants from National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Mental Health (NIMH).
Abbreviations used
- AKAP
A-kinase-anchoring protein
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- AMPAR
AMPA receptor
- 2-Br
2-bromopalmitate
- GRIP
glutamate-receptor-interacting protein
- MAGUK
membrane-associated guanylate kinase
- NMDA
N-methyl-D-aspartate
- NMDAR
NMDA receptor
- PAT
palmitoyl acyltransferase
- PSD
postsynaptic density
- SAP
synapse-associated protein
- shRNA
short hairpin RNA
- TARP
transmembrane AMPA receptor regulatory protein
References
- 1.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 3.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 4.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 6.Scholz R, Berberich S, Rathgeber L, Kolleker A, Kohr G, Kornau HC. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron. 2010;66:768–780. doi: 10.1016/j.neuron.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulangara K, Kropf M, Glauser L, Magnin S, Alberi S, Yersin A, Hirling H. Phosphorylation of glutamate receptor interacting protein 1 regulates surface expression of glutamate receptors. J Biol Chem. 2007;282:2395–2404. doi: 10.1074/jbc.M606471200. [DOI] [PubMed] [Google Scholar]
- 9.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 11.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 12.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–226. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas GM, Hayashi T, Chiu SL, Chen CM, Huganir RL. Palmitoylation by DHHC5/8 targets GRIP1 to dendritic endosomes to regulate AMPA-R trafficking. Neuron. 2012;73:482–496. doi: 10.1016/j.neuron.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSouza S, Fu J, States BA, Ziff EB. Differential palmitoylation directs the AMPA receptor-binding protein ABP to spines or to intracellular clusters. J Neurosci. 2002;22:3493–3503. doi: 10.1523/JNEUROSCI.22-09-03493.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, Bailey AO, Thompson JX, Roth AF, Drisdel RC, Mastro R, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noritake J, Fukata Y, Iwanaga T, Hosomi N, Tsutsumi R, Matsuda N, Tani H, Iwanari H, Mochizuki Y, Kodama T, et al. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol. 2009;186:147–160. doi: 10.1083/jcb.200903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 20.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uemura T, Mori H, Mishina M. Isolation and characterization of Golgi apparatus-specific GODZ with the DHHC zinc finger domain. Biochem Biophys Res Commun. 2002;296:492–496. doi: 10.1016/s0006-291x(02)00900-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Xiong W, Kojic L, Cynader MS. Subunit-selective palmitoylation regulates the intracellular trafficking of AMPA receptor. Eur J Neurosci. 2009;30:35–46. doi: 10.1111/j.1460-9568.2009.06788.x. [DOI] [PubMed] [Google Scholar]
- 24.Van Dolah DK, Mao LM, Shaffer C, Guo ML, Fibuch EE, Chu XP, Buch S, Wang JQ. Reversible palmitoylation regulates surface stability of AMPA receptors in the nucleus accumbens in response to cocaine in vivo. Biol Psychiatry. 2011;69:1035–1042. doi: 10.1016/j.biopsych.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 26.El-Husseini Ael D, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 27.Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 28.Li B, Otsu Y, Murphy TH, Raymond LA. Developmental decrease in NMDA receptor desensitization associated with shift to synapse and interaction with postsynaptic density-95. J Neurosci. 2003;23:11244–11254. doi: 10.1523/JNEUROSCI.23-35-11244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 30.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 32.El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. Ion channel clustering by membrane-associated guanylate kinases: differential regulation by N-terminal lipid and metal binding motifs. J Biol Chem. 2000;275:23904–23910. doi: 10.1074/jbc.M909919199. [DOI] [PubMed] [Google Scholar]
- 33.Firestein BL, Craven SE, Bredt DS. Postsynaptic targeting of MAGUKs mediated by distinct N-terminal domains. NeuroReport. 2000;11:3479–3484. doi: 10.1097/00001756-200011090-00016. [DOI] [PubMed] [Google Scholar]
- 34.Zhou F, Xue Y, Yao X, Xu Y. CSS-Palm: palmitoylation site prediction with a clustering and scoring strategy (CSS) Bioinformatics. 2006;22:894–896. doi: 10.1093/bioinformatics/btl013. [DOI] [PubMed] [Google Scholar]
- 35.Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 37.Yamazaki M, Fukaya M, Abe M, Ikeno K, Kakizaki T, Watanabe M, Sakimura K. Differential palmitoylation of two mouse glutamate receptor interacting protein 1 forms with different N-terminal sequences. Neurosci Lett. 2001;304:81–84. doi: 10.1016/s0304-3940(01)01766-9. [DOI] [PubMed] [Google Scholar]
- 38.Misra C, Restituito S, Ferreira J, Rameau GA, Fu J, Ziff EB. Regulation of synaptic structure and function by palmitoylated AMPA receptor binding protein. Mol Cell Neurosci. 2010;43:341–352. doi: 10.1016/j.mcn.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao L, Takamiya K, Thomas G, Lin DT, Huganir RL. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc Natl Acad Sci USA. 2010;107:19038–19043. doi: 10.1073/pnas.1013494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsuda K, Matsuda S, Gladding CM, Yuzaki M. Characterization of the δ2 glutamate receptor-binding protein delphilin: splicing variants with differential palmitoylation and an additional PDZ domain. J Biol Chem. 2006;281:25577–25587. doi: 10.1074/jbc.M602044200. [DOI] [PubMed] [Google Scholar]
- 41.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2:584–590. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 42.McCabe JB, Berthiaume LG. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol Biol Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasegawa M, Cuenda A, Spillantini MG, Thomas GM, Buee-Scherrer V, Cohen P, Goedert M. Stress-activated protein kinase-3 interacts with the PDZ domain of α1-syntrophin: a mechanism for specific substrate recognition. J Biol Chem. 1999;274:12626–12631. doi: 10.1074/jbc.274.18.12626. [DOI] [PubMed] [Google Scholar]
- 44.Thomas GM, Rumbaugh GR, Harrar DB, Huganir RL. Ribosomal S6 kinase 2 interacts with and phosphorylates PDZ domain-containing proteins and regulates AMPA receptor transmission. Proc Natl Acad Sci USA. 2005;102:15006–15011. doi: 10.1073/pnas.0507476102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukai J, Liu H, Burt RA, Swor DE, Lai WS, Karayiorgou M, Gogos JA. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Hu J, Hofer K, Wong AM, Cooper JD, Birnbaum SG, Hammer RE, Hofmann SL. DHHC5 interacts with PDZ domain 3 of post-synaptic density-95 (PSD-95) protein and plays a role in learning and memory. J Biol Chem. 2010;285:13022–13031. doi: 10.1074/jbc.M109.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keith DJ, Sanderson JL, Gibson ES, Woolfrey KM, Robertson HR, Olszewski K, Kang R, El-Husseini A, Dell’acqua ML. Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms. J Neurosci. 2012;32:7119–7136. doi: 10.1523/JNEUROSCI.0784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 50.Fukata Y, Dimitrov A, Noritake J, Vielemeyer O, Perez F, Fukata M. Visualization of endogenous palmitoylated PSD-95 reveals local palmitoyltransferase-induced nucleation of postsynaptic assembly. Neuroscience 2011 Meeting; Washington DC, U.S.A. 12–16 November 2011; 2011. Abstract 237. 20/D27. [Google Scholar]