Abstract
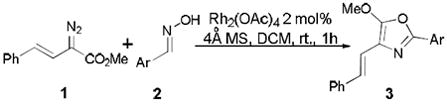
An efficient one-step synthesis of multi-functionalized oxazole derivatives is achieved in high yield by dirhodium(II)-catalyzed reactions of styryl diazoacetate with aryl oximes.
Oxazoles are widely distributed in nature, and many of them have shown biological activities.1 Because oxazoles are used as building blocks in organic synthesis2 for α,α-disubstituted amino acids,3 in cycloaddition reactions,4 and in the total synthesis of natural products,5 efficient methods for their syntheses continue to be of intense interest.6 Significant achievements in the synthesis of their core structures that are amenable to further substitutions have been reported,7 and several transition metal catalyzed methodologies for the functionalization of oxazoles have been developed.7c-7j Because these approaches require multistep syntheses, general synthetic processes for functionalized oxazoles having structural diversity and complexity continue to be needed.
Diazo compounds have been extensively studied during the last few decades,8 and several synthetic methodologies for oxazole formation have been reported,9 including oxazole syntheses from diazocarbonyl compounds with nitriles catalyzed by transition metal catalysts, Lewis acids or thermal conditions (Eq. 1).10 However, this transformation has been limited to diazoacetoacetates and diazoketones, and with ethyl diazoacetate the yield of the corresponding 5-alkoxyoxazoles (R1 = OR) is only 26~31%.10a Here we report our recent discovery of a surprisingly efficient dirhodium(II)-catalyzed reaction of styryl diazoacetate with aryl oximes to give 4-styryl-5-methoxyoxazoles directly in high yield under mild conditions (Eq. 2).
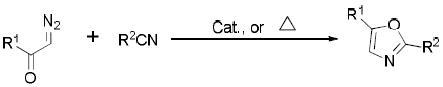 |
(1) |
The reaction between styryl diazoacetate 1 and the oxime of 4-chlorobenzaldehyde catalyzed by rhodium acetate yielded the multi-functionalized 4-styryl-5-methoxyoxazole 3a in 82% isolated yield when 4Å molecular sieves was used as an additive (Eq 2). The structure of the generated oxazole was confirmed by single-crystal X-ray diffraction analysis of its bromo-derivative.11 This process represents a significant improvement for the synthesis of 5-methoxyoxazoles from diazoacetates compared to previously reported reactions with nitriles (Eq. 1)10 and for the synthesis of 4-vinyl-5-methoxyoxazoles via a recently reported coupling strategy.7i In catalytic reactions with styryldiazoacetates the easily accessible oximes are more reactive than are nitriles and give the corresponding oxazoles in high yield. The reaction of styryl diazoacetate 1 with benzonitrile gives the corresponding oxazole in only 27 % isolated yield under the same reaction conditions.12
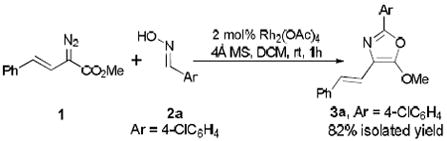 |
(2) |
Originally we thought that enoldiazoacetates 4 would undergo stepwise [3,3]-cycloaddition with oximes analogous to their asymmetric vinylogous reactions with hydrazones 5 catalyzed by Rh2(R-PTL)4 followed by diastereoselective Sc(OTf)3-catalyzed Mannich addition to form the corresponding tetrahydropyridazine derivatives (6, Scheme 1).13 However, instead of the expected six-membered outcome with enoldiazoacetates, rhodium acetate-catalyzed reactions of oximes occurred by a completely different processes.
Scheme 1.
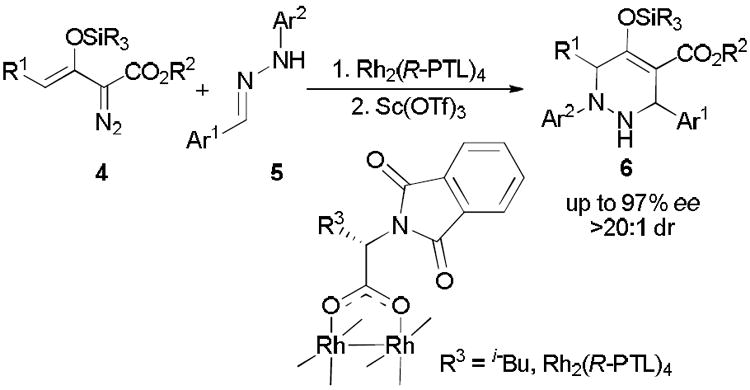
Stepwise formal [3+3]-cycloaddition of hydrazones with enoldiazoacetates
The reaction of enoldiazoacetate 4a with 4-chlorobenzaldehyde oxime 2a catalyzed by dirhodium(II) acetate gave succinate derivative 7a14 and TBS-substituted oxime 8a as major products (Eq. 3) without any evidence of an OH insertion product at the vinylogous position. This outcome is consistent with initial rhodium acetate catalyzed intramolecular conversion of enoldiazoacetate 4a to 2-TBSO-cyclopropenecarboxylate15 followed by oxime addition, ring opening, and TBS transfer (Scheme 2). The outcome described in Eq 3, in contrast with that of Scheme 1, suggests that hydrazones 5 are able to intercept the intermediate metal carbene prior to its intramolecular conversion to 2-TBSO-cyclopropenecarboxylate.
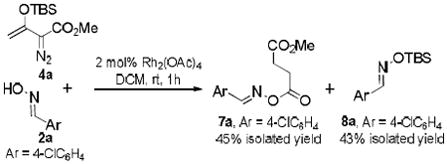 |
(3) |
Scheme 2.
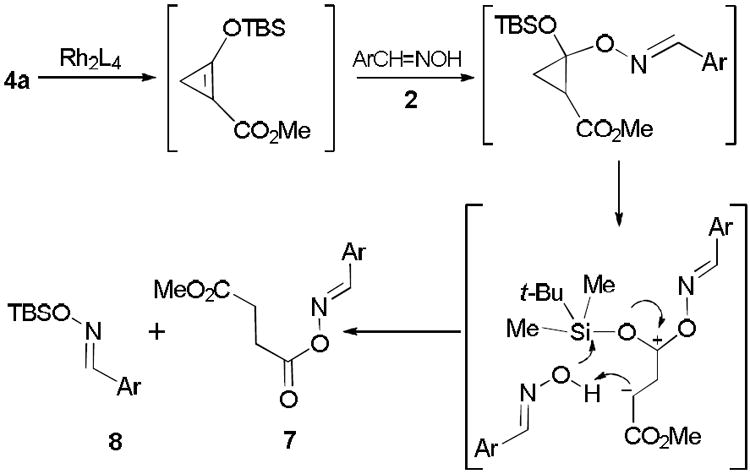
Pathway to products with enoldiazoacetates
The substrate scope for reactions of oximes with styryl diazoacetate 1 under optimized dirhodium(II)-catalyzed conditions has been determined, and the results are summarized in Table 1. All of the oxazole products were obtained in good to high yields, and 5-methoxyoxazoles 3 were the sole isolated reaction products. Both electron-deficient and electron-rich oximes give good to high yields of oxazoles. The position of the methyl substituent on the hydroxylamine’s aryl group has little influence on product yields (entries 7-9). The reaction of styryl diazoacetate 1 with 2-furyl and 2-naphthyl substrates also produced the corresponding oxazoles in 62% and 87% yield, respectively.
Table 1.
Dirhodium(II)-catalyzed oxazole synthesis reaction of styryl diazoacetate 1 with aryl oximesa
| |||
|---|---|---|---|
| entry | Ar (2) | 3 | yield (%)b |
| 1 | 4-ClC6H4 (2a) | 3a | 82 |
| 2 | 4-BrC6H4 (2b) | 3b | 89 |
| 3 | 4-FC6H4 (2c) | 3c | 71 |
| 4 | Ph (2d) | 3d | 83 |
| 5 | 4-NO2C6H4 (2e) | 3e | 91 |
| 6 | 4-MeOC6H4 (2f) | 3f | 67 |
| 7 | 4-MeC6H4 (2g) | 3g | 77 |
| 8 | 3-MeC6H4 (2h) | 3h | 71 |
| 9 | 2-MeC6H4 (2i) | 3i | 72 |
| 10 | 2-furyl (2j) | 3j | 62 |
| 11 | 2-naphthyl (2k) | 3k | 87 |
Reactions were carried out over 2 h on a 1.0 mmol scale: 1b (1.5 mmol), 2 (1.0 mmol), 4 Å MS (100 mg), in 3.0 mL DCM with Rh2(OAc)4 (2.0 mol%) at room temperature.
Isolated yield of 3 (based on limiting reagent 2).
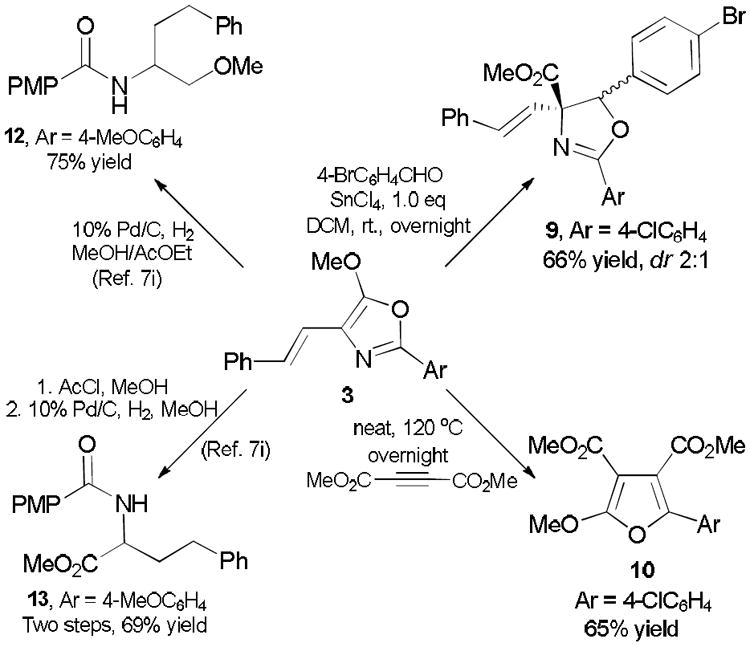
With these results in hand we investigated transformations of functionalized oxazole products to other synthetically interesting motifs (Scheme 4). A Suga-Ibata reaction of oxazole 3a with an aldehyde for the synthesis of oxazolines was performed.16 When the reaction was promoted by SnCl4, the desired addition product 9 was obtained in 66% isolated yield with 2:1 diastereoselectivity; this compound is a useful precursor of α-amino-β-hydroxyl carboxylic acids having a quaternary carbon center.17 We also employed oxazole 3a for 1,3-diplar cycloaddition reactions with dimethyl 2-butynedioate under thermal conditions.18 These reactions showed 100% conversion when dimethyl 2-butynedioate was the solvent, and furan derivative 10 was isolated as the major product accompanied by a small amount of hydrolyzed furan. Another demonstration of the utility of these 4-styryl-5-methoxyoxazole derivatives, reported by Antilla and coworkers,7i is the synthesis of functionalized amino alcohol 12 and amino acid 13 derivatives in high yield.
Scheme 4.
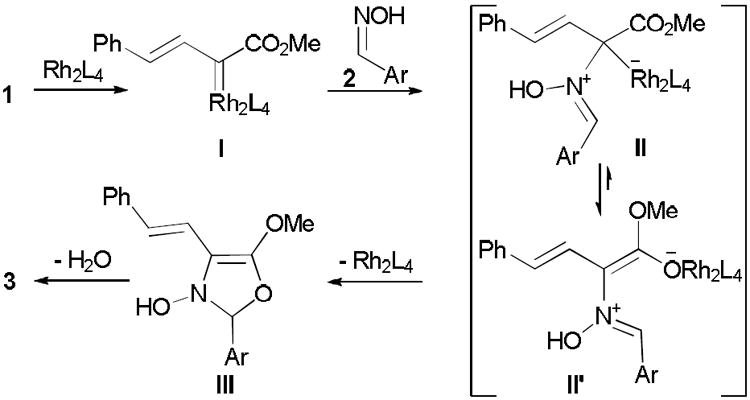
Proposed reaction mechanism for oxazole formation from styryldiazoacetate woth oximes
In summary, we have discovered an efficient dirhodium(II)-catalyzed synthesis of 4-styryl-5-methoxyoxazoles starting from styryl diazoacetate and oximes in high yield under mild conditions. A possible mechanism for formation of 4-styryl-5-methoxy-2-aryloxazoles (3) from catalytic reactions between 1 and 2 is described in Scheme 4. Dirihodium(II)-catalyzed dinitrogen extrusion from styryldiazoacetate (1) forms metal carbene which reacts with oximes and generates the azomethine yilde (II). Rapid equilibration of this intermediate to the corresponding enol anion (II’) followed by oxo-Mannich addition to produce the ring-closed structure (III), that with final aromatization by dehydration gives oxazole derivatives 3 in high yield. Additional investigations are underway to investigate the scope of these reactions with other vinyldiazoacetates and to ascertain where the crossover point occurs in intermolecular interception of the intermediate metal carbene versus its intramolecular conversion to cyclopropenecarboxylates.
Supplementary Material
Scheme 3.
Acknowledgments
MPD ais grateful to the National Institutes of Health (GM 465030) for their support of this research. WH thanks the National Science Foundation of China (20932003), and the MOST of China (2011CB808600).
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.a) Palmer DC, editor. Oxazoles: Synthesis, Reactions and Spectroscopy, Part A. John Wiley & Sons; Hoboken, NJ: 2003. [Google Scholar]; b) Palmer DC, editor. Oxazoles: Synthesis, Reactions and Spectroscopy, Part B. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]; c) Ikeda Y, Nonaka H, Furumai T, Onaka H, Igarashi Y. J Nat Prod. 2005;68:1061. doi: 10.1021/np050091j. [DOI] [PubMed] [Google Scholar]; d) Banks JC, Moody CJ. Tetrahedron Lett. 2009;50:3371. [Google Scholar]
- 2.a) Padwa A. In: Progress in Heterocyclic Chemistry. Suschitzky H, Scriven EFV, editors. Vol. 6. Pergamon Press; Oxford: 1994. pp. 56–73. [Google Scholar]; b) Lee YJ, Lee JY, Kim MJ, Kim TS, Park HG, Jew SS. Org Lett. 2005;7:1557. doi: 10.1021/ol050228x. [DOI] [PubMed] [Google Scholar]; c) Arp FO, Fu GC. J Am Chem Soc. 2006;128:14264. doi: 10.1021/ja0657859. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Vedejs E, Grissom JW. J Am Chem Soc. 1988;110:3238. [Google Scholar]
- 3.a) Shaw SA, Aleman P, Christy J, Kampf JW, Va P, Vedejs E. J Am Chem Soc. 2006;128:925. doi: 10.1021/ja056150x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Uraguchi D, Koshimoto K, Miyake S, Ooi T. Angew Chem Int Ed. 2010;49:5567. doi: 10.1002/anie.201002315. [DOI] [PubMed] [Google Scholar]; c) Joannesse C, Johnston CP, Concellón C, Simal C, Philp D, Smith AD. Angew Chem Int Ed. 2009;48:8914. doi: 10.1002/anie.200904333. [DOI] [PubMed] [Google Scholar]
- 4.a) Jacobi PA, Lee Kyungae. J Am Chem Soc. 2000;122:4295. [Google Scholar]; b) Sabot C, Oueis E, Brune X, Renard P. Chem Commun. 2012;48:768. doi: 10.1039/c1cc16562c. [DOI] [PubMed] [Google Scholar]; c) Badillo JJ, Arevalo GE, Fettinger JC, Franz AK. Org Lett. 2011;13:418. doi: 10.1021/ol1027305. [DOI] [PubMed] [Google Scholar]
- 5.a) Li P, Evans CD, Wu Y, Cao B, Hamel E, Joullie MM. J Am Chem Soc. 2008;130:2351. doi: 10.1021/ja710363p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Grimley JS, Sawayama AM, Tanaka H, Stohlmeyer MM, Woiwode TF, Wandless TJ. Angew Chem Int Ed. 2007;46:8157. doi: 10.1002/anie.200702537. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tanaka H, Sawayama AM, Wandless TJ. J Am Chem Soc. 2003;125:6864. doi: 10.1021/ja035429f. [DOI] [PubMed] [Google Scholar]; d) Wang Y, Janjic J, Kozmin SA. J Am Chem Soc. 2002;124:13670. doi: 10.1021/ja028428g. [DOI] [PubMed] [Google Scholar]; e) Smith AB, Minbiole KP, Verhoest PR, Schelhaas M. J Am Chem Soc. 2001;123:10942. doi: 10.1021/ja011604l. [DOI] [PubMed] [Google Scholar]
- 6.a) Zhu C, Yoshimura A, Sointsev PV, Ji L, Wei Y, Nemykin VN, Zhdankin V. Chem Commun. 2012 doi: 10.1039/C2CC34836E. Accepted Manuscript. [DOI] [PubMed] [Google Scholar]; b) Amaike K, Muto K, Yamaguchi J, Itami K. J Am Chem Soc. 2012;134:13573. doi: 10.1021/ja306062c. [DOI] [PubMed] [Google Scholar]; c) Cano I, Alvarez E, Nicasio MC, Pérez PJ. J Am Chem Soc. 2011;133:191. doi: 10.1021/ja109732s. [DOI] [PubMed] [Google Scholar]; d) He W, Li C, Zhang L. J Am Chem Soc. 2011;133:8482. doi: 10.1021/ja2029188. [DOI] [PubMed] [Google Scholar]
- 7.a) Williams DR, Fu L. Org Lett. 2010;12:808. doi: 10.1021/ol902833p. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bonne D, Dekhane M, Zhu J. Angew Chem Int Ed. 2007;46:2485. doi: 10.1002/anie.200605005. [DOI] [PubMed] [Google Scholar]; c) Kitahara M, Umeda N, Hirano K, Satoh T, Miura M. J Am Chem Soc. 2011;133:2160. doi: 10.1021/ja111401h. [DOI] [PubMed] [Google Scholar]; d) Besselièvre F, Mahuteau-Betzer F, Grierson DS, Piguel S. J Org Chem. 2008;73:3278. doi: 10.1021/jo7027135. [DOI] [PubMed] [Google Scholar]; e) Verrier C, Martin T, Hoarau C, Marsais F. J Org Chem. 2008;73:7383. doi: 10.1021/jo801093n. [DOI] [PubMed] [Google Scholar]; f) Miyasaka M, Hirano K, Satoh T, Miura M. J Org Chem. 2010;75:5421. doi: 10.1021/jo101214y. [DOI] [PubMed] [Google Scholar]; g) Besselièvre F, Piguel S. Angew Chem Int Ed. 2009;48:9553. doi: 10.1002/anie.200904776. [DOI] [PubMed] [Google Scholar]; h) Cho H, Joseph J, Chang S. Angew Chem Int Ed. 2010;49:9899. doi: 10.1002/anie.201005922. [DOI] [PubMed] [Google Scholar]; i) Cui S, Wojtas L, Antilla CJ. Org Lett. 2011;13:5040. doi: 10.1021/ol201865h. [DOI] [PubMed] [Google Scholar]; j) Besselièvre F, Piguel S, Mahuteau-Betzer F, Grierson DS. Org Lett. 2008;10:4029. doi: 10.1021/ol801512q. [DOI] [PubMed] [Google Scholar]
- 8.a) Doyle MP, McKervey MA, Ye T. Modern CatalyticMethods for Organic Synthesis with Diazo Compounds. John Wiley & Sins; New York: 1998. [Google Scholar]; b) Davies HM, Beckwith REJ. Chem Rev. 2003;103:2861. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]; (c) Padwa A, Weingarten MD. Chem Rev. 1996;96:223. doi: 10.1021/cr950022h. [DOI] [PubMed] [Google Scholar]; c) Xu X, Qian Y, Yang L, Hu W. Chem Commun. 2011;47:797. doi: 10.1039/c0cc03024d. [DOI] [PubMed] [Google Scholar]; d) Xu X, Zhou J, Yang L, Hu W. Chem Commun. 2008:6564. doi: 10.1039/b816104f. [DOI] [PubMed] [Google Scholar]
- 9.a) Shi B, Blake AJ, Lewis W, Campbell IB, Judkins BD, Moody CJ. J Org Chem. 2010;75:152. doi: 10.1021/jo902256r. [DOI] [PubMed] [Google Scholar]; b) Honey MA, Pasceri R, Lewis W, Moody CJ. J Org Chem. 2012;77:1396. doi: 10.1021/jo202201w. [DOI] [PubMed] [Google Scholar]; c) Austeri M, Rix D, Zeghida W, Lacour J. Org Lett. 2011;13:1394. doi: 10.1021/ol2000815. [DOI] [PubMed] [Google Scholar]; d) Linder J, Moody CJ. Chem Commun. 2007:1508. doi: 10.1039/b618160k. [DOI] [PubMed] [Google Scholar]; e) Shi B, Blake AJ, Campbell IB, Judkins BD, Moody CJ. Chem Commun. 2009:3291. doi: 10.1039/b903878g. [DOI] [PubMed] [Google Scholar]; f) Källström K, Hedberg C, Brandt P, Bayer A, Andersson PG. J Am Chem Soc. 2004;126:14308. doi: 10.1021/ja0464241. [DOI] [PubMed] [Google Scholar]
- 10.a) Doyle MP, Buhro WE, Davidson JG, Elliott RC, Hoekstra JW, Oppenhuizen M. J Org Chem. 1980;45:3657. [Google Scholar]; b) González-Bobes F, Fenster MDB, Kiau S, Kolla L, Kolotuchin S, Soumeillant M. Adv Synth Catal. 2008;350:813. [Google Scholar]; c) Lu L, Lu P, Ma S. Eur J Org Chem. 2007:676. [Google Scholar]; d) Clémençon IF, Ganem B. Tetrahedron. 2007;63:8665. [Google Scholar]
- 11.See supporting information for details. CCDC 895782 contains the supplementary crystallographic data for 3b. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12.See supporting information.
- 13.Xu X, Zavalij PJ, Doyle MP. Angew Chem Int Ed. 2012;51 doi: 10.1002/anie.201202525. anie.201203962. [DOI] [PubMed] [Google Scholar]
- 14.See supporting information for details and CCDC 895783 contains the supplementary crystallographic data for 7a. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15.Cyclopropene formation from enoldiazoacetates: Davies HML, Houser JH, Thornley C. J Org Chem. 1995;60:7529.; Davies HML, Ahmed G, Churchill MR. J Am Chem Soc. 1996;118:10774.; Xu X, Shabashov D, Zavalij PY, Doyle MP. J Org Chem. 2012;77:5313. doi: 10.1021/jo3006733.; Xu X, Shabashov D, Zavalij PY, Doyle MP. Org Lett. 2012;14:800. doi: 10.1021/ol203331r.
- 16.a) Suga H, Shi X, Ibata T. J Org Chem. 1993;58:7397. [Google Scholar]; b) Mitchell JM, Shaw JT. Angew Chem Int Ed. 2006;45:1722. doi: 10.1002/anie.200503341. [DOI] [PubMed] [Google Scholar]; c) Evans DA, Janey JM, Magomedow N, Tedrow JS. Angew Chem Int Ed. 2001;40:1884. [PubMed] [Google Scholar]; d) Huang Y, Ni L, Gan H, He Y, Xu J, Wu X, Yao H. Tetrahedron. 2011;67:2066. [Google Scholar]
- 17.a) Suga H, Fujieda H, Hirotau Y, Ibata T. J Org Chem. 1994;59:3359. [Google Scholar]; b) Suga H, Ikai K, Ibata T. J Org Chem. 1999;64:7040. [Google Scholar]; c) Griesbeck AG, Bondock S, Lex Johann. J Org Chem. 2003;68:9899. doi: 10.1021/jo034830h. [DOI] [PubMed] [Google Scholar]
- 18.a) Gotthardt H, Huisge R, Bayer H. J Am Chem Soc. 1970;92:4340. [Google Scholar]; b) Caesar JC, Griffiths DV, Griffiths PA, Tebby JC. J Chem Soc Perkin Trans 1. 1990:2329. [Google Scholar]; c) Sabot C, Oueis E, Brune X, Renard P. Chem Commun. 2012;48:768. doi: 10.1039/c1cc16562c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


