Abstract
Memory formation is known to be critically dependent upon the medial temporal lobe (MTL). Despite this well-characterized role, it remains unclear whether and how MTL encoding processes are affected by top-down goal states. Here, we examined the manner in which task demands at encoding affect MTL activity and its relation to subsequent memory performance. Participants were scanned using high-resolution neuroimaging of the MTL while engaging in two incidental encoding tasks: one that directed participants’ attention to stimulus distinctiveness, and the other requiring evaluation of similarities across stimuli. We hypothesized that attending to distinctiveness would lead to the formation of more detailed memories and would more effectively engage the hippocampal circuit than attending to similarity. In line with our hypotheses, higher rates of subsequent recollection were observed for stimuli studied under the Distinctiveness than Similarity task. Critically, within the hippocampus, CA1 and the subiculum demonstrated an interaction between memory performance and task such that a significant subsequent memory effect was found only when task goals required attention to stimulus distinctiveness. To this end, robust engagement of the hippocampal circuit may underlie the observed behavioral benefits of attending to distinctiveness. Taken together, these findings advance understanding of the effects of top-down intentional information on successful memory formation across subregions of the MTL.
Keywords: medial temporal lobe, hippocampus, functional MRI, memory, encoding, goal states
1. Introduction
A wealth of evidence indicates that our memories are affected not only by available sensory information, but also the cognitive operations performed on this information. As such, top-down processing is thought to play an important role in influencing what is encoded into memory. Prior studies have implicated prefrontal and parietal regions in mediating such goal-directed control and attentional resources during learning (e.g., Blumenfeld & Ranganath, 2007; Chun & Turk-Browne, 2007; Uncapher & Rugg, 2009). It remains unclear, however, how encoding processes in the medial temporal lobe (MTL)—a region known to play a critical role in episodic memory formation—are modulated by top-down influences.
Early efforts to address this issue focused on how selective attention modulates activity in domain-specific MTL cortex, demonstrating enhanced encoding activity when preferred-category features were selectively attended (Gazzaley, Cooney, McEvoy, Knight, & D'Esposito, 2005; Yi & Chun, 2005). More recently, Uncapher & Rugg (2009) reported subsequent memory effects in the hippocampus (i.e., greater activity for subsequent hits than misses) for features that were selectively attended during learning but not for unattended features. Studies investigating modulatory effects of deep vs. shallow processing, however, have not demonstrated differences in MTL subsequent memory effects according to task demands (Fletcher, Stephenson, Carpenter, Donovan, & Bullmorel, 2003; Schott et al., 2013). As such, reports of top-down influences on MTL encoding activity are mixed. Furthermore, the degree to which subregions of the MTL may be differentially affected by top-down influences remains unclear.
To address these questions, we evaluated MTL activity using high-resolution functional MRI (hr-fMRI) as participants engaged in two encoding tasks focusing attention on either stimulus distinctiveness or commonalities across stimuli. The phenomenon of better memory for distinctive than common events is well-documented. With respect to word frequency, for example, Glanzer and Adams (1990) found both higher hit rates and lower false alarm rates for rare words like kumquat relative to common words like apple. Similarly, enhanced memory performance has been shown for items within a list that differ from the list context, such as a number within a list of syllables (e.g., Hunt, 1995). Of direct relevance to the current study, researchers have also shown that instructions to participants during learning emphasizing stimulus distinctiveness lead to higher rates of subsequent recollection than those emphasizing similarities among stimuli (Mäntylä, 1997). Taken together, such findings suggest that encoding the distinctive features of an item enhances subsequent memory performance relative to encoding commonalities across items. Given the key role of the MTL in encoding episodic memories, the aim of the current study was to assess whether and how MTL encoding processes are influenced by such differences in task demands at encoding.
Using a modified version of the paradigm employed by Mäntylä (1997), we scanned participants as they attended to either stimulus distinctiveness or similarities across stimuli. We hypothesized that attending to distinctiveness would more effectively engage encoding processes within the hippocampal circuit than attending to similarities across stimuli. Specifically, we predicted that encoding memories with differing degrees of detail would lead to an interaction between encoding task and subsequent memory performance such that subsequent memory effects in the hippocampus would be greater for items encountered in the Distinctiveness task than in the Similarity task. Critically, our use of hr-fMRI allowed us to evaluate whether potential task-related differences in subsequent memory effects were subfield-specific or evident across the entirety of the hippocampal circuit. Prior hr-fMRI studies suggest that input structures of the hippocampal circuit, namely the dentate gyrus and CA2/3 region (DG/CA2/3), are selectively active during encoding and show a bias toward pattern separation, whereas output structures (CA1 and the subiculum) have been shown to be differentially active during retrieval and pattern completion (Bakker, Kirwan, Miller, & Stark, 2008; Carr, Viskontas, Engel, & Knowlton, 2010b; Eldridge, Engel, Zeineh, Bookheimer, & Knowlton, 2005; Olsen et al., 2009; Viskontas, Carr, Engel, & Knowlton, 2009; Zeineh, Engel, Thompson, & Bookheimer, 2003). Other hr-fMRI data, however, have demonstrated hippocampal encoding or novelty effects not just in DG/CA2/3 but across all subfields of the hippocampus (Chen, Olsen, Preston, Glover, & Wagner, 2011; Duncan, Ketz, Inati, & Davachi, 2012; Preston et al., 2010; Suthana, Ekstrom, Moshirvaziri, Knowlton, & Bookheimer, 2009; Wolosin, Zeithamova, & Preston, 2012; Zeineh, Engel, & Bookheimer, 2000), and are thus more consistent with extant models of hippocampal function which vest encoding and retrieval mechanisms within the same hippocampal subfields (e.g., O’Reilly and Rudy, 2001; Rolls, 1996). Given these latter findings, we hypothesized that attention to distinctiveness would lead to more effective hippocampal encoding than attention to similarity, as evidenced by a task by subsequent memory interaction across the entirety of the hippocampus.
2. Materials and Methods
Having modified the encoding tasks originally developed by Mäntylä (1997), we first conducted a behavioral experiment (Experiment 1) to ensure that attending to stimulus distinctiveness at encoding led to greater subsequent memory performance than attending to similarities across stimuli. A separate hr-fMRI experiment (Experiment 2) was then conducted in a different group of participants.
2.1 Experiment 1
2.1.1 Participants
Thirty two healthy young adults (24 female; 18 – 29 years of age, mean age = 20.87 ± 2.78 years) participated in Experiment 1 and were paid $8 per hour for their participation. All participants were fluent English speakers. The study was performed under a protocol approved by the UCLA Office for Protection of Research Participants.
2.1.2 Materials
All stimuli were presented via a Macintosh laptop computer using the MATLAB Psychophysics Toolbox. Stimuli consisted of 99 black and white photographs of young adults, 45 male and 44 female, with neutral facial expressions (Psychological Image Collection at Stirling, http://pics.psych.stir.ac.uk). Faces were divided into three sets of 33, such that 33 served as stimuli for the Distinctiveness task, 33 for the Similarity task, and the remaining 33 faces served as lures for a surprise recognition test. Sets were counterbalanced across participants such that each set was assigned to the distinctiveness, similarity, or lure condition an equal number of times.
2.1.3 Procedure
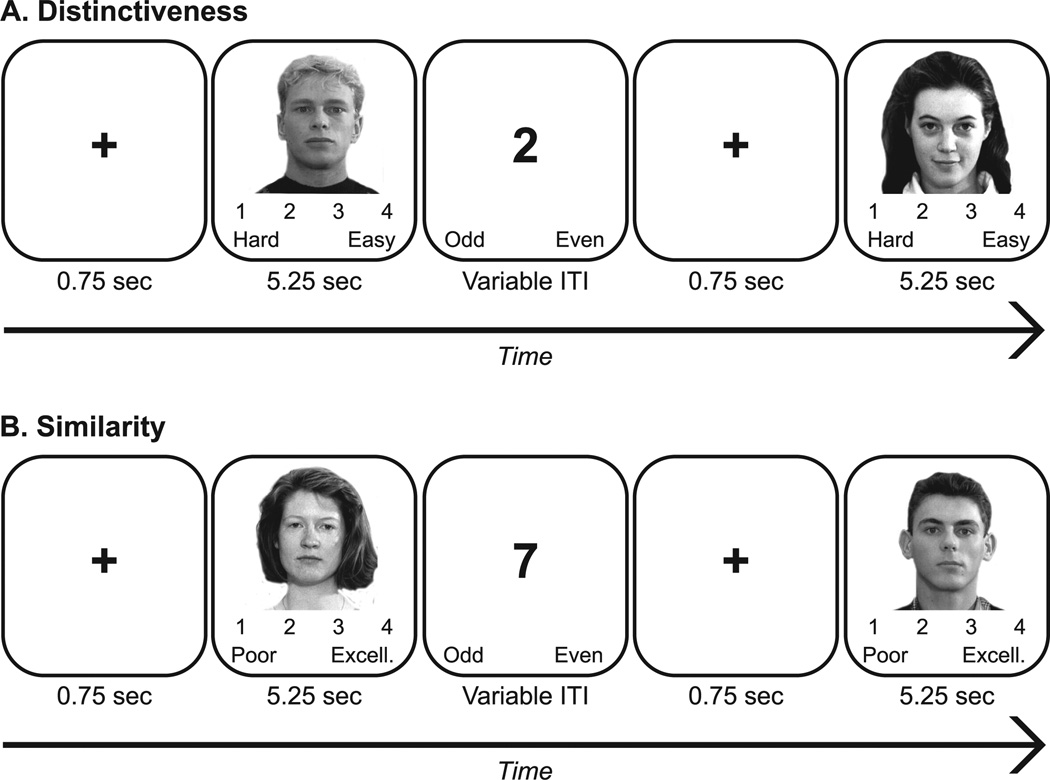
Having been recruited to perform a study of “face processing,” participants were unaware of any memory-related components of the experiment until performing a surprise recognition test. For the Distinctiveness, or “Taxi Driver”, task (Figure 1A), participants were told to imagine that they were chauffeurs picking up specific customers from the airport. As each customer’s photo appeared on the screen, participants were asked to make a subjective judgment regarding how easy it would be to find that particular customer in the crowd on a scale from 1–4, ranging from 1 = hard (customer has an average face; will blend in with the crowd), to 4 = easy (customer is very unique looking; will stand out of the crowd).
Figure 1.
Distinctiveness and Similarity tasks. During the Distinctiveness task (A), participants imagined themselves as chauffeurs and rated how easily a given “customer” could be picked out of a crowded airport on a scale from 1 to 4, ranging from 1 = hard to 4 = easy. During the Similarity task (B), participants imagined themselves as casting agents and rated how well each “actor” matched the director’s desired look on a scale from 1 to 4, ranging from 1 = poor match to 4 = excellent match. For Experiment 2, a variable inter-trial interval (ITI) was included during which participants made odd/even judgments.
In the Similarity, or “Casting Director”, task (Figure 1B), participants were told to imagine themselves as casting directors whose job it was to audition the lead male and female roles for a new film. They were informed that the director desired a particular look for each role, and that they should select actors matching this look for subsequent auditions. To this end, participants were first shown a sample male and female face fitting the desired look, and were then asked to rate a series of photos according to how well each actor matched the desired look on a scale from 1–4, ranging from 1 = poor match to 4 = excellent match.
The study employed a within-subjects design such that all participants completed both the Distinctiveness and Similarity tasks prior to performing the recognition memory test. Participants received instructions for both encoding tasks prior to beginning the experiment, and task order was counterbalanced across participants. For both tasks, trials lasted 6 sec each, beginning with a brief fixation cross (0.75 sec) followed by presentation of the face for 5.25 sec. Participants were asked to rate each face according to task instructions within this 5.25 sec period. Following completion of the second encoding task, participants performed a difficult maze for ten minutes followed by a surprise recognition test inclusive of faces from both encoding tasks. Participants first made studied/new judgments followed by remember/know judgments for those faces deemed studied. Participants were told to give a “remember” response when they remembered contextual details pertaining to when they originally viewed the face, and to give a “know” response when they confidently recognized a face in the absence of such details. This test was used to assess the extent to which successful recognition was based on episodic recollection or feelings of familiarity.
Results from each participant’s recognition test were used to sort trials into hits (studied faces that were successfully recognized), misses (studied faces that were forgotten), correct rejections (lure faces that were correctly judged as new) and false alarms (lure faces incorrectly judged as old). Hits and false alarms were further broken down according to “remember” and “know” responses. To examine whether task demands at encoding influenced subsequent memory performance, we conducted a 2-way repeated measures ANOVA examining hit rates for the two encoding tasks (distinctiveness, similarity) broken down according to memory quality (remember, know). Note that, because the recognition test included faces from both tasks, false alarms were not specific to a given task; thus, corrected hit rates were not used. Planned comparisons were conducted using paired, two-tailed t-tests (alpha level .05).
2.2 Experiment 2
2.2.1 Participants
Fifteen healthy, right handed individuals (8 female; 18 – 34 years of age, mean age = 23.67 ± 1.24 years) participated in Experiment 2. Imaging data from one participant were excluded due to excessive motion. Participants were fluent English speakers, and were paid $25 per hour for participation in the study. The study was performed under a protocol approved by the UCLA Office for Protection of Research Participants.
2.2.2 Materials
Stimuli were presented using the MATLAB Psychophysics Toolbox and were viewed via magnet-compatible goggles placed directly over the eyes (Resonance Technologies, Inc). Face stimuli were drawn from the same database used in Experiment 1, but a larger number of stimuli was used in Experiment 2 to allow for sufficient trial numbers for imaging analyses. To this end, stimuli consisted of 150 faces divided into three sets of 50, such that 50 served as stimuli for the Distinctiveness task, 50 for the Similarity task, and the remaining 50 faces served as lures for the surprise recognition test. Sets were counterbalanced across participants such that each set was assigned to the distinctiveness, similarity, or lure condition an equal number of times. Instructions to participants were identical to those of Experiment 1.
2.2.3 Procedure
As in Experiment 1, Experiment 2 employed a within-subjects design such that all participants completed both the Distinctiveness and Similarity tasks prior to the surprise recognition memory test, with task order counterbalanced across participants. Each task consisted of 50 face trials with odd-even baseline trials interspersed. During low-level tasks like passive fixation, participants may spontaneously engage in mnemonic processing, leading to hippocampal activation. The odd/even task has been shown to minimize hippocampal activity in comparison to passive fixation (Stark & Squire, 2001), and thus was chosen as the baseline task for the current study.
Each of the two encoding tasks was divided into two shorter runs of 25 face trials, such that participants performed both runs of one task followed by both runs of the other. Face trials lasted 6 sec each, beginning with a brief fixation cross (0.75 sec) followed by presentation of the face for 5.25 sec. Baseline trials were of variable duration, ranging from 3–18 sec in multiples of 3 sec, and consisted of a series of single digits presented for 600 msec each, with participants responding whether digits were odd or even. The order of face and baseline trials within each run was determined using a genetic algorithm (Wager & Nichols, 2003). Approximately 10 minutes following the last encoding run, participants performed a surprise recognition test inclusive of faces from both encoding tasks in which they made studied/new judgments followed by remember/know judgments for those faces deemed studied.
2.2.4 FMRI Data Acquisition and Pre-processing
Structural and functional imaging for Experiment 2 were performed using a 3T Siemens Allegra scanner. Structural images included (1) a sagittal localizer to identify the long axis of the hippocampus, (2) high-resolution T2 hippocampal images perpendicular to the long axis of the hippocampus (TR=4 s, TE=105 msec, 19 slices, voxel size 0.4 × 0.4 × 3 mm, 20 cm FOV) for subsequent subfield delineation, (3) high-resolution gradient EPI sequences coplanar with the functional images (TR=5 s, TE=66 msec, 19 slices, voxel size 1.6 × 1.6 × 3 mm, 20 cm FOV) to aid alignment of the high-resolution structural images with the functional images, and (4) an MP-RAGE (TR=2.3 s, TE=2.93 msec) for future volumetric analyses. Functional imaging of the MTL was conducted with high-resolution gradient echo EPI sequences, consisting of 19 slices perpendicular to the long axis of the hippocampus (TR=3 s, TE=39 msec, voxel size 1.6 × 1.6 × 3 mm, 20 cm FOV).
Pre-processing was performed using the FSL toolbox. Skulls were stripped using the Brain Extraction Tool (Smith, 2002), and functional images were realigned using McFLIRT to compensate for small head movement (Jenkinson, Bannister, Brady, & Smith, 2002). For participants with translational motion over 1mm, images were denoised using MELODIC (Beckmann & Smith, 2004). One subject displayed motion over 3mm, and thus was not included in any imaging analyses. Data were filtered with a high-pass cutoff of 75 sec, and were smoothed using a 2 mm FWHM smoothing kernel.
2.2.5 Response Classification
To examine the degree to which MTL encoding activity is modulated by goal state, we used results from each participant’s recognition test to back sort encoding trials into those that were subsequently recognized (hits) and those that were subsequently forgotten (misses). Importantly, this allowed for comparison of activity associated with successful memory formation in each of the two tasks in each region of interest. Furthermore, given that behavioral analyses for Experiment 2 revealed no significant difference in overall hit rate across the two tasks, subsequent memory analyses were not biased by unequal trial numbers in the two tasks. Finally, remember/know data from the recognition test were used to examine whether quality of memory for subsequent hits differed between the two tasks. Note, however, that there were insufficient trial numbers to perform additional analyses comparing subsequent remember and know hits for each task (specifically, 10 participants had fewer than 10 trials for one or more relevant conditions).
2.2.6 Regions of Interest and Timecourse Analyses
Anatomically defined regions of interest (ROIs) were created for each individual participant. Specifically, ROIs for hippocampal subfields (DG/CA2/3, CA1, and subiculum) and MTL cortical areas (entorhinal, perirhinal and parahippocampal) were defined in each participant according to landmarks visible on his or her high-resolution structural scan. The DG and CA2/3 fields are difficult to unambiguously separate given current functional resolution, and therefore these regions were collapsed into a single ROI (DG/CA2/3). Boundary definitions were guided by MTL atlases (Duvernoy, 2005; Insausti & Amaral, 2004) and specifications defined in structural MRI studies of the MTL (Insausti et al., 1998; Pruessner et al., 2002; 2000). Furthermore, because susceptibility artifacts may be seen in anterior regions of the MTL during EPI sequences, voxels that were not visible in the mean functional image were excluded from ROI analyses (Zeineh et al., 2000). All ROIs were prepared by the same observer (VAC) to maintain consistency.
Group timecourses were created using the summary statistics approach to the mixed effects model (Mumford & Nichols, 2006). First, timecourses for each condition were extracted from each anatomical ROI in each participant using a Finite Impulse Response (FIR) model, allowing for examination of differences across conditions in timecourse amplitude as well as shape. The design matrix in this model contained entries for subsequent hits and misses; odd-even baseline trials were left un-modeled. The underlying hemodynamic response for each trial type was estimated by averaging the signal at 3 sec bins beginning 3 sec prior to stimulus onset and ending 15 sec after stimulus onset. For a given task, weighted least squares was used to combine activation across the two encoding runs. Finally, timecourses across all participants were averaged to create a group timecourse for each ROI.
To evaluate subsequent memory effects in the MTL under differing goal states, we conducted a 3-way repeated measures ANOVA examining encoding activity related to encoding task (distinctiveness, similarity), subsequent memory performance (hit, miss), and timecourse bin (3–6 sec, 9–12 sec). The latter factor was included to allow for examination of differences in timecourse shape, given that a number of studies have shown that shape can vary considerably across regions (see, e.g., Buckner, Koutstaal, Schacter, Wagner, & Rosen, 1998). Specifically, we were interested in whether subsequent memory effects would be seen (a) early in the timecourse, (b) late in the timecourse, or (c) throughout the entirety of the timecourse. Based upon a generic timecourse created by averaging across all participants, ROIs, and conditions, we selected 3–6 sec to represent early timecourse activity, and 9–12 sec to represent late timecourse activity. Finally, planned comparisons were conducted using paired, two-tailed t-tests (alpha level .05).
3. Results
3.1 Experiment 1
3.1.1 Encoding Task Ratings
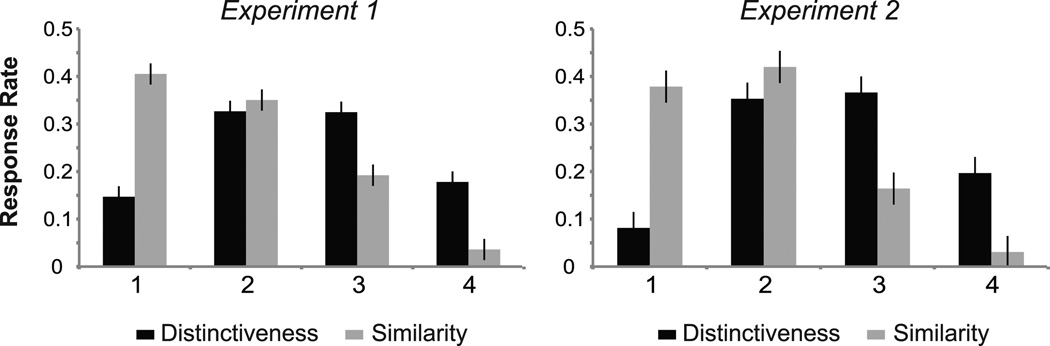
To verify that participants engaged in different encoding processes under differing goal states, we compared response rates along each task’s respective 4-point scale. A repeated measures ANOVA revealed a significant main effect of response option (1 through 4) (F(1,31) = 34.05, p < .001) but no effect of task (distinctiveness, similarity) (p > .3). A significant two-way interaction between encoding task and response option (F(3,93) = 36.23, p < .001) was found such that participants’ patterns of responding differed between the two encoding tasks (Figure 2). When judging how easy it would be to pick a given customer from the crowd in the Distinctiveness task, participants’ responses clustered around 2 (fairly hard) and 3 (fairly easy), with few faces rated at the extremes of the scale. When asked how well each actor matched the desired look in the Similarity task, participants’ responses clustered around 1 (poor) and 2 (average) for approximately 75% of trials, with few faces rated at the upper end of the scale. Such response patterns suggest that subjects processed faces differently in the Distinctiveness and Similarity tasks.
Figure 2.
Distinctiveness and similarity ratings during encoding. In the Distinctiveness task, ratings ranged from 1 = hard to pick out of a crowd (not distinctive) to 4 = easy to pick out of a crowd (very distinctive). For the Similarity task, ratings ranged from 1 = poor match (dissimilar) to 4 = excellent match (very similar). A significant two-way interaction between encoding task and response option was found for both Experiment 1 and 2, suggesting that participants processed faces differently in each of the two encoding tasks.
3.1.2 Effect of Goal on Subsequent Memory
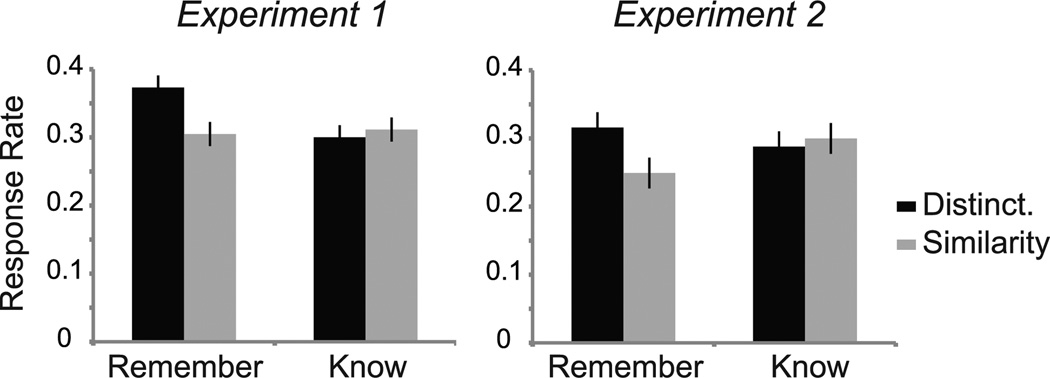
To determine whether the two encoding strategies led to differences in subsequent episodic recollection, a repeated measures ANOVA examining the relationship between encoding task (distinctiveness, similarity) and quality of memory (remember, know) was performed. This analysis revealed a significant main effect of task (F(1,31) = 4.70, p = .038) such that, collapsed across memory quality, the hit rate for the Distinctiveness task was higher than that of the Similarity task. No main effect of memory quality was found (p > .3). Analyses further revealed a significant interaction between task and memory quality (F(1,31) = 5.07, p = .032) such that a higher percentage of remember hits was found for the Distinctiveness than Similarity task (t(31) = 2.92, p = .007); there was no significant difference in the percentage of know hits for the two tasks (p > .5) (Figure 3). Importantly, the order in which the two tasks were performed did not significantly influence subsequent memory performance (no main effect of task order, p > .5, nor interactions involving task order, p values > .5). As noted in section 2.1.3, corrected hit rates were not used given that false alarms were not specific to a given task. Nonetheless, we include false alarm rates here for completeness: Participants responded to foils significantly more often with a “Know” response (10.17% ± 0.48%) than a “Remember” response (3.32% ± 0.23%) (t(31) = 5.44, p < .001).
Figure 3.
Effect of encoding task on subsequent memory performance. Experiment 1: A significant main effect of encoding task was found (p = .038) such that the subsequent hit rate for the Distinctiveness task was higher than that of the Similarity task. Analyses further revealed a significant interaction between encoding task and subsequent memory quality (p = .032), with a higher percentage of subsequent remember hits for the Distinctiveness than Similarity task (p = .007). Experiment 2: A trend toward a significant interaction between encoding task and subsequent memory quality was found (p = .10), with a higher percentage of subsequent remember hits for faces studied under the Distinctiveness than Similarity task (p < .05).
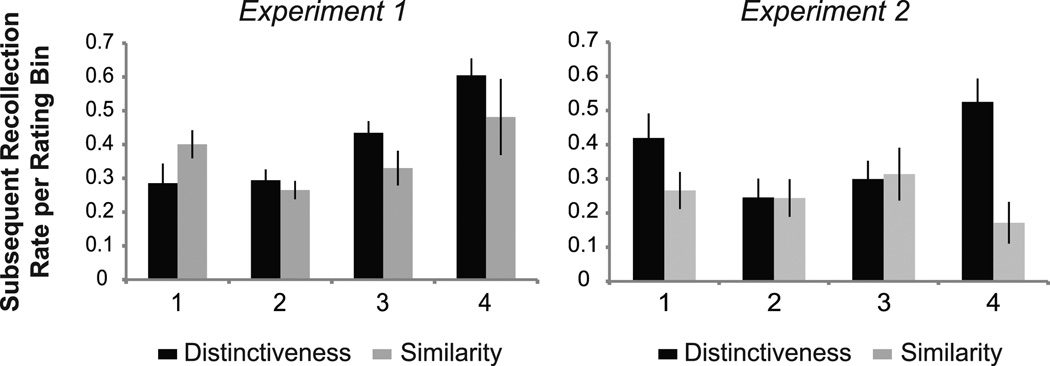
To further explore the relationship between encoding task and subsequent memory, we next examined the extent to which Distinctiveness and Similarity ratings at encoding related to subsequent memory performance. For a given encoding task, faces were first binned according to rating and then further subdivided into subsequent remember, know, and miss trials. In this way, the proportion of subsequently recollected faces for each rating bin could be calculated. Given that not all participants used each rating option during encoding—leading to an unbalanced data set, linear mixed modeling was used to evaluate the relationship between encoding task, task ratings, and subsequent rates of recollection. Specifically, this analysis tested whether increasing rates of subsequent recollection were found with increasing Distinctiveness or Similarity ratings. Results revealed a main effect of rating (X2 (1) = 14.758, p < .001) as well as a task x rating interaction (X2 (1) = 6.293, p = .012), such that a linear effect was found for the Distinctiveness task (X2 (1) = 21.644, p < .001) but not the Similarity task (p > .5) (Figure 4). That is, faces judged as more distinctive at encoding were associated with higher subsequent rates of recollection, whereas in the Similarity task increasing similarity ratings were not associated with higher subsequent rates of recollection.
Figure 4.
Effect of task ratings on subsequent rates of recollection. For each task and for each rating bin, the proportion of subsequently recollected trials was calculated. Experiment 1: A significant interaction was found between encoding task and rating (p = .012) such that subsequent rates of recollection increased with increasing ratings in the Distinctiveness task but not the Similarity task. Experiment 2: No main effects of rating nor interaction between encoding task and rating was found.
3.2 Experiment 2
3.2.1 Behavioral Results
As in Experiment 1, a repeated measures ANOVA revealed a significant main effect of response option (1 through 4) (F(1,14) = 19.96, p < .001), but no effect of task (distinctiveness, similarity) (p > .1). A significant two-way interaction was found between encoding task and response option (F(3,42) = 23.55, p < .001), such that participants’ patterns of responding differed between the two encoding tasks in manner similar to that of Experiment 1 (Figure 2). To determine whether the two encoding strategies led to differences in subsequent episodic recollection as in Experiment 1, a repeated measures ANOVA examining the relationship between encoding task (distinctiveness, similarity) and quality of memory (remember, know) was performed. This analysis revealed no main effects of task (p > .1) or memory quality (p > .8), but did reveal a trend toward a significant interaction between task and memory quality (F(1,14) = 3.05, p = .10) such that a higher percentage of remember hits was found for faces studied under the Distinctiveness than Similarity task (t(14) = 2.20, p < .05). There was no significant difference in the percentage of know hits for the two tasks (p > .6) (Figure 3). As in Experiment 1, participants responded to foils significantly more often with a “Know” response (11.47% ± 1.25%) than a “Remember” response (1.87% ± 0.30%) (t(14) = 3.97, p = .001). No effects of task order on subsequent memory performance were found (no main effect of task order, p > .1, nor interactions involving task order, p values > .5).
Next, mixed modeling was used to examine the extent to which Distinctiveness and Similarity ratings at encoding related to subsequent memory performance. Results revealed no main effect of rating on subsequent rates of recollection (p > .2), nor an interaction between task and rating (p > .4) (Figure 4).
Finally, to assess whether our fMRI results could be attributed to differences in time on task, encoding trials for each task were back sorted into hits and misses, and reaction times for each condition (see Table 1) were subjected to a repeated measures ANOVA. Results revealed no main effect of task (p > .2) or memory performance (p > .5) on reaction time, nor was there an interaction between task and memory performance (p > .3).
Table 1.
| Subsequent Hit | Subsequent Miss | |
|---|---|---|
| Distinctiveness | 2798 ± 126 msec | 2805 ± 132 msec |
| Similarity | 2843 ± 149 msec | 2959 ± 166 msec |
Reaction times during encoding as a function of task and subsequent memory performance.
Note: Data reflect reaction times and standard error of the mean for Experiment 2.
3.2.2 Imaging Results
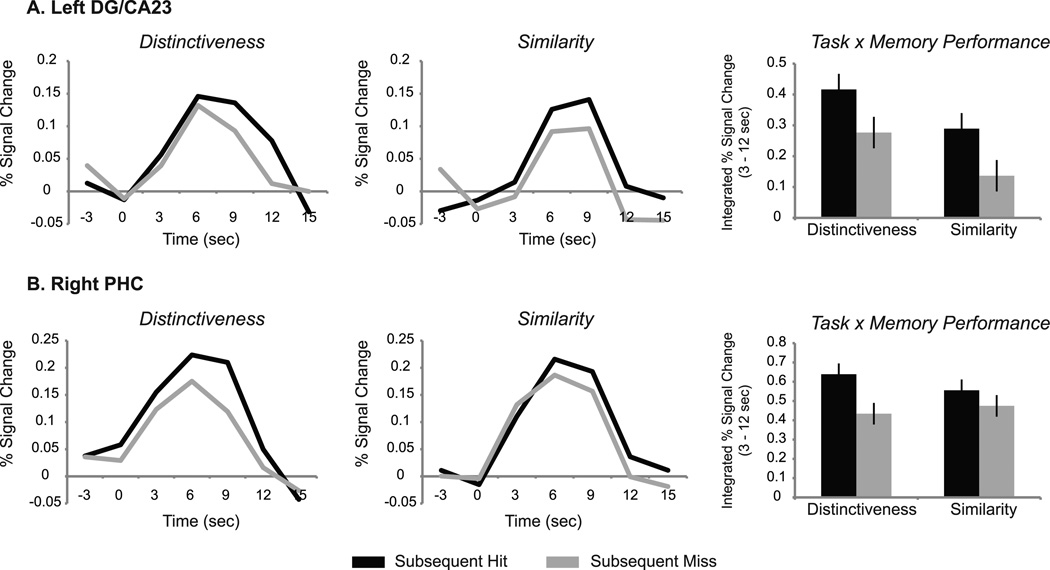
A repeated measures ANOVA was conducted in each ROI to examine encoding activity associated with encoding task (distinctiveness, similarity), subsequent memory performance (hit, miss), and timecourse bin (3–6 sec, 9–12 sec). Analyses of DG/CA2/3 and PHC data revealed no main effect of timecourse bin nor any two or three-way interactions involving timecourse bin (DG/CA2/3: p values > .4; PHC: p values > .2); thus, analyses for both regions were calculated using integrated signal change across the entire 3–12 sec period. Findings in left DG/CA2/3 revealed a significant main effect of memory performance (F(1,13) = 12.60, p = .004) such that collapsed across task, activity associated with subsequent hits was greater than that for misses (Figure 5A). However, no significant interaction between encoding task and memory performance (p > .9), nor a main effect of task (p > .3) was seen. Similarly, in right PHC, analyses revealed a main effect of memory performance (F(1,13) = 15.64, p = .002) such that activity associated with subsequent hits was greater than that for misses for both tasks (Figure 5B). No significant interaction between encoding task and memory performance (p > .2) nor a main effect of task was found (p > .8).
Figure 5.
Main effect of subsequent memory. Timecourses represent encoding activity associated with subsequent hits and misses for the Distinctiveness and Similarity tasks. Bar graphs represent integrated percent signal change from 3–12 sec. Encoding activity in left DG/CA2/3 (A) and right parahippocampal cortex (B) revealed a main effect of subsequent memory such that collapsed across task, activity associated with subsequent hits was greater than that for misses (left DG/CA2/3, p = .004; right parahippocampal cortex, p = .002). DG: dentate gyrus. PHC: parahippocampal cortex.
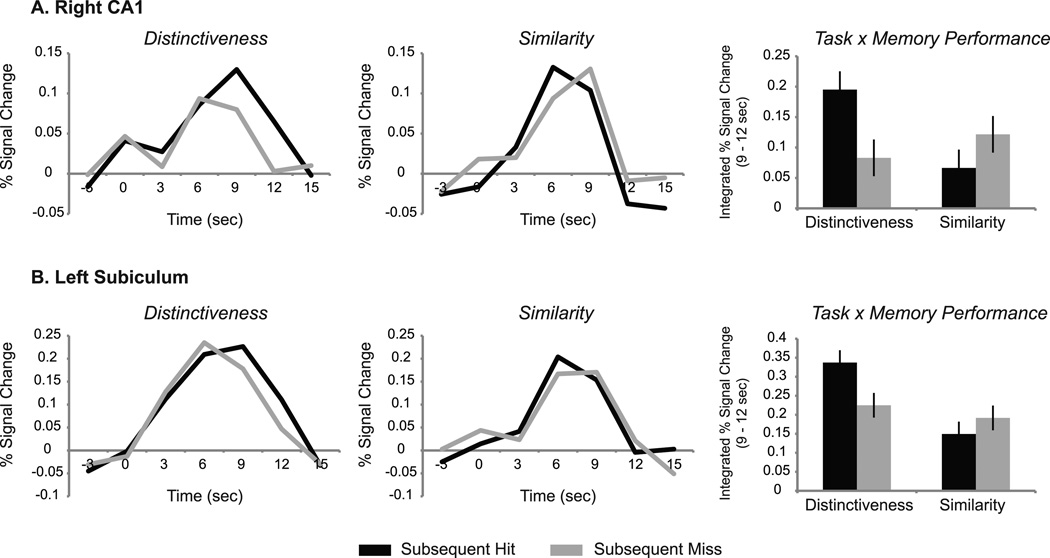
A different pattern of results was found in right CA1, which demonstrated no main effects of encoding task, memory performance, or timecourse bin (p values > .2), but did reveal a significant three-way interaction between these factors (F(1,13) = 4.99, p = .044). Specifically, no significant main effects or interactions were found early in the timecourse (3–6 sec; p values > 0.1). Later in the timecourse (9–12 sec), however, a two-way interaction was found between task and memory performance (F(1,13) = 7.73, p = .016) such that activity associated with subsequent hits was greater than that for misses in the Distinctiveness task (t(13) = 2.11, p = .05), whereas in the Similarity task activity associated with subsequent misses was numerically but not significantly greater than that for hits (p > .2) (Figure 6A). Stated more succinctly, late in the timecourse right CA1 activity revealed a greater subsequent memory effect for the Distinctiveness than the Similarity task. When this analysis was repeated using the entire timecourse (3–12 sec), the two-way interaction between task and memory performance was no longer significant (p > 0.1). Thus, the influence of the Distinctiveness task on encoding activity appeared to be driven by activation late in the timecourse in CA1.
Figure 6.
Encoding task by subsequent memory interaction. Timecourses represent encoding activity associated with subsequent hits and misses for the Distinctiveness and Similarity tasks. Bar graphs represent integrated percent signal change from 9–12 sec. Encoding activity in right CA1 (A) and left subiculum (B) revealed a significant interaction between encoding task and subsequent memory late in the timecourse, such that greater subsequent memory effects were observed for the Distinctiveness than the Similarity task (right CA1, p = .016; left subiculum, p = .047).
Similarly, in the left subiculum, no main effects of encoding task, memory performance, or timecourse bin (p values > .2) were found, but results revealed a significant three-way between these factors (F(1,13) = 4.79, p = .047). Specifically, no significant results were found early in the timecourse (p values > 0.1), but late in the timecourse a two-way interaction was found between task and memory performance (F(1,13) = 5.70, p = .033; see Figure 6B). As in CA1, activity associated with subsequent hits was greater than that for misses in the Distinctiveness task (t(13) = 2.17, p < .05), but not the Similarity task (p > .5). When this analysis was repeated using the entire timecourse, the two-way interaction between task and memory performance was no longer significant (p > 0.6). Finally, no significant results were found in perirhinal or entorhinal cortices.
To directly evaluate whether top-down influences on MTL activity differed between regions, we conducted three-way repeated measures ANOVAs for each pairwise combination of regions, with factors region, encoding task (distinctiveness, similarity), and subsequent memory performance (hit, miss), focusing specifically on activity late in the timecourse. As expected based on the results reported above, analyses revealed a main effect of memory when comparing left DG/CA2/3 and right PHC (F(1,13) = 12.46, p = .004), but no task x memory interaction (p > .6) nor any significant interactions involving region (p values > .4). Conversely, comparisons between right CA1 and left subiculum revealed a significant task x memory interaction (F(1,13) = 10.72, p = .006) but no main effect of memory (p > .3) nor any significant interactions involving region (p values > .2). Moreover, a trend towards a significant region x task x memory interaction was found for comparisons between left DG/CA2/3 and right CA1 (F(1,13) = 4.32, p = .058), left DG/CA2/3 and left subiculum (F(1,13) = 3.26, p = .094), and right PHC and right CA1 (F(1,13) = 4.39, p = .056). These results raise the possibility that encoding activity in MTL subregions is differentially affected by task demands, such that subicular and CA1 encoding activity may be influenced more strongly by top-down goal states than DG/CA2/3 or PHC.
4. Discussion
The current study examined the manner in which task demands during learning affect MTL activity and its relation to subsequent memory performance. Using a paradigm in which participants’ attention was directed toward either item distinctiveness or similarities among items, we found that attending to distinctiveness was associated with enhanced subsequent recollection relative to attending to similarity. Imaging results demonstrated robust subsequent memory effects across the MTL. Critically, in CA1 and the subiculum, significant subsequent memory effects were only observed when task goals required attention to stimulus distinctiveness, indicating more effective recruitment of the whole hippocampal circuit during learning when attending to distinctiveness than similarity.
4.1 Distinctiveness and subsequent memory
Consistent with previous studies (e.g., Glanzer & Adams, 1990; Hunt, 1995), we found that attending to distinctiveness plays a key role in the formation of rich episodic memories, and that stimuli judged as more distinctive are better remembered than those judged as more typical.
4.2 Mechanisms of hippocampal encoding
Results from Experiment 2 revealed increased activity for subsequent hits relative to misses in several regions throughout the MTL: Left DG/CA2/3, right CA1, left subiculum and right PHC. Subsequent memory effects in DG/CA2/3 and PHC did not differ according to task demands, such that these regions were recruited to an equivalent degree during successful encoding regardless of goal state. In CA1 and the subiculum, however, activity was associated with subsequent memory success only in the Distinctiveness task. Such findings suggest that top-down goal states differentially emphasizing stimulus distinctiveness modulate the degree to which hippocampal activity supports successful memory formation.
Motivated by recent hr-fMRI findings demonstrating encoding effects not only in DG/CA2/3 but across all subfields of the hippocampal circuit (see Carr, Rissman, & Wagner, 2010a for a review), we explored the hypothesis that attending to distinctiveness would more effectively recruit hippocampal encoding processes across the entirety of the hippocampus than attending to similarity. Our results largely support this hypothesis with the exception of activity patterns in DG/CA2/3—subsequent memory effects in this region did not differ according to task demands. One interpretation of these results is that encoding processes in DG/CA2/3 are less susceptible to top-down influences than in CA1 or the subiculum. It bears noting, however, that when the pattern of activity in DG/CA2/3 was directly compared to that of CA1 and the subiculum, differences between subfields only reached trend levels of significance. Furthermore, given that anatomical ROIs were used, it is possible that portions of DG/CA2/3 may have exhibited a task x subsequent memory interaction as well, but that such effects were not detected when averaging across the entirety of the ROI. To this end, caution is warranted in interpreting regional differences among hippocampal subfields in the degree to which top-down goal states affect encoding activity. It may be that the behavioral benefits of attending to distinctiveness depend upon robust engagement of the entire hippocampal circuit such that each region plays a critical role in supporting the formation of rich, vivid memories.
Supporting this view, computational models of MTL function posit that during encoding, overlapping activity patterns from entorhinal cortex are passed to CA3 as separable, distinct patterns via the DG (e.g., Norman & O'Reilly, 2003). These disparate patterns are then thought to be bound together into a conjunctive representation within recurrent connections of CA3, while simultaneously, another representation of entorhinal activity is formed in CA1 via direct perforant path connections. This CA1 representation is thought to be invertible in that CA1 both receives feed-forward projections from entorhinal cortex and projects back to entorhinal cortex either directly or via the subiculum. Importantly, during learning CA1 receives input not only from entorhinal cortex, but from CA3 as well. This co-activation is thought to create a key link between the pattern-separated representations of CA3 and overlapping entorhinal representations, allowing CA1 to serve as a “translator” during subsequent retrieval processes (Hasselmo, Wyble, & Wallenstein, 1996; Norman & O'Reilly, 2003; O'Reilly & McClelland, 1994; Rolls & Kesner, 2006). Thus, such models suggest that successful encoding depends upon linking the pattern-separated representation in CA3 to the invertible representation of entorhinal input within CA1 and the subiculum, facilitating future retrieval processes.
Given that the current results demonstrate robust hippocampal engagement across the entire circuit during the Distinctiveness but not Similarity task, and that subsequent memory performance was superior for the Distinctiveness task, our findings are in agreement with computational models of hippocampal function highlighting the importance of the entire hippocampal circuit in successful memory formation. Our findings in CA1 and the subiculum also lend support to prior hr-fMRI studies suggesting a role for these regions in memory formation and not simply memory retrieval. Such studies have demonstrated mismatch detection in CA1 (Chen et al., 2011; Duncan et al., 2012), performance-related activity during spatial learning in CA1 (Suthana et al., 2009), novelty encoding in the subiculum (Bakker et al., 2008; Preston et al., 2010; Zeineh et al., 2000), and subsequent memory effects in the subiculum (Wolosin et al., 2012).
In Experiment 1, faces encountered in the Distinctiveness task were more often subsequently recollected than those encountered in the Similarity task, an effect which reached trend levels of significance in Experiment 2. Previous work employing explicit encoding tasks has shown greater hippocampal encoding activity for subsequently recollected than familiar items (e.g., Eldridge et al., 2005; Carr et al., 2009), a finding thought to reflect differences in quality among memory representations. Here, we used different incidental encoding tasks to influence the quality of encoded memory representations. It is possible that when participants attend to distinctiveness under incidental learning conditions, they are engaging similar neural processes as when intentionally and effectively memorizing items. As such, encoding conditions that enhance subsequent recollection may engage the hippocampus in a similar manner.
Interestingly, our observed pattern of results in CA1 and the subiculum was due to a late hit-miss difference in activation specific to the Distinctiveness task. When attending to distinctiveness, CA1 and subicular activity associated with subsequent hits returned to baseline more slowly than for subsequent misses, whereas attention to similarity led to an equivalently rapid return to baseline for both subsequent hits and misses. These results raise the possibility that successful memory formation when attending to distinctiveness resulted in more sustained neural activity in CA1 and the subiculum. However, given that the blood oxygenation level dependent (BOLD) signal measured by fMRI is an indirect measure of neuronal function, it is important to note that regional differences in timecourse shape may also reflect differences in the vascular networks supporting each region, or regional differences in metabolism.
4.3 Top-down modulation of hippocampal activity
There have been several studies exploring the influence of top-down goal states on hippocampal activity during memory retrieval. In a study of recognition memory in which task demands were manipulated, Dudukovic & Wagner (2007) demonstrated that hippocampal activation was influenced by retrieval goals rather than the perceptual novelty of test probes. Similarly, when evaluating hippocampal responses to test probes that either matched or did not match previous targets, Duncan, Curtis, & Davachi (2009) demonstrated that hippocampal sensitivity to perceptual mismatch was not obligatory but rather modulated by task goals. Of particular relevance to the current study, Hashimoto et al. (2012) demonstrated that when recognition judgments were based upon perceptual novelty, hippocampal responses to foils were larger than when recognition judgments were based upon semantic novelty, such that attending to perceptual differences led to greater novelty responses than generalizing across differences.
The current study extends such work by exploring top-down influences on hippocampal processes during encoding, enabling us to evaluate the relationship between task demands, hippocampal activity, and subsequent memory. Our findings provide novel evidence that focusing attention on stimulus distinctiveness during learning modulates the degree to which hippocampal subfield activity is associated with subsequent memory success.
4.4 Top-down modulation of MTL cortical activity
Analyses of MTL cortical activity revealed a main effect of subsequent memory in PHC, but no task x subsequent memory interaction, indicating that top-down goal states did not significantly affect encoding activity in this region. One interpretation of these findings is that the cortex is specialized to encode regularities in the environment by assigning them overlapping representations (Norman & O'Reilly, 2003; O'Reilly & McClelland, 1994), and as such is biased to form non-specific memories regardless of top-down influences. It bears noting, however, that when the pattern of activity in PHC was directly compared to that of CA1 and the subiculum, differences between subfields only reached trend levels of significance. Finally, it is likely that the observed influence of task demands on hippocampal encoding activity likely resulted from interactions with a variety of cortical areas outside the MTL. Thus, an important topic for future study is to understand the nature of such hippocampal-cortical interactions by evaluating, for example, functional connectivity between fronto-parietal areas and the hippocampus.
4.5 Conclusion
Using hr-fMRI and a paradigm in which participants’ attention was directed toward either item distinctiveness or similarities among items, we demonstrated that hippocampal encoding activity was better predictive of subsequent memory success when attending to distinctiveness than similarity. Such findings provide evidence that goal states differentially modulate hippocampal activity during learning, and that recruitment of the entire hippocampal circuit during successful encoding may underlie the behavioral benefits of attending to distinctiveness. Taken together, these findings advance understanding of the effects of top-down goal states on MTL processing during learning.
Highlights
Examined influence of top-down goal states on hippocampal encoding activity
Instructions to attend to stimulus distinctiveness or similarity during learning
Increased rates of subsequent recollection for distinctiveness task
More effective recruitment of hippocampal circuitry for distinctiveness task
Advances understanding of top-down influences on hippocampal subfield activity
Acknowledgments
This work was made possible by grants from the National Science Foundation (NSF BCS 0848246, awarded to BJK), the National Institutes of Health (T32 MH15795, awarded to VAC), Achievement Rewards for College Scientists (awarded to VAC), and the University of California, Los Angeles (Dissertation Year Fellowship, awarded to VAC). We thank Karen LaRocque and Serra Favila for providing helpful feedback regarding the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern Separation in the Human Hippocampal CA3 and Dentate Gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23(2) doi: 10.1109/TMI.2003.822821. 137–. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal Cortex and Long-Term Memory Encoding: An Integrative Review of Findings from Neuropsychology and Neuroimaging. The Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Buckner R, Koutstaal W, Schacter D, Wagner A, Rosen B. Functional-Anatomic Study of Episodic Retrieval Using fMRI I. Retrieval Effort versus Retrieval Success. NeuroImage. 1998;7(3):151–162. doi: 10.1006/nimg.1998.0327. [DOI] [PubMed] [Google Scholar]
- Carr VA, Rissman J, Wagner AD. Imaging the Human Medial Temporal Lobe with High-Resolution fMRI. Neuron. 2010a;65(3):298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Viskontas IV, Engel SA, Knowlton BJ. Neural activity in the hippocampus and perirhinal cortex during encoding is associated with the durability of episodic memory. Journal of Cognitive Neuroscience. 2010b;22(11):2652–2662. doi: 10.1162/jocn.2009.21381. [DOI] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learning & Memory. 2011;18(8):523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Current Opinion in Neurobiology. 2007;17(2):177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Dudukovic NM, Wagner AD. Goal-dependent modulation of declarative memory: neural correlates of temporal recency decisions and novelty detection. Neuropsychologia. 2007;45(11):2608–2620. doi: 10.1016/j.neuropsychologia.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Duncan K, Curtis C, Davachi L. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. The Journal of Neuroscience. 2009;29(1):131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22(3):389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Section with MRI. Third Edition. New York: Springer; 2005. [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. The Journal of Neuroscience. 2005;25(13):3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CME, Carpenter TA, Donovan T, Bullmorel ET. Regional brain activations predicting subsequent memory success: an event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39(4–5):1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D’Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. Journal of Cognitive Neuroscience. 2005;17(3):507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Glanzer M, Adams JK. The mirror effect in recognition memory: data and theory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16(1):5–16. doi: 10.1037//0278-7393.16.1.5. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Abe N, Ueno A, Fujii T, Takahashi S, Mori E. Changing the criteria for old/new recognition judgments can modulate activity in the anterior hippocampus. Hippocampus. 2012;22(2):141–148. doi: 10.1002/hipo.20878. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6(6):693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hunt RR. The subtlety of distinctiveness: What von Restorff really did. Psychonomic Bulletin & Review. 1995;2(1):105–112. doi: 10.3758/BF03214414. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral D. Hippocampal formation. In: Paxinos G, Jurgen KM, editors. The human nervous system. Vol. 2. San Diego: Elsevier Academic Press; 2004. pp. 871–914. [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR American Journal of Neuroradiology. 1998;19(4):659–671. [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Mäntylä T. Recollections of faces: remembering differences and knowing similarities. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1997;23(5):1203–1216. doi: 10.1037//0278-7393.23.5.1203. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Nichols T. Modeling and inference of multisubject fMRI data. IEEE Engineering in Medicine and Biology Magazine. 2006;25(2):42–51. doi: 10.1109/memb.2006.1607668. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychological Review. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4(6):661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JDE, Wagner AD. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. The Journal of Neuroscience. 2009;29(38):11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of Content-sensitive Subsequent Memory Responses in Human Medial Temporal Lobe. Journal of Cognitive Neuroscience. 2010;22:156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12(12):1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schott BH, Wüstenberg T, Wimber M, Fenker DB, Zierhut KC, Seidenbecher CI, et al. The relationship between level of processing and hippocampal-cortical functional connectivity during episodic memory formation in humans. Human Brain Mapping. 2013;34(2):407–424. doi: 10.1002/hbm.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, Bookheimer SY. Human hippocampal CA1 involvement during allocentric encoding of spatial information. The Journal of Neuroscience. 2009;29(34):10512–10519. doi: 10.1523/JNEUROSCI.0621-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher M, Rugg M. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. The Journal of Neuroscience. 2009;29(25):8270–8279. doi: 10.1523/JNEUROSCI.1043-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Carr VA, Engel SA, Knowlton BJ. The neural correlates of recollection: hippocampal activation declines as episodic memory fades. Hippocampus. 2009;19(3):265–272. doi: 10.1002/hipo.20503. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. Journal of Cognitive Neuroscience. 2012;24(7):1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D-J, Chun MM. Attentional modulation of learning-related repetition attenuation effects in human parahippocampal cortex. The Journal of Neuroscience. 2005;25(14):3593–3600. doi: 10.1523/JNEUROSCI.4677-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage. 2000;11(6 Pt 1):668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299(5606):577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]