Abstract
Previous research in our laboratory showed that retinol inhibited all-trans retinoic acid (ATRA)-resistant human colon cancer cell invasion via a retinoic acid receptor (RAR)-independent mechanism in vitro. The objective of the current study was to determine if dietary vitamin A supplementation inhibited metastasis of ATRA-resistant colon cancer cells in a nude mouse xenograft model. Female nude mice (BALB/cAnNCr-nu/nu, n=14 per group) consumed a control diet (2400 IU retinyl palmitate/kg diet) or a vitamin A supplemented diet (200,000 IU retinyl palmitate/kg diet) for one month prior to tumor cell injection to preload the liver with vitamin A. HCT-116, ATRA-resistant, human colon cancer cells were intrasplenically injected. Mice continued to consume their respective diets for 5 wks following surgery. Consumption of supplemental vitamin A decreased hepatic metastatic multiplicity to 17% of control. Hepatic and splenic retinol and retinyl ester concentrations were significantly higher in the mice supplemented with vitamin A when compared to mice consuming the control diet. Supplemental vitamin A did not decrease body weight, feed intake or cause toxicity. Thus, supplemental dietary vitamin A may decrease the overall number of hepatic metastasis resulting from colon cancer.
Keywords: colon cancer, vitamin A, chemoprevention, metastasis
Introduction
Death due to colorectal cancer is generally caused by distant metastasis rather than the primary tumor itself (1). Many cancers metastasize to specific organs [For a review please see: (2)]. For example, colon cancer mainly metastasizes to the liver and lungs (2). The retinoids, a group of compounds consisting of vitamin A, its natural metabolites and several synthetic compounds, have also been shown to inhibit metastasis in a variety of in vivo model systems (3-13). Most previous work concerns the efficacy of all-trans retinoic acid (ATRA) to prevent tumor metastasis. However, the phenomenon of ATRA-resistance limits the efficacy of this compound as a cancer therapeutic. ATRA resistance occurs in most tumor types during their progression and is defined as the inability of cells to growth inhibit or differentiate in response to treatment with ATRA. This resistance is due to loss of retinoic acid receptor (RAR) expression following promoter methylation [for a review see: (14)].
In addition to the phenomenon of ATRA-resistance, the diet contains almost no ATRA (15). Rather, the diet contains preformed vitamin A as retinyl esters, such as retinyl palmitate, in animal-derived food sources. Dietary retinyl palmitate has been shown to decrease malignant melanoma metastasis in mice (9). Retinyl esters are cleaved by lipases, yielding retinol and fatty acids in the intestinal lumen. Once absorbed, retinol is re-esterified and transported to the liver, the major site of vitamin A storage, via chylomicrons. Hepatic retinol levels increase in response to supplementation and values in excess of 90 μM have been reported (16). Therefore, dietary vitamin A supplementation can increase retinol levels in the colon and liver, potentially affecting both primary colon tumors and those that have metastasized to the liver. Indeed, retinol decreased hepatic metastases in a hamster model of pancreatic ductal carcinoma (5).
Previously, we showed that retinol decreased the growth and invasion of ATRA-resistant human colon cancer cells via a novel retinoic acid receptor (RAR)-independent mechanism in vitro (17-19). Our laboratory has also shown that retinol decreases matrixmetalloproteinase (MMP) 2 and 9 as well as phosphatidylinositol 3-kinase (PI3K) activity in cultured human metastatic, ATRA-resistant colon cancer cell lines (17,19). A non-ATRA-resistant, or ATRA-sensitive, cell line was not used in these previous studies or the current work because, to our knowledge, there are no metastatic ATRA-sensitive cell lines. Mouse xenograft studies utilizing intrasplenic injection or orthotopic implantation of colon tumor cells to generate hepatic metastases have underscored the importance of these enzymes in the metastatic process [for reviews please see: (20-22)]. Specifically, MMPs are a family of proteolytic enzymes associated with tissue remodeling processes that occur during tumor invasion and metastasis (23-25). Also, activation of PI3K is associated with increased cell invasion and tumor metastasis (26-28). Akt is a serine-protein kinase, downstream of PI3K. Akt activation via phosphorylation, a direct consequence of PI3K signaling, has been shown to coincide with metastasis both in vitro and in vivo (29-31).
The objective of the present study was to determine if dietary vitamin A supplementation decreased the hepatic metastases of colon tumor cells in vivo. Our model utilized intrasplenic injection of HCT-116 human colon carcinoma cells into female BALB/cAnNCr-nu/nu athymic mice to generate hepatic metastases. These mice consumed control or vitamin A-supplemented diets for one month prior to tumor injection and five weeks following injection to mimic a chemopreventive scenario. Hepatic tumor incidence and multiplicity, hepatic, splenic and serum retinoid concentrations, as well as MMP -2, -9, phosphorylated and total Akt levels, indicative of PI3K activity, were determined at sacrifice. Our data show that hepatic preloading resulted elevated hepatic and splenic retinol and retinyl ester concentrations and a marked decrease in tumor multiplicity.
Materials and Methods
Tissue Culture
The human colorectal carcinoma cell line HCT-116 was obtained from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco's Modified Eagle's Medium in a humidified atmosphere at 37°C with 5% CO2. Media were supplemented with 10% fetal bovine serum and antibiotics (1000 U/mL penicillin and 1000 μg/mL streptomycin).
Animal Studies
Animals were housed at the University of Texas at Austin. This animal study was performed in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory animal under assurance number A4107-01 and with the University of Texas at Austin IACUC protocol number 05060201.
Purified AIN-76 rodent diet was modified to contain different concentrations of retinyl palmitate and irradiated (Research Diets, Inc, New Brunswick, NJ). Diet composition is shown in Table 1. The control diet contained 2,400 U vitamin A/kg diet as retinyl palmitate meeting the National Research Council (NRC) mouse vitamin A requirement (32). The vitamin A supplemented diet contained 200,000 IU vitamin A/kg diet as retinyl palmitate. Diets were stored at 4 °C in opaque containers to prevent degradation of vitamin A. Diets and water were consumed ad libitum. Fresh diets were provided every other day and feed consumption was recorded at this time. The mice were weighed weekly.
Table 1. Diet composition.
| Dietary vitamin A (IU/kg) | ||||
|---|---|---|---|---|
|
| ||||
| 2400 | 200,000 | |||
|
| ||||
| gm% | kcal% | gm% | kcal% | |
| Protein | 20 | 21 | 20 | 21 |
| Carbohydrate | 66 | 68 | 66 | 68 |
| Fat | 5 | 12 | 5 | 12 |
|
| ||||
| kcal/gm, Total | 3.90 | 100.0 | 3.90 | 100.0 |
|
| ||||
| Ingredient | gm | kcal | gm | kcal |
|
| ||||
| Casein | 200 | 800 | 200 | 800 |
| DL-Methionine | 3 | 12 | 3 | 12 |
| Corn Starch | 150 | 600 | 150 | 600 |
| Sucrose | 500 | 2000 | 500 | 2000 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Corn oil | 50 | 450 | 50 | 450 |
| Mineral Mix S10001 | 35 | 0 | 35 | 0 |
| Vitamin Mix V13001, no added Vitamin A | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 0 | 2 | 0 |
| Retinyl Palmitate, 250,000 IU/gm | 0.0096 | 0 | 0.8 | 0 |
|
| ||||
| Total | 1000.0596 | 3902 | 1000.85 | 3902 |
Female BALB/cAnNCr-nu/nu athymic mice (n=14 per group; strain code 01B70, NCI-Frederick, Frederick, Maryland) aged 6 to 8 weeks consumed either the 2,400 IU vitamin A/kg control diet or a diet containing 200,000 IU vitamin A/kg diet as retinyl palmitate for one month prior to intrasplenic injection. Metastasized liver tumors were generated by intrasplenic injection of 2 × 106 HCT-116 cells in 50 μl of magnesium and calcium free Hank's buffered saline solution (HBSS) as described by (33-35).
Following surgery, the mice consumed their respective diets for five more weeks prior to sacrifice. Livers were excised at sacrifice and liver metastatic incidence and multiplicity determined visually. Half of each liver was fixed in 10% formalin for immunohistochemical and pathological analysis. The remaining half of each liver and the entire spleen were snap frozen in liquid nitrogen. These liver and spleen samples along with serum samples were stored at −80C prior to high-pressure liquid chromatographic (HPLC) determination of retinoid levels.
Upon necropsy, the number of metastatic tumors per liver ranged from greater than 100 to fewer then 10. Because it was difficult to accurately determine the number of tumors when there were more than 50 tumors per liver, we developed a scoring system to quantify tumor multiplicity, similar to the system in (36), as shown in Table 2.
Table 2. Tumor multiplicity scores.
| Number of tumors per liver | Score |
|---|---|
| 1 to 10 | 1 |
| 11 to 20 | 2 |
| 21 to 30 | 3 |
| 31 to 40 | 4 |
| 41 to 50 | 5 |
| 51 to 60 | 6 |
| 61 to 70 | 7 |
| 71 to 80 | 8 |
| 81 to 90 | 9 |
| 91 to 100 | 10 |
| Over 101 | 11 |
Immunohistochemistry
Formalin-fixed liver portions were paraffin-embedded prior to immunohistochemistry. Hematoxylin and eosin stained sections were also examined for signs of liver toxicity by a veterinary pathologist. All immunohistochemistry was performed in the Histology and Tissue Processing Facility Core at MD Anderson, Science Park, Smithville, TX. Cytokeratin (CK) 20 (sc-17113, Santa Cruz Biotechnology, Santa Cruz, CA), a protein expressed in human goblet cells and enterocytes (37), was used to detect metastasized human colon cancer cells in paraffin-embedded liver sections. MMP-2 (AB19167, Chemicon, Temecula, CA), MMP-9 (AB16306-50, Abcam, Cambridge, MA), total Akt (#4691, Cell Signaling, Beverly, MA), and phospho-Akt (Ser 473) (sc-7985-R, Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were also used for immunohistochemistry. Three consecutive 4 μm sections were stained with CK20, MMP-2, and MMP-9 or CK20, total Akt, and phospho-Akt in this order. Tissue sections were deparaffinized in xylene and rehydrated in descending ethanol series to water. Next, endogenous peroxidase was blocked by incubating with 3% hydrogen peroxide for 10 min. Antigen retrieval was performed by heating tissue sections in 10 mM citrate buffer (pH 6.0) for 10 min in a microwave followed by a 20 min cool down. Non-specific antibody binding was blocked with Biocare Blocking Reagent (BS966M, Biocare, Phoenix, AZ) for 10 min. A 1:100 dilution of CK20, a 1:100 dilution of MMP-2, a 1:500 dilution of MMP-9, a 1:100 dilution of total Akt and a 1:50 dilution of phospho-Akt were used for primary antibody incubation. The samples were then incubated with horseradish peroxidase conjugated anti-rabbit (RMR622, Biocare, Concord, CA) or anti-goat (GHP516L, Biocare, Concord, CA) secondary antibodies. The slides were the counterstained, mounted and observed by light microscopy. Staining intensity was determined using NIH Image. Phospho-Akt levels were normalized for total Akt levels. All immunohistochemistry was performed by the Histology & Tissue Processing Facility Core in the University of Texas M.D. Anderson Cancer Center, Smithville, TX.
High Pressure Liquid Chromatography
Serum was isolated from whole blood as described in (38). Sera, livers, and spleens were quantitatively assayed for retinoid content via HPLC as described (39). Briefly, 100 μl of serum and thawed, homogenized tissue samples were adjusted to a total volume 500 μl with PBS and the retinoids extracted with 350 μl of a 50:50 (v/v) solution of acetonitrile/butanol followed by 300 μl of a saturated K2HPO4 solution. Following thorough mixing and centrifugation, the upper phase was aspirated. Each sample (180 μl) was loaded onto an analytical 5-μm reverse-phase C18 column (Grace Vydac, Deerfield, IL). Retinoids were eluted using a three mobile phase gradient system (40,41) at a flow rate of 1.5 mL/min on a Waters Empower System (Waters Corporation, Milford, MA). Serum and tissue retinoids were identified by matches in elution time to known standards as described (39). The concentrations of the retinoids were calculated from the area under each peak detected at a wavelength of 340 nm compared to a known amount of standard. The levels of retinoids were normalized to sample volume (serum) and wet tissue weight (spleen and liver).
Statistical Analysis
Values shown are the mean ± SEM for n = 14 unless otherwise indicated. All statistical analyses were performed using SPSS (Apache Software Foundation, Wilmington, DE, version 17.0 for Macintosh). The effects of vitamin A supplementation of liver on tumor multiplicity score, liver and body weight, serum and tissue retinoid concentrations, and phospho-Akt and total Akt levels were analyzed using a two-tailed T-test for unequal variance. Results were considered significantly different at P < 0.05.
Results
Dietary vitamin A supplementation decreases liver metastatic multiplicity
All-trans retinoic acid-resistant human colorectal cancer cells were intrasplenically injected to generate liver metastases in mice consuming vitamin A-sufficient (control group) or vitamin A-supplemented diets (VAS group). All mice exhibited liver metastases, regardless of diet. Therefore, we determined metastasic multiplicity, defined here as the number of externally visible metastatic tumors per liver, per mouse. Tumor identity was confirmed by positive CK-20 staining (Fig. 1B). Five out of the 14 mice consuming the vitamin A supplemented diet (VAS group) exhibited more then 50 tumors per liver (Fig. 1A, left), making the number of tumors difficult to accurately quantify. To overcome this, we developed a scoring system (Table 2) similar to that used in (36) to quantify the number of hepatic metastatic tumors. In contrast to the control group, no mice in the VAS group had more than 50 metastases per liver (Fig. 1A, right). Supplementation with 200,000 IU vitamin A/kg diet significantly reduced tumor multiplicity from an average score of 4.4 ± 1.2 in control mice to 1.5 ± 0.3 in mice supplemented with vitamin A (P = 0.04) (Fig. 1C). These scores reflect an approximate tumor multiplicity of 56.6 ± 22.0 and 9.9 ± 3.8 hepatic metastasic tumors per mouse in the VAS and control groups, respectively. Tumor size and morphology were not affected by treatment. Metastatic tumors in both groups ranged from 1-6 mm in diameter. Liver weight did not differ significantly between dietary groups (1.6 ± 0.3 g versus 1.2 ± 0.1 g; for control and VAS groups, respectively; P= 0.16). These data indicate that dietary vitamin A supplementation prior to tumor cell injection, reduces the number of metastases per liver but not the size of each individual metastatic tumor. Overall, this reduction in the number of tumors per liver resulted in a lower tumor load per liver in mice supplemented with 200,000 IU vitamin A/kg diet when compared to control.
Fig 1. Effect of vitamin A supplementation on tumor multiplicity.
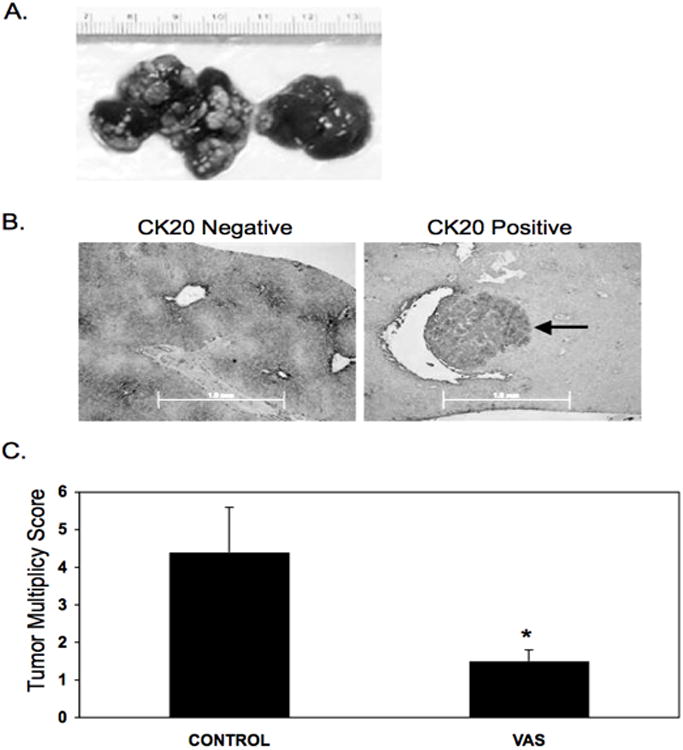
BALB/cAnNCr-nu/nu female mice, aged 6 to 8 wks, were randomly divided into two groups. The first group consumed the control diet, containing 2,400 IU vitamin A/kg diet. The second group consumed a diet supplemented with 200,000 IU vitamin A/kg diet. The mice consumed these diets ad libitum for one month prior to surgery. To generate metastases, the mice were intrasplenically injected with 2 × 106 HCT-116 cells in 50 μl of magnesium and calcium-free HBSS. Following surgery, the mice continued to consume their respective diets for five weeks. After five weeks, all mice were sacrificed and their livers examined visually for the presence and number of metastases. (A) Livers representing tumor multiplicity scores of 11 (left) and 1 (right). (B) Representative slides showing negative (left) and positive (right) staining for CK20. Arrow indicates metastatic tumor. Scale bar is 1.0 mm. (C) Data shown are hepatic tumor score per mouse, mean ± SEM, n=14. Scoring scheme is shown in Table 2. *Significantly different from control (P < 0.05).
After consuming the diets for nine weeks, there were no significant differences in body weight between control mice (21.2 ± 0.5 g) and those consuming 200,000 IU vitamin A/kg diet (20.2 ± 0.5 g). Also, there were no differences in food intake between the control and vitamin A-supplemented groups (data not shown). Finally, vitamin A toxicity results in skin irritation and hepatic cirrhosis. Neither toxicity symptom was observed in mice consuming supplemental vitamin A. Taken together, these data show that high levels of vitamin A supplementation do not adversely affect body weight and food intake or cause toxicity in mice.
Dietary vitamin A supplementation increases hepatic and splenic retinol and retinyl ester concentrations
To determine if vitamin A supplementation for nine weeks increased tissue and serum retinoid levels we examined ATRA, retinol and retinyl ester concentrations using HPLC (Fig 2. and Table 3). All-trans retinoic acid was not detected in either tissue or serum (Fig. 2). Hepatic retinol levels were elevated in mice consuming the vitamin A-supplemented diet by almost 8-fold when compared to mice consuming the control diet (1,759.0 ± 332.3 vs 231.2 ± 57.1 pmol/mg tissue, respectively; P < 0.01) (Table 3). Hepatic retinyl ester levels also exhibited a large, approximately 35-fold increase, in mice consuming the vitamin A-supplemented diet (13,928.7 ± 1,313.5 vs 398.4 ± 95.6 pmol/mg tissue, respectively; P < 0.01). Consumption of a vitamin A-supplemented diet also resulted in more than a 12-fold increase in splenic retinol concentration (126.9 ± 18.9 vs 10.1 ± 1.5 pmol/mg tissue respectively; P < 0.01) and a 26-fold elevation in splenic retinyl ester levels (7.3 ± 1.6 vs 189.9 ± 55.0 pmol/mg tissue respectively; P = 0.01) (Table 3). Specifically, serum retinol levels tended to be increased by dietary vitamin A supplementation (P = 0.10). Serum retinol concentrations were 1.2 ± 0.3 μM and 3.0 ± 0.9 μM for mice consuming the control and VAS supplemented diets, respectively (n=5 per group). These data indicate that both hepatic and splenic retinol and retinyl ester levels are increased following consumption of a vitamin A-supplemented diet. We hypothesize that this increase in tissue retinol levels is responsible for the decreased metastatic incidence.
Fig 2. Retinoids detected in tissue and serum of control and vitamin A-supplemented mice.
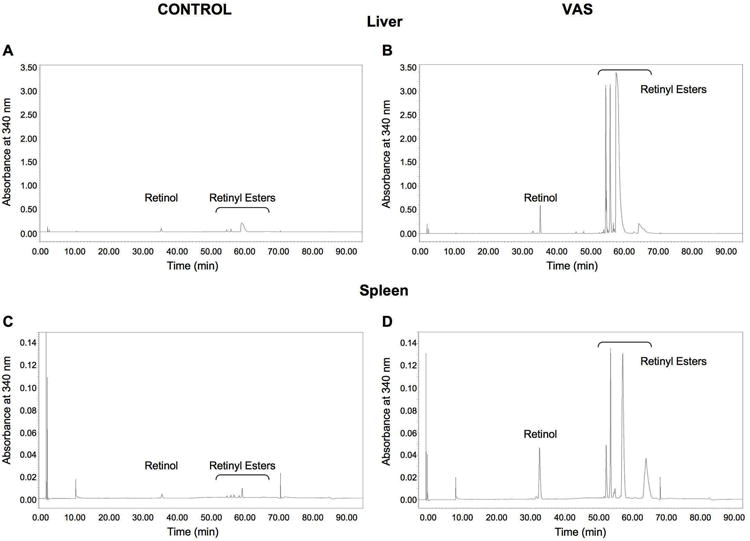
Retinol and retinyl esters but not ATRA or RAL were detected in the livers (A, B) and spleens (C, D) of control (left column) and vitamin A-supplemented (right column) mice. The results from one representative mouse per group and tissue are shown. Tissue sample weights are as follows 128.0 mg (A), 101.0 mg (B), 150.0 mg (C), 137.0 mg (D). Note y-axis scale differences between (A, B) and (C, D).
Table 3.
Tissue retinol and retinyl ester concentrations.
| Retinol (pmol/mg tissue wt weight) | Retinyl esters (pmol/mg tissue wt weight) | |||
|---|---|---|---|---|
|
| ||||
| Tissue | Control Diet | VAS Diet | Control Diet | VAS Diet |
| Liver | 231 ± 57 | 1,760 ± 330* | 398 ± 96 | 13,900 ± 1,310* |
| Spleen | 10 ± 2 | 127 ± 19* | 7 ± 2 | 190 ± 55* |
Tissue concentrations of retinoids were determined via HPLC. Data are presented as mean ± SEM, n = 14 mice per diet group. VAS, vitamin A supplemented. Astericks indicate significantly different from mice consuming the control diet.
P < 0.01.
Dietary vitamin A supplementation did not decrease MMP or phospho-Akt levels in metastatic tumors
In a previous study, we showed that retinol decreased MMP-2, -9 and PI3K activity resulting in inhibition of human colon cancer cell invasion in vitro (17,19). To determine if the activity of these enzymes were decreased by dietary vitamin A supplementation, immunohistochemistry for MMP -2, -9, total Akt, and phospho-Akt was performed on liver metastases. Phospho-Akt corrected for total Akt represents PI3K activity. These four antibodies were pre-examined for positive staining in different tissues (data not shown). Liver metastases introduced by intrasplenic injection of human colon cancer cells did not contain detectable levels of MMP-2 and -9 (Fig. 3B and C). Metastasized tumors were positive for total and phospho-Akt. However, vitamin A supplementation did not change the level of total or phospho-Akt (Fig. 3D to F). We hypothesize that the lack of MMP -2 and -9 activity and an affect of vitamin A supplementation on PI3K activity may be due to the fact that the livers were harvested at sacrifice, long after metastatic tumor cell invasion.
Fig 3. Immunohistochemical analysis of MMP-2, MMP-9, phospho-Akt and total Akt levels in liver sections.
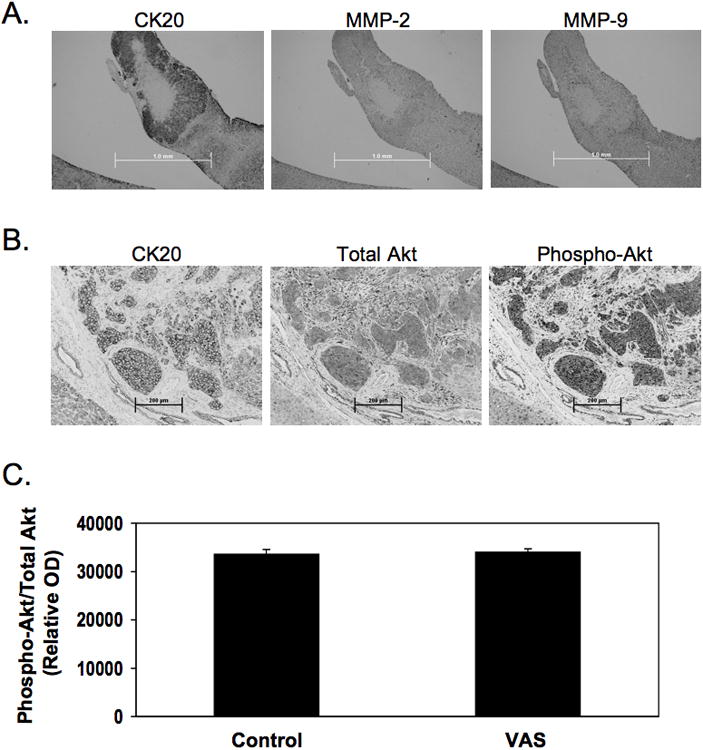
Consecutive slides of liver metastatic tumors were analyzed for CK-20, MMP-2, and MMP-9 protein (A). In addition, consecutive slides of liver metastasis sample were analyzed for with CK20, total Akt, and phospho-Akt protein levels (B). One representative sample is shown. Scale bar in (A) indicates 1.0 mm. Scale bar in (B) indicates 200 μm. The level of active Akt was not altered by vitamin A supplementation (C). Data shown are mean ± SEM, n=5.
Discussion
The liver is the major storage site for vitamin A and the target organ for colon cancer metastasis. Previously, we showed that retinol decreased ATRA-resistant colon cancer cell growth and invasion as well as MMP -2, -9 and PI3K activity in vitro (17-19). Here, we demonstrate that consumption of a diet containing 200,000 IU vitamin A/kg diet for one month prior to tumor injection, effectively pre-loading the liver with vitamin A, and five weeks following tumor injection decreased the multiplicity of liver metastases in nude mice intrasplenically injected with ATRA-resistant human colon cancer cells (Fig. 1C). In addition, dietary vitamin A supplementation resulted in large increases in both hepatic and splenic retinol and retinyl ester concentrations (Table 2). Given our previous work showing that retinol inhibits the invasion of these cells in vitro (17). We hypothesize that these elevated tissue retinoid levels, specifically retinol, were responsible for the decrease in metastatic multiplicity observed.
Retinoids have also been shown to inhibit metastasis in a variety of model systems. ATRA has been shown to inhibit metastasis in numerous studies (4,7,8,10-12,42,43); however the diet contains little ATRA (15). Retinyl palmitate is the most common dietary retinoid and has been shown to decrease malignant melanoma metastasis in mice (9). Retinyl palmitate, and all retinyl esters, are hydrolyzed to retinol in the intestinal lumen. Retinol has also been shown to inhibit hepatic metastases in a hamster model of pancreatic ductal carcinoma (5). While the current study uses a xenograft model, previous work indicates that dietary vitamin A is effective in a carcinogenesis model. Specifically, Delage et al (44) showed that dietary supplementation with 200,000 IU vitamin A/kg diet as retinyl palmitate, the same level used in the current study, inhibits high fat diet-induced aberrant crypt foci. Similar effects should be seen in other carginogenesis models following dietary vitamin A supplementation. It should be noted, however, that invasion and metastasis, the focus of the current study, rarely occur in mouse carcinogenesis models [as reviewed in (45)]. Importantly, no previous work has examined the effect of dietary vitamin A supplementation on colorectal cancer metastasis despite the ability of supplementation to increase intestinal lumen and hepatic retinoid levels.
The current study employed a chemoprevention model. Specifically, mice consumed their respective diets for one month prior to tumor injection. Our intent was to increase the liver retinoid concentrations before splenic tumor cell injection. We hypothesized elevated hepatic retinol levels would decrease metastatic incidence as well as multiplicity. However, tumor incidence in this study was 100% regardless of diet. Interestingly, splenic retinol and retinyl ester concentrations were also increased by supplemental dietary vitamin A. This increase in splenic retinoid levels may have decreased, but not eliminated, the number of metastatic cell leaving the spleen, resulting in decreased metastatic multiplicity. Previously, we showed that retinol decreased the activity of the metastasis-promoting enzymes MMP -2, -9, and PI3K (17,46). However, due to elevated concentrations of retinol in the spleen as a result of vitamin A supplementation, it is possible that any tumor cells shed by the spleen may be resistant to retinol. We hypothesize that these retinol-resistant cells would be capable of establishing hepatic metastases despite elevated hepatic retinol concentrations. The lack of a difference in tumor size between the treatment groups may also indicate that cells in the hepatic metastases are be resistant to the growth-inhibitory effect of retinol we observed in a previous in vitro study (18). Retinol-resistance combined with end-point sampling may be account for the lack of effect of dietary vitamin A supplementation on MMP-2, MMP-9 and PI3K activity in the hepatic metastases (Fig. 3). Future work will examine the effect of dietary vitamin A supplementation on MMP -2 and -9 and PI3K activity closer to the time of tumor cell injection. The activity of these proteins, particularly the MMPs, should be elevated early in the metastatic process because they are involved in both extra- and intra-vasation.
As mentioned previously, dietary vitamin A supplementation increased hepatic retinol and retinyl ester levels (Table 2). In the current study, hepatic retinol levels following consumption of both the control and VAS diets were higher than those observed by (16,47,48). This difference may be due to different lengths of time spent consuming their respective diets, different strains of mice, or another unknown factor. Hepatic retinyl ester levels in the current study were higher then those observed by (48), for mice consuming 250,000 IU vitamin A/kg diet for 6 wks; however mice in the current study consumed their respective diets for a total of 11 wks prior to sacrifice, which may account for this difference. Hepatic retinyl ester levels in mice consuming the control diet in the current study are similar to those observed by other groups (47,48) when the amount of dietary vitamin A in each control diet is taken into account [for example, (36) fed 4500 IU vitamin A/kg diet and observed retinyl ester concentrations between 800 and 1000 pmol/mg tissue].
Dietary vitamin A supplementation also increased splenic retinol and retinyl ester levels (Table 2). Although the current study is the first, to our knowledge, to quantify splenic retinoids, the role of vitamin A in immune function is well established [for reviews see: (49,50)]. It is interesting to consider that the ability of dietary vitamin A levels to alter splenic retinol and retinyl ester concentrations may, in part, confer the effects of vitamin A on the immune response.
The recommended daily allowance (RDA) levels for vitamin A is 700 mcg RAE (retinol activity units) or 2333 IU/day for female adult humans (over age 14) (51). Daily ingestion of over 100,000 IU (33.3× the RDA) for more than six months is considered toxic [For a review please see; (52)]. In addition, the smallest daily vitamin A uptake reported to cause human liver cirrhosis was 25,000 IU (10× the RDA) for six years (53). The recommended vitamin A dietary level from the NRC for mice is 2,400 IU of vitamin A/kg of diet (32). The diets used in this study contained 2,400 (1× NRC) and 200,000 (80× NRC) IU vitamin A/kg diet. Therefore, although we observed no toxicity in the current study, if the NRC recommendation for mice is proportional to the RDA for humans, the level of vitamin A supplementation used in this study may result in vitamin A toxicity in humans. Although not examined here, it is possible that a lower level of vitamin A supplementation would show the same effect as 200,000 IU/kg vitamin A supplementation and decrease metastasic multiplicity. Future studies will determine if lower levels of supplemental vitamin A, which would be better tolerated by human patients, are also effective for prevention of metastases.
In conclusion, the present study shows that dietary vitamin A supplementation prior to tumor injection decreases the multiplicity of hepatic metastases of colorectal cancer. Tumor size was not affected, thus the combination of reduced tumor number with a lack of change in tumor size indicates that the tumor load was lower per liver in mice consuming supplemental vitamin A. We also show that hepatic and, surprisingly, splenic retinol and retinyl ester levels are increased following vitamin A supplementation. To our knowledge, this study is the first to show that dietary vitamin A supplementation inhibits colon cancer metastasis to the liver in a mouse model. This is also the first study to show that splenic retinoid levels can be increased by dietary vitamin A supplementation, indicating the spleen can also store retinoids, albeit to a lesser extent than the liver. Taken together, these data suggest that dietary vitamin A supplementation may prove useful for reducing the number of metastatic tumors that develop, and thus the overall amount of cancerous tissue per liver in patients prone to colorectal cancer metastasis.
Acknowledgments
This research was supported by NIH grant #1R21CA120414-01A1 to ML and NIEHS Center Grant ES 07784. The authors thank Erik Wilder, Kally O'Reilly and Louis Doan for their assistance with the mouse surgery and Dr. Susan Fischer and Dr. Kaoru Kiguchi, of the MD Anderson Cancer Center, University of Texas Smithville, TX, for surgical training. We would also like to thank Dr. Claudio Conti, DVM, University of Texas MD Anderson Cancer Center, Smithville, TX, for his pathological analyses of the liver sections.
References
- 1.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 2.Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin Exp Metastasis. 2008;25:335–344. doi: 10.1007/s10585-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 3.Barroga EF, Kadosawa T, Okumura M, Fujinaga T. Inhibitory effects of 22-oxa-calcitriol and all- trans retinoic acid on the growth of a canine osteosarcoma derived cell-line in vivo and its pulmonary metastasis in vivo. Res Vet Sci. 2000;68:79–87. doi: 10.1053/rvsc.1999.0360. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Alonso I, Palomares T, Alonso-Varona A, Castro B, Del Olmo M, et al. Effects of all-trans retinoic acid on tumor recurrence and metastasis. Rev Esp Enferm Dig. 2005;97:240–248. doi: 10.4321/s1130-01082005000400004. [DOI] [PubMed] [Google Scholar]
- 5.Heukamp I, Kilian M, Gregor J, Neumann A, Jacobi C, et al. Effects of the antioxidative vitamins A, C, and E on liver metastasis and intrametastatic lipid peroxidation in BOP-induced pancreatic cancer in syrian hamsters. Pancreatology. 2005;5:403–409. doi: 10.1159/000086541. [DOI] [PubMed] [Google Scholar]
- 6.O'Dwyer PJ, Ravikumar TS, McCabe DP, Steele G., Jr Effect of 13-cis-retinoic acid on tumor prevention, tumor growth, and metastasis in experimental colon cancer. J Surg Res. 1987;43:550–557. doi: 10.1016/0022-4804(87)90130-2. [DOI] [PubMed] [Google Scholar]
- 7.Qiao W, Chen Y, Chen Z, Chen F, Su W. Effects of retinoic acid on metastasis and its related proteins in gastric cancer cells in vivo and in vitro. Acta Pharmacol Sin. 2002;23:835–841. [PubMed] [Google Scholar]
- 8.Suzuki S, Kawakami S, Chansri N, Yamashita F, Hashida M. Inhibition of pulmonary metastasis in mice by all-trans retinoic acid incorporated in cationic liposomes. J Control Release. 2006;116:58–63. doi: 10.1016/j.jconrel.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Weinzweig J, Tattini C, Lynch S, Zienowicz R, Weinzweig N, et al. Investigation of the growth and metastasis of malignant melanoma in a murine model: the role of supplemental vitamin A. Plastic and reconstructive surgery. 2003;112:152–158. doi: 10.1097/01.PRS.0000066008.40176.EF. [DOI] [PubMed] [Google Scholar]
- 10.Wu Q, Chen YQ, Chen ZM, Chen F, Su WJ. Effects of retinoic acid on metastasis and its related proteins in gastric cancer cells in vivo and in vitro. Acta Pharmacol Sin. 2002;23:835–841. [PubMed] [Google Scholar]
- 11.Charoensit P, Kawakami S, Higuchi Y, Yamashita F, Hashida M. Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 2010;17:512–522. doi: 10.1038/cgt.2010.12. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Chan SY, Ho PC. Comparison of the in vitro and in vivo effects of retinoids either alone or in combination with cisplatin and 5-fluorouracil on tumor development and metastasis of melanoma. Cancer Chemother Pharmacol. 2008;63:167–174. doi: 10.1007/s00280-008-0763-1. [DOI] [PubMed] [Google Scholar]
- 13.Orienti I, Zuccari G, Bergamante V, Carosio R, Gotti R, et al. Fenretinide-polyvinylalcohol conjugates: new systems allowing fenretinide intravenous administration. Biomacromolecules. 2007;8:3258–3262. doi: 10.1021/bm7005592. [DOI] [PubMed] [Google Scholar]
- 14.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Ann Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 15.Vogel S, Gamble MV, Blaner WS. Biosynthesis, absorption, metabolism and transport of retinoids. In: Nau H, Blaner WS, editors. Retinoids: the biochemical and molecular basis of vitamin A and retinoid action. Berlin: Springer-Verlag; 1999. pp. 31–95. [Google Scholar]
- 16.Garcia AL, Ruhl R, Schweigert FJ. Retinoid concentrations in the mouse during postnatal development and after maternal vitamin A supplementation. Ann Nutr Metab. 2005;49:333–341. doi: 10.1159/000087697. [DOI] [PubMed] [Google Scholar]
- 17.Park EY, Wilder ET, Lane MA. Retinol inhibits the invasion of retinoic acid-resistant colon cancer cells in vitro and decreases matrix metalloproteinase mRNA, protein, and activity levels. Nutr Cancer. 2007;57:66–77. doi: 10.1080/01635580701268238. [DOI] [PubMed] [Google Scholar]
- 18.Park EY, Dillard A, Williams EA, Wilder ET, Pepper MR, et al. Retinol inhibits the growth of all-trans-retinoic acid-sensitive and all-trans-retinoic acid-resistant colon cancer cells through a retinoic acid receptor-independent mechanism. Cancer Res. 2005;65:9923–9933. doi: 10.1158/0008-5472.CAN-05-1604. [DOI] [PubMed] [Google Scholar]
- 19.Park EY, Wilder ET, Chipuk JE, Lane MA. Retinol decreases phosphatidylinositol 3-kinase activity in colon cancer cells. Mol Carcinog. 2008;47:264–274. doi: 10.1002/mc.20381. [DOI] [PubMed] [Google Scholar]
- 20.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 22.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 23.Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 24.Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991;26:230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(1):S42–51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 26.Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 27.Shaw LM, Rabinovitz I, Wang HH, Toker A, Mercurio AM. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 28.Stephens L, Williams R, Hawkins P. Phosphoinositide 3-kinases as drug targets in cancer. Curr Opin Pharmacol. 2005;5:357–365. doi: 10.1016/j.coph.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Kim S, Koh H, Yoon SO, Chung AS, et al. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- 30.Qiao M, Iglehart JD, Pardee AB. Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer Res. 2007;67:5293–5299. doi: 10.1158/0008-5472.CAN-07-0877. [DOI] [PubMed] [Google Scholar]
- 31.Nam SY, Lee HS, Jung GA, Choi J, Cho SJ, et al. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111:1105–1113. doi: 10.1111/j.1600-0463.2003.apm1111205.x. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. Nutrient Requirements of Laboratory Animals. 4th. Washington, DC: National Academy Press; 1995. [Google Scholar]
- 33.Zirvi KA, Keogh JP, Slomiany A, Slomiany BL. Transglutaminase activity in human colorectal carcinomas of differing metastatic potential. Cancer Lett. 1991;60:85–92. doi: 10.1016/0304-3835(91)90052-j. [DOI] [PubMed] [Google Scholar]
- 34.Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, et al. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, et al. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- 36.Giavazzi R, Campbell DE, Jessup JM, Cleary K, Fidler IJ. Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res. 1986;46:1928–1933. [PubMed] [Google Scholar]
- 37.Wildi S, Kleeff J, Maruyama H, Maurer CA, Friess H, et al. Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res. 1999;5:2840–2847. [PubMed] [Google Scholar]
- 38.Williams EA, Perkins SN, Smith NC, Hursting SD, Lane MA. Carbohydrate versus energy restriction: effects on weight loss, body composition and metabolism. Ann Nutr Metab. 2007;51:232–243. doi: 10.1159/000104143. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 40.Chen AC, Guo X, Derguini F, Gudas LJ. Human breast cancer cells and normal mammary epithelial cells: retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res. 1997;57:4642–4651. [PubMed] [Google Scholar]
- 41.Guo X, Gudas LJ. Metabolism of all-trans-retinol in normal human cell strains and squamous cell carcinoma (SCC) lines from the oral cavity and skin: reduced esterification of retinol in SCC lines. Cancer Res. 1998;58:166–176. [PubMed] [Google Scholar]
- 42.Benbow U, Schoenermark MP, Orndorff KA, Givan AL, Brinckerhoff CE. Human breast cancer cells activate procollagenase-1 and invade type I collagen: invasion is inhibited by all-trans retinoic acid. Clin Exp Metastasis. 1999;17:231–238. doi: 10.1023/a:1006639214618. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Zang C, Fenner MH, Possinger K, Elstner E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat. 2003;79:63–74. doi: 10.1023/a:1023366117157. [DOI] [PubMed] [Google Scholar]
- 44.Delage B, Groubet R, Pallet V, Bairras C, Higueret P, et al. Vitamin A prevents high fat diet-induced ACF development and modifies the pattern of expression of peroxisome proliferator and retinoic acid receptor mRNA. Nutr Cancer. 2004;48:28–36. doi: 10.1207/s15327914nc4801_5. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–96. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park EY, Chiptuk J, Lane MA. Retinol decreases phosphatidylinositol 3-kinase (PI3K) activity by inhibiting PI3K and phosphatidylinositol interaction. Mol Carcinog. 2008;47:264–274. doi: 10.1002/mc.20381. [DOI] [PubMed] [Google Scholar]
- 47.Raila J, Willnow TE, Schweigert FJ. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr. 2005;135:2512–2516. doi: 10.1093/jn/135.11.2512. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Tang XH, Gudas LJ. Homeostasis of retinol in lecithin: retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem Pharmacol. 2008;75:2316–2324. doi: 10.1016/j.bcp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pino-Lagos K, Guo Y, Noelle RJ. Retinoic acid: a key player in immunity. Biofactors. 1002;36:430–436. doi: 10.1002/biof.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duriancik DM, Lackey DE, Hoag KA. Vitamin A as a regulator of antigen presenting cells. J Nutr. 1395;140:1395–1399. doi: 10.3945/jn.110.124461. [DOI] [PubMed] [Google Scholar]
- 51.US Department of Agriculture. US Department of Health and Human Services: Dietary Guidelines for Americans, 2010. 7th. Washington, DC: 2010. [Google Scholar]
- 52.Penniston K, Tanumihardjo S. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 53.Hathcock JN. Vitamins and minerals: efficacy and safety. Am J Clin Nutr. 1997;66:427–437. doi: 10.1093/ajcn/66.2.427. [DOI] [PubMed] [Google Scholar]


