Abstract
Candida albicans is a major human pathogen whose treatment is challenging due to antifungal drug toxicity, drug resistance and paucity of antifungal agents available. Myrocin (MYR) inhibits sphingosine synthesis, a precursor of sphingolipids, an important cell membrane and signaling molecule component. MYR also has dual immune suppressive and antifungal properties, potentially modulating mammalian immunity and simultaneously reducing fungal infection risk. Wax moth (Galleria mellonella) larvae, alternatives to mice, were used to establish if MYR suppressed insect immunity and increased survival of C. albicans-infected insects. MYR effects were studied in vivo and in vitro, and compared alone and combined with those of approved antifungal drugs, fluconazole (FLC) and amphotericin B (AMPH). Insect immune defenses failed to inhibit C. albicans with high mortalities. In insects pretreated with the drug followed by C. albicans inoculation, MYR+C. albicans significantly increased mortality to 93% from 67% with C. albicans alone 48 h post-infection whilst AMPH+C. albicans and FLC+C. albicans only showed 26% and 0% mortalities, respectively. MYR combinations with other antifungal drugs in vivo also enhanced larval mortalities, contrasting the synergistic antifungal effect of the MYR+AMPH combination in vitro. MYR treatment influenced immunity and stress management gene expression during C. albicans pathogenesis, modulating transcripts putatively associated with signal transduction/regulation of cytokines, I-kappaB kinase/NF-kappaB cascade, G-protein coupled receptor and inflammation. In contrast, all stress management gene expression was down-regulated in FLC and AMPH pretreated C. albicans -infected insects. Results are discussed with their implications for clinical use of MYR to treat sphingolipid-associated disorders.
Introduction
Myriocin (2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxoicos-6-enoic acid, MYR) is a metabolite of the insect pathogenic fungus Isaria sinclairii. It is a sphingosine analog with immunosuppressive properties, more potent than cyclosporine [1]. MYR also known as ISP-1 and thermozymocidin, has antifungal antibiotic properties [2]. Fingolimod (FTY720) is a novel immune regulatory drug (trade name Gilenya, Novartis) derived from MYR which has been approved for treating multiple sclerosis, a chronic autoimmune disease that affects millions of people worldwide [3]. Besides immune modulation, MYR depletes sphingolipids from cells, by inhibiting the enzyme serine palmitoyltransferase which catalyses the formation of sphingosine, a precursor of sphingolipids [4]. MYR is also an agonist of the sphingosine 1 phosphate receptor [5]. Sphingolipids produce the outer leaflet of the plasma membrane and have a role in protecting the cell surface against harmful environmental factors. Sphingolipid metabolites, such as ceramide and sphingosine-1-phosphate, are important mediators in the signaling cascades involved in apoptosis, proliferation, and stress responses [6]. Since MYR shows promise in treating a number of major diseases additional to multiple sclerosis, including diabetes, cardiovascular disease [7], [8], certain cancers [9], photoreceptor degeneration in retinitis pigmentosa [10] and artherosclerosis [11], it is anticipated that the use of MYR and its derivatives will increase. Therefore, it is imperative that we understand the full potential of the drug including antagonism and synergy with other therapeutics.
Candida species are ranked amongst the most common causative agents of invasive fungal infections, with Candida albicans being the most frequently isolated species in hospitals worldwide [12]. Treating candidiasis can be costly and can extend a patient's stay in hospital [13]. The incidence of invasive candidiasis is increasing due to the growing number of immune compromised and debilitated patients, the mortality attributable to candidiasis remaining high [14]. It is unclear if the immune suppressive properties of MYR might increase the risk to Candida infections or if the antifungal properties provide sufficient protection to reduce the use of other antifungal drugs. Identifying synergistic compounds for combination antifungal therapy offers a promising antifungal strategy [15].
Typically, mammalian model systems are used (e.g. a murine infection model) to evaluate new therapeutics, but these experiments are time-consuming, costly and require full ethical consideration. The larvae of the greater wax moth, Galleria mellonella, have emerged as a surrogate alternative model host for human pathogens including fungi [16]. G. mellonella is particularly suited to investigating fungal virulence, evaluating the efficacy of antibiotics and studying immune defense responses, including elements of innate immunity shared by both humans and insects [17], [18]. Correlations have been established previously between the pathogenicity of microbes, such as C. albicans, Aspergillus fumigatus, Bacillus thuringiensis and Pseudomonas aeruginosa, in Galleria and mice [19], [20]. Furthermore, the efficacy of licensed antimicrobial agents has been evaluated in G. mellonella, demonstrating remarkable correlation between in vitro susceptibility testing results and in vivo drug efficacy in both insects and mammals [16].
The aim of this study was to determine if MYR, because of its dual (immune suppression and antifungal) attributes, suppressed the insect immune system and concomitantly prevented candidiasis, or if by weakening the immune system it increased susceptibility to infection. Furthermore, we wanted to establish if MYR combinations with current antifungal agents, amphotericin B (AMPH) and fluconazole (FLC), worked synergistically and increased host survival. In addition, we investigated which elements of the insect immune and stress management system could be used as early warning indicators of stress or infection. The findings of this work could have important clinical implications for prophylaxis of mycoses in immune-compromised patients caused by C. albicans and other fungal pathogens, including strains resistant to current antifungal drugs.
Materials and Methods
Insect rearing
G. mellonella were reared in strict isolation at 28°C, 60% relative humidity, with a 12∶12 h light∶dark cycle. Larvae were maintained on an artificial diet (containing organic wheat bran meal, bees wax, honey, ground rat food, and glycerin) [21].
Culture and maintenance of Candida albicans
The yeast form of Candida albicans SC5314 was produced on yeast potato extract agar at 37°C. Individual colonies were harvested at 24 h post-incubation, washed twice in phosphate buffer saline (PBS) pH 7, before determining cell numbers in a hemocytometer.
Antifungal effect of myrocin on Candida albicans growth in vitro
To investigate the antifungal effect in vitro of MYR on C. albicans growth, the antifungal susceptibility tests for MYR, AMPH and FLC were performed in the presence of C. albicans. Minimum inhibitory concentrations (MICs) were determined by using the modified broth microdilution method of the Clinical and Laboratory Standards Institute [22]. The antifungal efficacy of MYR, FLC, and AMPH were tested at concentrations ranging from 16 to 0.03 µg/ml for MYR and AMPH and 64-0.01 µg/ml for FLC. The MIC was defined as the lowest concentration of antifungal agent at which growth was inhibited by 80% compared with that of the growth in the control well.
Drug interactions
The in vitro drug interactions were studied by a chequerboard microdilution method [23] which consists of a two dimensional array of serial concentrations of the test compounds. For the combination testing, each drug (MYR, FLC and AMPH) was serially diluted with the corresponding solvents and then the dilution scheme was performed in order to obtain the correct final concentration. A total of 50 µl of each concentration of the compound was added to columns 1 to 10, and then 50 µl of each concentration of MYR was added (ranging from 4 to 0.03 µg/ml) to rows A to G. Columns 11 and 12 were drug free controls. A fractional inhibitory concentration index (FICI) established the type of interaction which ranged from synergistic (FICI≤0.5), antagonism (FICI>4.0) and no interaction (FICI 0.5–4.0).
Susceptibility of G. mellonella larvae to Candida albicans infection
Fifth instar G. mellonella larvae were used to investigate Candida pathogenesis and the effect of MYR during fungal infection. Twenty larvae were randomly chosen for each assay group, and all experiments were repeated three times. Each insect was injected with 20 µl of 105 Candida cells, as described previously [24], incubated at 37°C and larval survival was recorded at, 24 h and 48 h post-injection. The larvae were considered dead when they showed no response to probing with a pipette tip. In addition, to show the potency of MYR as an immunosupressant during Candida infection, the insects were infected with C. albicans after pre-treatment with 10 times higher concentration of MYR (0.5 µg/ml) in comparison with the dose of MYR (0.05 µg/ml) used in the remained of the experiments. Larval survival curves were created using GraphPad Prism v5.0 (GraphPad Software, San Diego California USA).
Effect of myriocin and antifungal agents on G. mellonella survival
Galleria larvae were pre-injected independently or in combination with 10 µl of 0.05 µg/ml MYR and 10 µl of the antifungal agents (AMPH or FLC) at a concentration of 250 µg/ml, then 1 h later injected with 105 C. albicans cells. Survival rates were recorded at 24 h and 48 h post treatment. Control groups consisted of drug-free/PBS injected insects, non-injected, and drug only groups of larvae. Experiments were repeated three times (n = 20 each larval group).
Recovery of Candida albicans from infected G. mellonella larvae
To investigate the effect of larval response and drug treatment on Candida growth recovery of fungal cells was determined. Candida was recovered from the fat body and hemolymph of insects (n = 10) 24 h and 48 h post injection with the above drug combinations or PBS (control). Hemolymph was collected in pre-chilled plastic tubes containing anticoagulant to which phenylthiourea (1 mg/ml) had been added to prevent melanisation. The anticoagulant comprised 62 mM NaCl, 100 mM glucose, 10 mM EDTA, 30 mM sodium citrate and 26 mM citric acid (pH 4.6) [25]. Fat body from infected larvae was homogenised in PBS and serially diluted. Aliquots (100 µl) of the fat body and hemolymph from each treatment were plated onto yeast extract potato dextrose (YEPD) agar plates containing streptomycin and ampicillin 1 mg/ml and incubated at 37°C for 48 h before recording the colony forming units (CFUs).
Impact of MYR on the G. mellonella hemogram
Hemograms were determined to monitor the MYR effect on the cellular defences of G. mellonella. Galleria larvae were injected with C. albicans 1 h after treatment with MYR, AMPH or FLC drugs alone or in combination. Hemolymph was then recovered from 20 larvae, 24 h and 48 h post treatment, as described above. To determine the total hemocyte counts, 5 µl of hemolymph were mixed with 10 µl anticoagulant and phenylthiourea and numbers of cells counted using a hemocytometer.
Effects of G. mellonella larval hemolymph and recombinant antimicrobial peptides (AMPs) on the in vitro growth of C. albicans
Larval hemolymph (n = 20), 24 h post treatment, was collected from different treatment groups (PBS injected, Candida alone, MYR, MYR-CA, AMPH-CA, FLC-CA, MYR-AMPH-CA, MYR-FLC-CA groups). A 10 µl aliquot of hemolymph (50 µl) and anticoagulant (10 µl) was spotted onto yeast minimal medium agar plates containing streptomycin and ampicillin (1 mg/ml) inoculated with 106 or 105 Candida cells. The cultures were incubated at 37°C for 48 h and inhibition zones (mm) recorded.
The antifungal properties of recombinant cecropin D and gallerimycin were determined using a turbidimetric assay [22]. The antimicrobial peptides cecropin D (Sequence: ENFFKEIERAGQRIRDAIISAAPAVETLAQAQKIIKGGD) and gallerimycin (Sequence: GVTITVKPPFPGCVFYECIANCRSRGYKNGGYCTINGCQCLR) from G. mellonella were produced by custom synthesis (Coring System Diagnostix, Gernsheim, Germany). Cysteine-free peptides were purified by reversed phase HPLC to >75% purity. Cysteine-containing peptides were used as raw products without further purification. Cecropin D and gallerimycin were dissolved in DMSO and used to determine antifungal activity. Candida cells suspended in yeast minimal medium were used at a final concentration of 2.5×104 cells/ml. The recombinant AMPs at a final concentration of 500 µg/ml and fungal cells were incubated in 96-well microtitre plates (Greiner, UK) at 37°C and growth was determined spectrophotometrically at 600 nm. Readings were taken every 15 min over a 24 h period. Growth rate was calculated using the formula Y = N0+C*exp(-exp((2.7*μ/C)*(Lag-X)+1)) (N0 = initial number of cells; C = difference between initial and final cell numbers; Lag = time delayed before growth; μ = maximum specific growth rate).
Impact of MYR on phenoloxidase (PO), Lysozyme, Superoxide dismutase (SOD) and malondialdehyde (MDA) activities
PO, lysozyme and oxidative stress management are important elements in insect immunity. PO, lysozyme, SOD and MDA activity were assayed as outlined below with each experiment being repeated three times unless otherwise indicated.
PO activity in the hemolymph was measured after 24 h post treatment in the following groups: insects injected with PBS, Candida alone, MYR, MYR-CA, AMPH-CA, FLC-CA, MYR-AMPH-CA, or MYR-FLC-CA. Cell-free hemolymph plasma samples from groups of 10 larvae were collected from treated and control insects. Hemolymph plasma fractions were prepared by collecting 10 µl of hemolymph from an incision made on the third proleg of each larva. This was diluted with 20 µl PBS and centrifuged at 500 g for 5 min at 4°C to remove the hemocyte pellet. The supernatants of the hemolymph plasma were then used for spectrophotometric analysis of PO enzymatic activity and protein concentration in a modification of the method described by Ashida [26]. Five microlitres of plasma were added to wells of a flat-bottomed 96-well microtitre plate containing 200 µl of 2 mg/ml L-DOPA (L-3,4-dihyroxyphenylalanine dissolved in sterile pyrogen-free water). After 30 min at 28°C, the absorbance was quantified at 490 nm using a plate reader (BMG Labtech, Germany). The units of PO activity were expressed as the change in absorbance at 490 nm per 1 min per mg of protein. The protein concentration of the samples was estimated by the Bradford method using BSA as the standard.
Lysozyme activity of hemolymph plasma from groups of 10 larvae was determined by a zone-of-clearance assay by using a substrate of freeze-dried Micrococcus luteus as a substrate suspended in agarose. The radius of the digested zone was compared with a standard curve made with egg white lysozyme (EWL) and expressed as an EWL equivalent (mg/ml).
Superoxide dismutase activity in the hemolymph from groups of 10 larvae was measured after 24 h post injection with PBS, Candida alone, MYR, MYR-CA, AMPH-CA, FLC-CA, MYR-AMPH-CA, or MYR-FLC-CA. Superoxide dismutase assay was performed as previously described [27].
Lipid peroxidation was determined using a modified–2-thiobarbituric acid (TBA) assay which quantifies the end product, malondialdehyde (MDA) [28]. Samples from groups of 10 larvae were tested.
Implants inserted into G. mellonella larvae infected with Candida albicans
The encapsulation response is one of the frontline defences during pathogen invasion. Encapsulation responses were determined by implanting a 2 mm long, 0.5 mm diameter piece of white nylon monofilament through a perforation in the ventral segment of the cuticle of larvae infected with C. albicans and/or MYR, AMPH, or FLC. Implants were removed from the body cavity after 2 h, and then photographed from three angles. The degree of the melanization was quantified by image Pro software by measuring the coloration of all surface areas of each implant, and then comparing these values with that of a control implant prior to implantation (without melanization).
Effect of MYR on the transcription of immunity and stress-related genes in G. mellonella infected with Candida albicans
Both AMPs and stress management genes play a major role in insect responses to pathogens [29]. The expression of 17 immunity and stress management genes was quantified in larvae treated with MYR, FLC, AMPH and C. albicans combinations, as outlined earlier. Fat body was recovered from at least 3 larvae per treatment at 24 and 48 h post-treatment. RNA was extracted from RNAlater stabilized fat body using a RNeasy Plus mini kit according to the manufacturer's instructions (Qiagen). RNA extracts were quantified spectrophometrically then reverse transcribed to cDNA using a qScript™ cDNA SuperMix (Quanta Bioscience). The cDNA quantity was checked and normalised using a reference gene PCR of 1/50 dilutions for each sample measured against a standard curve, and sufficient cDNA of similar concentration for each sample diluted to amplify all genes. Samples were quality checked for consistency between values for the two reference genes used: 18 s rRNA (AF286298) and elongation factor 1-alpha (EF1) (AF423811). Expression was then measured in the normalised samples using the Rotor-Gene 6000 (Corbett Research), with Rotor-Gene SYBR Green PCR mix (Qiagen), relative to these two reference genes. Cycling conditions were 95°C 5 min then 42 cycles of: 95°C 5 sec, annealing 10 sec, 72°C 20 sec. An initial touchdown of 1°C per cycle from 65°C for the first 5 cycles, resulted in optimal amplification for all loci. HRM analysis performed at the end of each run allowed each PCR to be checked for the presence of the expected product. All reactions were performed in triplicate, and optimal threshold values and reaction efficiencies calculated from 7-point serial dilutions of mixed cDNA from fungal- infected insects. Fold change values were calculated using the ΔΔCt method for each locus. The ΔΔCt for each sample was determined by subtracting the measured Ct value from the Ct value of each reference or ‘housekeeping’ gene. ΔΔCts were then converted to relative copy numbers with the formula 2ΔΔΔCt. Fold changes were also calculated using reaction efficiencies using the Pffafl equation. Values showed similar trends for both reference genes and for each method of calculation: ΔΔCt values for 18 s are shown. Primers were designed from published G. mellonella sequences (NCBI) or from coding sequence where high homology protein sequences could be identified from an EST library [29], and are given in Table 1. For hsp90, a primer designed to the conserved 3′UTR region found in Lepidoptera [30] was paired with a degenerate primer designed from an alignment of 8 lepidopteran hsp90 sequences (GU230738, AB214972, AB060275, EF197936, GU230737, AF254880, GU230739, AB206477) using CODEHOP [31]. Details of the genes/contigs used are provided in the supplementary text (Text S1). Transcription data for all the treatments was presented in the form of heatmaps. Each cell in the heatmap shows mean gene expression fold changes relative to the uninjected larval control (basal expression). Specific color codes were used illustrate degrees of gene expression change relative to the PBS control, and relative to the C. albicans infected group at 24 h and 48 h.
Table 1. Gene loci and primers used to analyse gene expression in Galleria mellonella larvae.
| Locus | Sequence reference | Putative Function/Process | Forward primer | Reverse primer |
| 18 s | AF286298 | Housekeeping | CACATCCAAGGAAGGCAG | AGTGTACTCATTCCGATTACGA |
| EF1: Elongation factor 1-Alpha (Ef-1a) | AF423811 | Housekeeping | AACCTCCTTACAGTGAATCC | ATGTTATCTCCGTGCCAG |
| GAL: Gallerimycin | AF453824 | Antimicrobial peptide | GAAGTCTACAGAATCACACGA | ATCGAAGACATTGACATCCA |
| GLIO: Galiomicin | AY528421 | Antimicrobial peptide | GTGCGACGAATTACACCTC | TACTCGCACCAACAATTGAC |
| GLV: Gloverin-like protein | AF394588 | Antimicrobial peptide | AGATGCACGGTCCTACAG | GATCGTAGGTGCCTTGTG |
| CER D: Cecropin D | Contig 19824 | Antimicrobial peptide | CTGCGCCATGTTCTTCA | TCGCATCTCTGATCCTCTG |
| 6tox | AF394584 | Antimicrobial peptide | GCGAACTGCGAAGAATTATC | TGTCTGTCTTGAGTTGCATATTG |
| HSP 90 | See methods | Molecular chaperone/stress response | GCRTCVCGYATGGAGGAAGT | GAACTAAATCAGTCTTTGG |
| TSF: Transferrin precursor | AY364430 | Siderophore/antimicrobial peptide | CGTAGCAGTCATCAAGAAGG | CGCACTCACTAGAACTGG |
| 18w: 18 wheeler | Contig14234 | Toll Receptor | CACTCGATTTAGGCAACAACA | TCCGAGACGATCAACACTTC |
| IMPI: Inducible metalloproteinase inhibitor | AY330624 | Metalloproteinase | TAGTAAGCAGTAGCATAGTCC | GCCATCTTCACAGTAGCA |
| C1-Contig 17373 | Contig 17373 | Glutathione peroxidase activity/Response to oxidative stress, Phospholipase A2 activity | CCACACTGTGAGGCAACATT | GTTTGCTTAGCACGGTCACA |
| C2- Contig 03093 | Contig 03093 | Peroxiredoxin activity/Response to oxidative stress | CTGACAATGACCGTGCACTT | TCTACGGGTGTAGCGACCTT |
| C3- Contig 15265 | Contig 15265 | G-protein coupled receptor activity/Stress response | CACACTGCAGGGCTTGTTTA | CCGTCCATCCTGACGTCTAT |
| C4- Contig 20595 | Contig 20595 | G-protein coupled receptor activity/Stress response | GCACATGACGTTAAGCCAGA | CCATTCCTGATCGCAACTTT |
| C5- Contig 21310 | Contig 21310 | Hsp protein binding/Stress response | GCAGCCTTAACGACCTGTTC | GTACACCTCAACCCCAGGAA |
| C6- Contig 1327 | Contig 1327 | Superoxide-generating NADPH/Oxidase activity/inflammatory response | GCTTGACATTGAGCTGTCCA | CCGTCCAATCACCTTTGACT |
| C7- Contig 15362 | Contig 15362 | Signal transducer/Regulation of cytokines, I-kappa B kinase/NF-kappa B cascade | CGAGCTAAAGACAGGCGATT | TCACCTGCGGTTGAATCATA |
| C8- Contig 19101 | Contig 19101 | Protein binding/Phagocytosis | ATTGCTAGCCAGGTTCAGGA | AGCTATTTGGCGGAAACTCA |
Primers were designed from published G. mellonella sequences (NCBI) or from coding sequences where high homology protein sequences could be identified from an EST library.
Statistical Analysis
All experiments were repeated three times with the data presented as the mean of those experiments. Statistical analysis of the data was performed using Prism v5.0 for Mac OS X (GraphPad software, San Diego California USA, www.graphpad.com). Data sets were tested for normal distribution using the Kolmogorov-Smirnov method. Deviation of outlier data was assessed by the Extreme studentized deviate method. Survival rates were compared using the Mantel-Cox test. Student's t test was used to analyze encapsulation response, and one-way ANOVA with Tukey's post tests were used for all other experiments. Significance levels were set at <0.05.
Ethics Statement
No permits were required for the described study, which complied with all relevant regulations. The field studies did not involve endangered or protected species.
Results
MYR inhibits C. albicans growth in vitro
Interactions between MYR and the other antifungal agents were studied using a chequerboard microdilution assay. The minimum inhibitory concentration (MIC) for MYR in the presence of Candida cells at 48 h was 0.12 µg/ml compared with 2 µg/ml and 0.25 µg/ml for AMPH and FLC, respectively (Table 2). The MYR+AMPH combination was synergistic in inhibiting Candida growth at 0.25 µg/ml and 0.03 µg/ml, respectively, whereas the MYR combination with FLC was antagonistic with MICs of 16 µg/ml and 0.12 µg/ml, respectively (Table 2).
Table 2. In vitro activity of antifungal drug combinations against Candida albicans.
| Standard MIC (µg/ml) | Combinatio MIC (µg/ml)/MYR (µg/ml) | FICI | Interpretation | |
| MYR | 0.12 | / | ||
| AMPH | 2 | 0.25/0.03 | 0.3 | synergism |
| FLC | 0.25 | 16/0.12 | 65 | antagonism |
Antifungal activity and interactions between drugs were determined using minimum inhibitory concentrations (MIC) and chequerboard microdilution assays, respectively. MIC was defined as the lowest concentration of antifungal agent resulting in an 80% inhibition of fungal growth compared with the control. Interactions based on FICI values were defined as: synergistic (FICI<0.5), antagonistic (FICI>4.0) and no interaction (FICI 0.5–4.0). MYR = myriocin; AMPH = amphotericin; FLC = fluconazole; MIC = minimum inhibitory concentration; FICI = fractional inhibitory concentration index.
MYR reduces G. mellonella survival during C. albicans infection
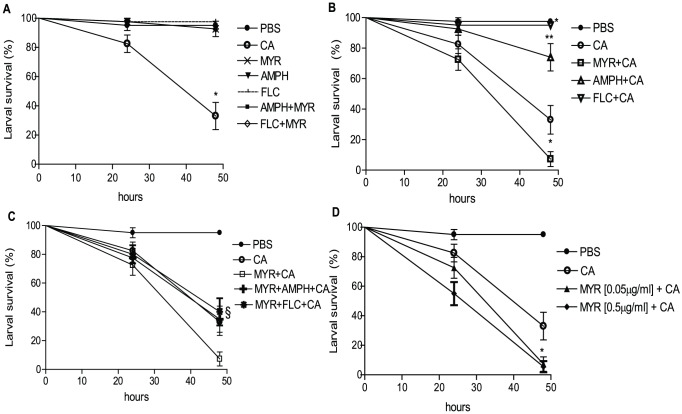
Larvae of G. mellonella injected with 105 Candida yeast cells resulted in survival rates of 82% and 33% at 24 h and 48 h, respectively, compared with 97% survival of larvae injected with PBS alone (p<0.0001; Figure 1A). MYR injection alone showed no apparent toxic effect that decreased larval survival below that of the PBS control at 48 h (Figure 1A). Larvae injected with the antifungal agents AMPH or FLC alone, also resulted in 97% larval survival at 48 h (Figure 1A). However, when MYR was injected into larvae which were subsequently infected with C. albicans, MYR treatment was lethal, reducing survival rates to 70% and 7% at 24 h and 48 h, respectively, and were significantly lower than C. albicans alone (p = 0.001; Figure 1B). In contrast, AMPH or FLC pre-treatments had a prophylactic effect, increasing larval survival rates, after fungal injection, for AMPH to 92% and 74% (p = 0.003) and for FLC to 100% at 24 h and 48 h, respectively (p<0.001; Figure 1B). In addition, the survival rates of insects infected with C. albicans after pre-treatment with MYR combined with either AMPH or FLC, were similar (p = 0.8) to the group infected with Candida alone (Figure 1C). However combined groups (MYR+AMPH+CA and MYR+FLC+CA) showed significant increased survival rates (p = 0.03 and p = 0.01; Figure 1C), respectively, when compared with MYR+CA group. Therefore, the anti-C. albicans synergy observed in vitro between MYR and AMPH (Table 2) was not observed in G. mellonella larvae infected with C. albicans. Increasing the MYR concentration ten-fold (to 0.5 µg/ml) did not improve antifungal prophylaxis in C. albicans infected larvae and instead mortality increased significantly at 24 h (p<0.001; Figure 1D) and at 0.05 µg/ml MYR concentration (p = 0.04) when compared with larvae from the CA group.
Figure 1. Effects of the antifungal drugs myriocin, amphotericin B, and fluconazole on Galleria mellonella survival 24 and 48 h post-treatment.
(A) Comparison of the survival rates of G. mellonella larvae following injection with PBS, or antifungal drugs (MYR, FLC, or AMPH) alone or in combination, or with C. albicans alone. The survival rate of the Candida-infected group was significantly lower than the PBS control group (*p<0.0001, n = 20) and also compared with all other groups. (B) Comparison of the survival rates of G. mellonella larvae following injection with PBS, or C. albicans alone, or the antifungal drug followed by C. albicans. Pre-treatment of larvae with MYR, or AMPH or FLC had significant impacts on survival rates when compared with CA group (*p<0.001 and **p = 0.003, n = 20). (C) Comparison of the survival rates of G. mellonella larvae following injection with PBS, or C. albicans alone or with MYR combined with either AMPH or FLC followed by C. albicans. Larvae from combined groups (MYR+AMPH+CA and MYR+FLC+CA) showed increased survival rates (§ p = 0.03,and p = 0.01, n = 20), respectively, when compared with MYR+CA group. (D) Comparison of the survival rates of G. mellonella larvae following injection with PBS, or C. albicans alone or with two different concentrations of MYR followed by C. albicans. Effect of MYR at higher concentration (0.5 µg/ml) decreased the survival rate when compared with CA group (*p<0.001, n = 20). PBS = injected buffer; CA = Candida albicans; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole.
C. albicans recovered from G. mellonella larvae treated with MYR
To determine the cause of death of the G. mellonella larvae at 24 h, we examined the fat body and hemolymph of C. albicans infected insects treated with different combinations of antifungals (Figure 2). Candida cells were recovered from all infected insects with the highest numbers recorded from the fat body of the group injected solely with C. albicans followed by the MYR+FLC group (Figure 2). Total Candida CFUs recovered from the fat body and hemolymph were lowest in insects treated with either AMPH or MYR+AMPH combined (Figure 2). Candida CFUs recovered from the fat body of the CA group were significantly higher than all the other groups (p<0.01). In contrast, CFUs recovered from the hemolymph of MYR+CA group were significantly higher (p<0.001) than all the other groups. In addition, only in the MYR+CA group were the CFUs recovered from hemolymph significantly higher than from the fat body (p<0.001). At 48 h, the CFUs recovered from the fat body were significantly higher (p<0.001) than at 24 h in the MYR+C. albicans treatment (data not shown).
Figure 2. Recovery of Candida albicans from infected Galleria mellonella larvae.
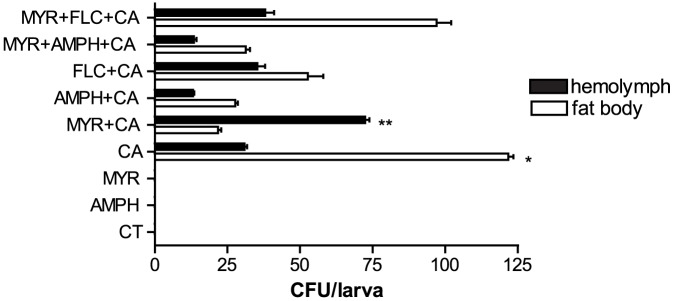
The number of C. albicans CFUs recovered from G. mellonella larval fat body and hemolymph 24 h post-drug treatment and infection. CFUs recovered from the fat body of the CA group were significantly higher than all the other groups (*p<0.01, n = 10). In contrast, CFUs recovered from the hemolymph of MYR+CA group were significantly higher (**p<0.001, n = 10) than all the other groups. In addition, only in the MYR+CA group were the CFUs recovered from hemolymph significantly higher than from the fat body (**p<0.001, n = 10). The drug treatments and abbreviations are as in Figure 1. Error bars indicate standard errors. Each experiment was triplicated.
MYR impacts on the G. mellonella hemogram
Injecting larvae with PBS resulted in a significant increase in hemocyte numbers compared with the non-injected control (p<0.001; Figure 3). When larvae were injected solely with MYR, FLC or AMPH, a significant decrease (p<0.001) in the hemocyte numbers was observed in comparison to the PBS injected controls (Figure 3). This reduction was particularly marked with AMPH and AMPH+MYR with counts significantly lower than all the other groups (p<0.001; Fig. 3). Following treatment solely with C. albicans, hemocyte numbers fell significantly (p<0.001; Figure 3) and remained low even in the presence of MYR, FLC, or AMPH used alone or in combination (MYR+FLC, MYR+AMPH; p<0.001; Figure 3).
Figure 3. Impact of myriocin and antifungal drugs on the Galleria mellonella hemograms.
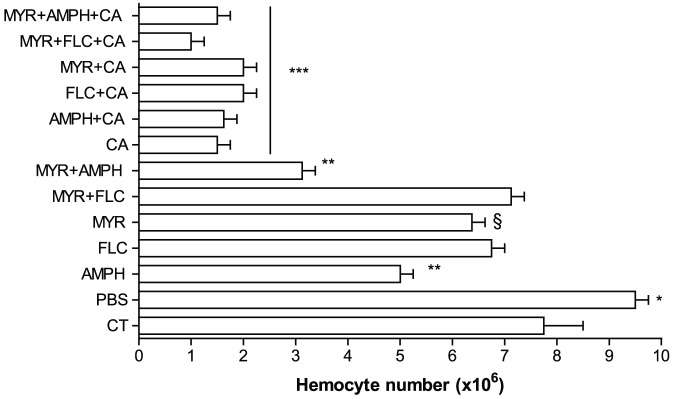
Hemocyte numbers (cells/ml) of G. mellonella larvae were determined 24 h after injection with C. albicans and/or drugs. The PBS group showed a significant increase in hemocyte numbers (*p<0.001, n = 20) and all the other groups showed a decrease of the hemocytes in comparison with control (CT). In particular, AMPH and MYR+AMPH groups showed decreased numbers (**p<0.001, n = 20). In all the groups where Candida was present, the hemocyte numbers were lower (***p<0.0001, n = 20). Controls were uninjected (CT) or PBS injected larvae. The drug treatments and abbreviations are as in Figure 1. Error bars indicate standard errors. Each experiment was triplicated.
G. mellonella larval hemolymph and recombinant antimicrobial peptides (AMPs) do not affect growth of C. albicans in vitro
Hemolymph from larvae of G. mellonella pre-injected with PBS (control), antifungal drugs or C. albicans was tested after 24 h and was found to have no inhibitory effect on C. albicans growth in vitro (data not shown). The recombinant AMPs, cecropin D and gallerimycin were also tested in vitro against C. albicans and only the latter exhibited anti-Candida activity at 100 µg/ml (p<0.001; Figure 4).
Figure 4. Effect of Galleria mellonella recombinant antimicrobial peptides on the in vitro growth of Candida albicans.
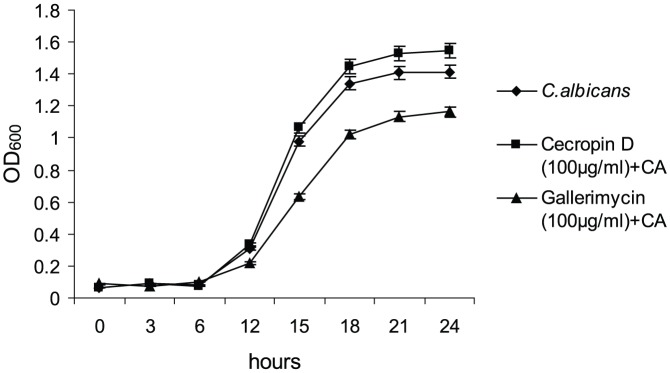
The growth of C. albicans in vitro was monitored spectrometrically over 24 h to determine the inhibitory effect of recombinant antimicrobial peptides cecropin D and gallerimycin. The maximum growth rate for C. albicans was 0.11, for galleriomycin and cecropin D were 0.06 and 0.08, respectively. Error bars indicate standard errors. Each experiment was triplicated.
Impact of MYR on phenoloxidase (PO), lysozyme, encapsulation, superoxide dismutase (SOD) and malondialdehyde (MDA) activity
The effect of antifungal drugs on major immune components of G. mellonella, including PO, lysozyme and reactive oxygen species, were monitored. PO and lysozyme play a major role in insect humoral immune responses to invading organisms. Larvae injected with MYR or FLC alone showed no changes (p>0.05) in the PO activity in the hemolymph when compared with the control groups whereas AMPH alone significantly reduced PO activity (p<0.001; Figure 5). PO activity was also significantly lower (p<0.001) in all the treatments in which C. albicans was present as well as with the MYR+FLC combination in the absence of the pathogen (p<0.001; Figure 5). In addition, cellular host response was investigated by the encapsulation process during infection. Melanotic encapsulation of plastic implants inserted into C. albicans-infected Galleria larvae was quantified using Image Pro software by measuring the coloration of the area of the implants. Encapsulation was significantly reduced (p<0.05; Figure 6A), with and without MYR, AMPH and FLC, and showed only limited melanisation compared with untreated and PBS control insects (Figure 6B). All the treatments induced lysozyme activity. The highest activity was with C. albicans alone (p<0.001; Figure S1), then FLC with (p<0.001; Figure S1) and without fungus (p<0.0001; Figure S1), and then the MYR+FLC combination with C. albicans (p = 0.001; Figure S1). Lysozyme activity was higher in insects treated with Candida when compared with MYR or C. albicans plus MYR treatments (p<0.001; Figure S1). SOD activity was slightly depressed in the MYR+FLC group (p>0.05; Figure S2) than the PBS control, although this was not statistically significant. A marked increase in SOD activity was detected in the AMPH+CA and MYR+FLC+CA groups (p<0.01; Figure S2). The MDA content, except for MYR alone (p = 0.2), was higher in all treatments compared with the PBS and un-injected control groups, particularly in the presence of C. albicans (p = 0.001–0.03; Figure S3).
Figure 5. Impact of myriocin and antifungal drugs on phenoloxidase (PO) activity during Candida albicans pathogenesis.
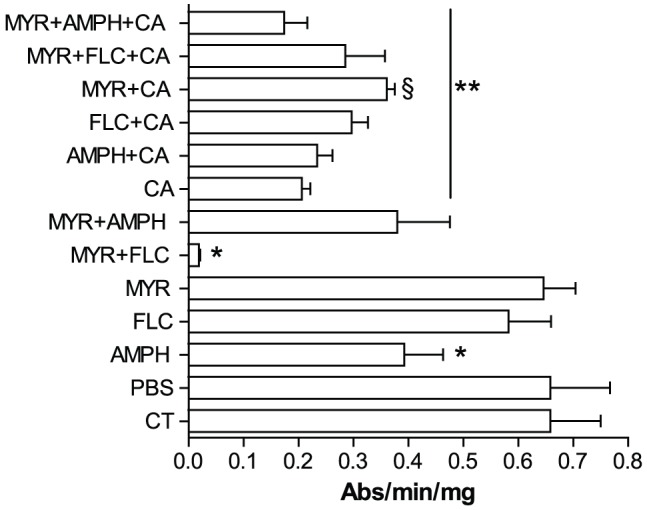
The in vitro PO activity of G. mellonella larval hemolymph was measured at 24 h during fungal pathogenesis and drug treatments. Significant decreases of PO activity were detected in the groups treated with AMPH and MYR+FLC (*p<0.001, n = 10) when compared with the controls. In addition, all the treatments in which Candida was present, a significant decrease of PO activity was observed when compared with the control groups (**p<0.001, n = 10). However larvae from MYR+CA group showed higher PO activity (§ p<0.05) when compared with CA group alone. Controls were uninjected (CT) or PBS injected larvae. The drug treatments and abbreviations are as in Figure 1. Error bars indicate standard errors. Each experiment was triplicated.
Figure 6. Encapsulation response of G. mellonella was decreased during Candida pathogenesis.
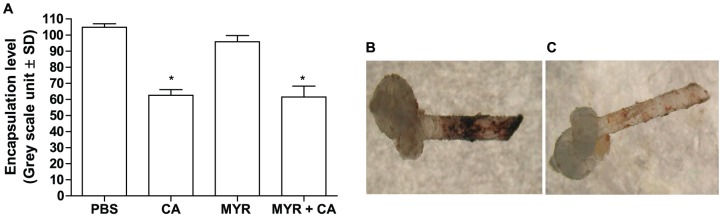
Melanization of the implants was quantified to determine if encapsulation was affected during Candida pathogenesis after implants were introduced into the left pro-leg of larvae 24 h post- infection. Two hours after insertion of implants, during C. albicans pathogenesis (n = 5, triplicated), the degree of the melanization was quantified using Image Pro software by first measuring the coloration (gray value) of all areas of each implant, and then comparing these values with intact implants (not inserted). Decreased encapsulation responses were observed in larvae infected with Candida with or without pre treatment with MYR compared with PBS injected larvae (Figure A, *p<0.05). Plastic implants inserted into C. albicans-infected Galleria larvae showed markedly reduced melanization illustrated in the Figure C compared with PBS control insects (Figure B).
MYR impacts on immunity and stress management genes during C. albicans pathogenesis
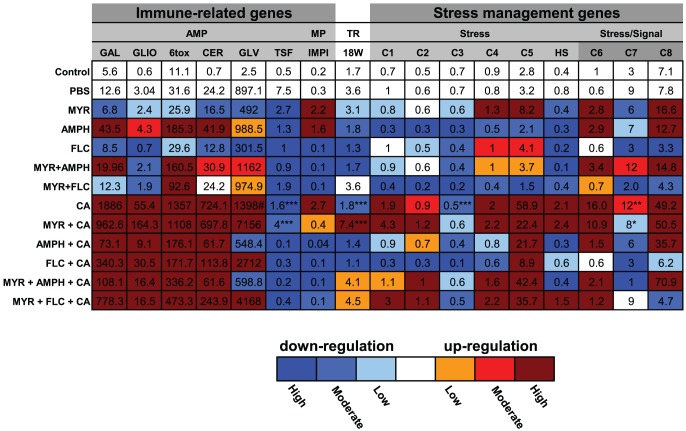
The effect of MYR treatment on a total of 17 genes was investigated. Differences in expression of AMPs (n = 6 genes), IMPI, 18W genes and putative stress management genes (n = 9 genes) were measured between drug-treated and PBS-injected groups (Figure 7). The colour gradient illustrated in the heat map (Figure 7) shows the corresponding percentage of down or up-regulation of the treatment groups in relation to the PBS controls.
Figure 7. Gene expression comparison between PBS control and treated groups in Galleria mellonella.
Expression of 17 gene transcripts in response to antifungal drugs and C. albicans 24 h post treatment. Mean value in each cell of the heat-map corresponds to gene expression fold change relative to uninjected larvae control (basal expression). Color gradient was used to visualize the gene expression comparison between treated groups and PBS injected control. The color gradients correspond to 3 different levels of gene expression relative to PBS injected control. A light color represents low gene expression i.e.0–25% of that expressed by the PBS injected control; intermediate color to moderate gene expression i.e. 25%–50% of PBS injected control; dark color to high gene expression, above 50% of PBS injected control. Among the 17 gene transcripts investigated during Candida pathogenesis (CA group) in the Galleria larvae only 3 genes TSF, 18W and C3 showed significant down-regulation (***p<0.001) in comparison with PBS injected control. The remaining gene transcripts in this group were significantly up-regulated (p<0.001). When the effect of MYR on the gene expression, during Candida pathogenesis (MYR+CA group), was compared with PBS control down-regulation profile on TSF (p<0.001) was found. Contrasting 18W and C7 transcripts results from CA and MYR+CA groups showed opposite gene-regulation profiles when compared to PBS control (***p<0.001, **p<0.05, *p>0.05). MYR = myriocin; AMPH = amphotericin; FLC = fluconazole, AMP = antimicrobial peptide; MP = metalloproteinase; TR = Toll receptor; GAL = gallerimycin; GLIO = galiomicin, 6tox; CER D = cecropin D; GLV = gloverin; TSF = transferrin; IMPI = Inducible metalloproteinase inhibitor; 18W = 18 wheeler; HS = heat shock protein 90; Contig codes for putative stress management genes Contig 17373 (C1); Contig 03093 (C2); Contig 15265 (C3); Contig 20595 (C4); Contig 21310 (C5); Contig 1327 (C6); Contig15362 (C7); Contig19101 (C8); #×10.
In the MYR alone group, all AMPs (Gallerimycin, Galiomicin, 6tox, Cecropin D, Gloverin, Transferrin) and 18W were down-regulated to different degrees when compared with the PBS injected group, while the IMPI gene showed up-regulation (p<0.001; Figure 7). However only Transferrin and IMPI showed statistical differences (p<0.001). Among the stress management genes Contigs 17373 (C1) (p>0.05), 15265 (C3) (p<0.01), 15362 (C7) (p<0.01) and hsp90 (p>0.05) were down-regulated while Contigs 20595 (C4) (p>0.05), 21310 (C5) (p<0.05), 1327 (C6) (p<0.01) and 19101 (C8) (p<0.01) were up-regulated in comparison with the PBS controls (Figure 7). Therefore, among the 17 genes investigated, 11 genes were down-regulated and 5 genes were up-regulated by MYR-treatment alone.
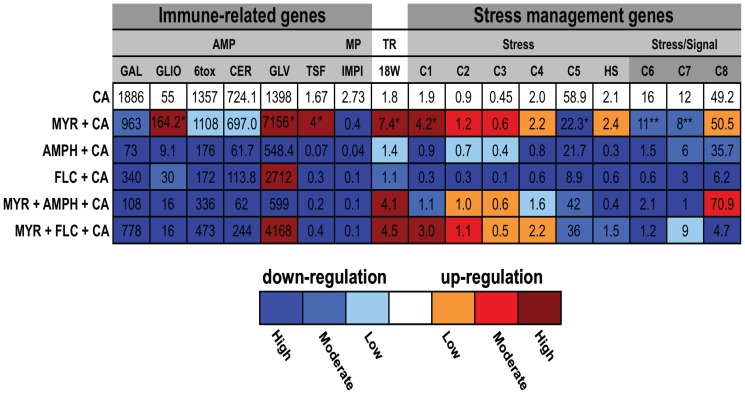
When the antifungal drugs AMPH and FLC were tested on the insects different effects on gene expression were observed. AMPH treatment alone resulted in down-regulation of 9/17 genes and up-regulation of 8/17 genes compared with the PBS controls (Figure 7). Interestingly, virtually all (7/9) stress-management genes were down-regulated by AMPH treatment alone. FLC alone down-regulated 13/17 genes and up-regulated of only 2 genes among the 17 genes investigated (Figure 7). In general, MYR plus either AMPH or FLC caused changes in gene regulation to varying degrees when compared with drug treatments alone (Figure 7). With Candida infection alone, 14/17 genes (Gallerimycin, Galiomicin, 6tox, Cecropin D, Gloverin, IMPI, hsp90 and Contigs 17373 (C1), 03093 (C7), 20595 (C4), 21310 (C5), 1327 (C6), 15362 (C7), 19101 (C8)) were up-regulated with only Transferrin, 18W, and Contig 15265 (C3) down-regulated in comparison to the PBS controls (p<0.001; Figure 7). Pre-treatment with MYR followed by Candida infection also caused up-regulation of the same set of genes except that 18W and Contig 15362 (C7) which were up and down-regulated, respectively (Figure 7). Pre-treatment with MYR+AMPH or MYR+FLC combinations prior to C. albicans resulted in up-regulation of the majority of the AMP genes (Figure 7). These two groups also showed different levels of modulation of the stress management genes (Figure 7). Interesting results were obtained comparing fold changes of the genes between the C. albicans alone infected larvae with and without the antifungals. With the MYR+Candida group, 10/17 genes (p<0.001; Galiomicin, Gloverin, Transferrin, 18W, hsp90), (p<0.05 Contig 17373 (C1); p>0.05 Contig 15265 (C3); p<0.01 Contigs 20595 (C4), 03093 (C7), and 19101 (C8)) were up-regulated while the remaining genes were down-regulated compared with C. albicans alone group (Figure 8). The same 5 stress related genes were up-regulated in the two groups but had significantly higher expression in the MYR+Candida group (p = 0.001). In addition, the stress-signaling related genes Contigs 1327 (C6) and 15362 (C7) were down-regulated in the MYR+Candida compared with the Candida alone treatments (p<0.05; Figure 8). In contrast to the MYR+C. albicans treatment compared with the C. albicans alone, pre-treatment with AMPH or FLC or MYR+AMPH or MYR+FLC combinations prior to C. albicans resulted in down-regulation of the majority of the AMP genes whilst different levels of modulation were observed for the stress management genes (Figure 8). At 48 h, in general, for all treatments, there was a marked down-regulation, to different degrees, of all gene transcripts from the Galleria larvae (Figure S4 and S5).
Figure 8. Gene expression comparison between C. albicans alone and drug-treated groups in Galleria mellonella.
Gene expression comparing C. albicans infected larvae group with and without antifungals. Expression of 17 gene transcripts in response to C. albicans and antifungal drugs 24 h post treatment. The value in each heat-map cell corresponds to the mean gene expression fold change relative to the uninjected larvae control (basal expression). To visualize the change in the gene expression a color gradient was used. The color gradients correspond to 3 different levels of gene expression relative to CA group. A light color represents low gene expression i.e.0–25% of that expressed by C. albicans alone; intermediate color to moderate gene expression i.e. 25%–50% of C. albicans alone; dark color to high gene expression, above 50% of C. albicans alone. The effect of MYR during Candida pathogenesis (MYR+CA group) in the Galleria larvae in comparison with CA group, showed a significant increase (*p<0.001) in the level of gene expression of 3 antimicrobial peptides GLIO, GVL and TSF, toll receptor 18W and stress related gene C1. On the other hand this treatment also caused a significant decrease of gene expression of IMPI, C5 (*p<0.001), C6 and C7 (**p<0.05). Abbreviations listed in Figure 7.
Discussion
In the present study, we show that neither MYR, FLC nor AMPH alone or in combination were harmful to the model insect, G. mellonella. AMPH and FLC are designed to target fungal not animal cells whereas MYR primarily targets mammalian cells and its role as an antifungal has not been fully characterized. MYR in combination with C. albicans resulted in a remarkable 93% larval mortality after 48 h. This is in contrast to AMPH and FLC with C. albicans which only caused 26% and 0% mortalities, respectively, after 48 h. Similarly, the combination of MYR with the other drugs enhanced larval mortalities. This did not correspond with the synergistic or antagonistic effects of these drug combinations in the in vitro test against Candida. A preliminary study with Galleria larvae injected with the entomopathogen fungus Metarhizium anisopliae, used in biological control [32], showed an increased mortality when larvae were pre-treated with MYR (unpublished data). This result is similar to the present study using Candida which suggests that MYR has a general immunosupressive effect on the host. Furthermore, both fungi were susceptible to MYR in vitro (unpublished data).
In addition, although there was no obvious antifungal prophylaxis observed in vivo using MYR with C. albicans, a preliminary study with the topical application (i.e. main infection route) of M. anisopliae on the Galleria larvae pre-treated with MYR was performed and an antifungal prophylactic effect was observed so that survival rates were increased (unpublished data). Therefore, there is the possibility that future derivatives of MYR could reduce the risk of mycoses in immune-compromised patients. There are many examples where drugs have dual functions leading to improved health care and reduced cost. For example, in the 1990s when a new HIV protease inhibitor was introduced for HIV combined therapy, this inhibitor also inhibited Candida pathogenicity related proteases leading to a significant decrease in candidiasis in HIV immunodeficient patients [12]. Another potential application of myriocin could be for topical antifungal treatment as in superficial candidiasis, in particular, in patients with chronic mucocutaneous candidiasis (CMC) who suffer from a selective immune deficiency with increased susceptibility to superficial candidal infections [33]. It would also be interesting to test the susceptibility to MYR of other aetiological agents of fungal disease in CMC patients, such as dermatophyte infections, i.e. Trichophyton and Epidermophyton, histoplasmosis [33].
In G. mellonella larvae treated with the different combinations of drugs and C. albicans, the humoral and cellular defenses failed to control the infection or protect the host from increased mortality. There was no obvious correlation between levels of activity of PO, SOD, MDA, lysozyme, AMPs or hemograms with larval survival when infected with C. albicans. For example, the total hemocyte counts were similar for combinations of C. albicans with MYR or FLC yet larval survival times were markedly different. However, an important finding was the impact of MYR on the Candida colonization profile, where an increased number of circulating Candida CFUs were observed in the hemolymph of the MYR+CA group than the CA group alone; these cells may rapidly deplete nutrients and release toxic compounds accelerating death. Furthermore, this finding combined with larval hemocytopenia could contribute to decreased larval survival.
Furthermore, no in vitro antifungal activity of hemolymph from larvae pre-infected with C. albicans was detected. The AMPs, even though their genes were highly upregulated, failed to inhibit C. albicans, probably due to the peptides being degraded by the fungal proteases. Recombinant gallerimycin, which is known to have antifungal activity, had limited inhibition of C. albicans in vitro even when used at relatively high concentrations.
The hemocytopenia, recorded in all treatments with C. albicans, probably results from either an abortive attempt to encapsulate the fungus or from cell lysis. Since the hemocytes are a source of PO this would also partially explain why there was a corresponding decline in the levels of plasma PO activity and a reduced encapsulation response. Dunphy et al. (2003) also noted that pathogenic C. albicans reduce nodule formation by damaging the hemocytes, allowing the fungus to colonise the host [34]. Recovery of C. albicans from G. mellonella larvae revealed that the number of CFUs from the hemolymph was significantly higher than from the fat body only with the MYR treatment. Since MYR has been shown in vitro to kill C. albicans then in vivo the drug could either have been inactivated in some way or removed from the hemolymph before it could make contact with the fungus. MYR is a structural analogue of sphingosine [4], a precursor of important signaling molecules and an essential component of eukaryotes cell membranes, which can readily bind to and increase the permeability of cell membranes [35]. Thus, in the present paper, injection of MYR prior to the C. albicans may have resulted in the drug binding to the inner surface of the hemocoel, and particularly to the extensive fat body, so that drug dilution in the hemolymph occurred and reduced the killing of the fungus to give the increase in CFUs and enhanced larval death recorded. In contrast, AMPH and FLC have different modes of antifungal activity to MYR as they target ergosterol, a key component of fungal cell membranes [36]. Following injection of AMPH and FLU into G. mellonella hemocoel, and in contrast to MYR, these drugs, because of their ergosterol specificity, would have remained at higher levels than MYR and available to kill C. albicans. This would then explain the higher survival rates of larvae with these drugs and the lower levels of C. albicans recorded in the hemolymph. Alternatively, exposure of C. albicans in the hemolymph to sub-MIC levels of MYR could have increased the lethality of the fungal infection by, for example, enhancing fungal protease secretion. However, pre-treating the G. mellonella larvae with a MYR concentration ten-fold higher (from 0.05 µg/ml to 0.5 µg/ml) did not improve antifungal prophylaxis in C. albicans infected larvae and instead mortality increased significantly at 24 h. This indicates that the fungal pathogenicity is not enhanced by sub-MIC levels of MYR.
Regarding the expression of the immune and stress management genes, all drug combinations tested affected to various degrees all 17 genes examined. The AMP genes were significantly up-regulated by AMPH alone, MYR plus AMPH and by all drug combinations in the presence of C. albicans. However, as stated earlier, this upregulation of the AMP genes had no obvious effect on the outcome of the infection. It is possible that the AMPs play a role in protecting the host from secondary infections by opportunistic microbial pathogens. Particularly noteworthy was the effect of the C. albicans alone and the MYR+C. albicans combination on the upregulation of the stress management genes. Although five stress management genes were up-regulated in the two groups, this expression was significantly higher in the MYR+C. albicans combination. This may be due to a number of factors such as MYR increasing the permeability of the insect cell membranes and/or interfering with cell signaling and inducing oxidative stress all of which are well documented for MYR in vertebrate systems [21]. The stress induced by MYR, together with enhanced survival of C. albicans circulating in the hemolymph in the presence of reduced levels of this drug, probably combine to increase the mortality of insects receiving the MYR-C. albicans treatment. In addition, stress management genes were also upregulated in C. albicans infected insects pre-treated with combinations of MYR with FLC or AMPH, which only marginally improved survival compared with C. albicans alone. These observations provide further evidence that MYR was the cause of the stress which was exacerbated by C. albicans. In marked contrast with the MYR combination with C. albicans, expression of all the stress management genes was downregulated in C. albicans infected insects pre-treated with FLC or AMPH suggesting fungal inhibition which correlates with increased larval survival. Finally, in the MYR alone and the MYR+C. albicans groups, Contig 15362 (C7) was downregulated and this gene has a putative role in signal transduction/cytokine regulation, I-kappaB kinase/NF-kappaB cascade and inflammatory responses [29].
The present study, using the Galleria model, demonstrates for the first time, an intricate interplay between immune and stress management genes in response to pathogens and therapeutics. Such interplay is well documented in humans [37], [38] so that the opportunity exists to identify common mechanisms involved in disease pathology in these disparate groups. The findings of the present study may have clinical implications in the use of MYR, or its analogues, to treat various sphingolipid-associated disorders. The dual immunosuppressant and antifungal properties of MYR failed to be translated into the G. mellonella model host during Candida infection. Furthermore, MYR stresses and impacts on the host and exacerbates C. albicans infection leading to enhanced host mortality. This increased host mortality highlights the potential clinical implication and health risks since G. mellonella has proven to be an effective surrogate for the murine model for evaluating microbial pathogenicity and drug therapeutics. However, in the present study, MYR was applied early in infection but candidiasis may cause hyperinflammatory responses at later infection stages, so that application of MYR or its analogues at these later stages may be beneficial and dampen hyperinflammatory responses. It would therefore also be very useful to study late stage infections in the G. mellonella model. Many correlations for G. mellonella and mice have been established for the pathogenicity of microbes, including C. albicans, Aspergillus fumigatus, Pseudomonas aeruginosa, and Yersinia pseudotuberculosis [19], [20], [39] as well as in the efficacy of licensed drugs [40]. It is known that C. albicans has its own mechanisms, including proteases, for suppressing the immune response of the Galleria larvae [41], and the upregulation by MYR of the stress genes is an interesting observation. Surprisingly, stress-related genes are not only involved in protecting cells from environmental stressors and pathogens [42] but can also be toxic under certain circumstances. For example, HSP90 which is upregulated by MYR+C. albicans if nitrated can induce cell death in other systems [43]. Thus MYR is not overtly suppressing Galleria immunity but covertly doing so by upregulating the stress genes, some of which participate in killing the insect.
Finally, this is one of the first times, as far as we are aware, that a therapeutic-pathogen combination has been found to be more harmful than the pathogen alone. Most therapeutics, including FLC and AMPH, targeting a range of bacteria and fungi in insect models, generally increase survival [44], [45]. Furthermore, the antifungal synergies observed with MYR in vitro were not translated in the C. albicans infected insect host. However, antifungal drugs have been shown to work synergistically with other therapeutics as reported between AMPH and 5-flucytosine against Cryptococcus neoformans in G. mellonella [16] and between FLC and an hsp90 inhibitor against C. albicans in G. mellonella. Most often, the synergies detected in the G. mellonella model can be reproduced in mammals [37]. This alternative model demonstrated to be an attractive tool to study therapeutic prophylaxis of antifungal drugs. The increased mortalities of the G. mellonella hosts following MYR plus C. albicans treatment are now the subject of further investigation.
Supporting Information
(DOC)
Lysozyme activity. The lysozyme activity in the hemolymph of Galleria mellonella larvae was measured 24 h after injection with antifungal drugs with and without Candida albicans. All the treatments induced lysozyme activity to different levels. AMPH, FLC and MYR levels compared with PBS injected group were higher (* p<0.01, n = 20). CA and FLC+CA groups compared with PBS injected groups showed markedly increased lysozyme activity (**p<0.001, n = 20). MYR+AMPH, MYR+FLC, AMPH+CA and MYR+CA groups did not show statistical differences when compared with PBS inject group (***p>0.05, n = 20). PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was triplicated.
(EPS)
Superoxide dismutase (SOD) activity. SOD activity (antioxidant defense response) in the hemolymph of G. mellonella larvae was measured 24 h after injection with antifungal drugs with and without C. albicans. MYR+FLC treatment resulted in a slightly decreased SOD activity (Mean±SD 0.46±0.026, *p>0.05, n = 20) than the PBS control, although this was not statistically significant. A marked increase in SOD activity was detected in the AMPH+CA and MYR+FLC+CA groups (**p<0.01, n = 20) when compared with PBS control. PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was done in triplicate.
(EPS)
Malondialdehyde (MDA) activity. The MDA content in the hemolymph of G. mellonella larvae was measured 24 h after injection with antifungal drugs followed by C. albicans. The FLC+CA and MYR+FLC+CA groups showed the greatest MDA activity (*p<0.05, n = 20) when compared with the PBS control. PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was done triplicate.
(EPS)
Gene expression comparison between PBS control and treated groups in Galleria mellonella . Expression of 13 gene transcripts in response to C. albicans and antifungal drugs 48 h post treatment. Mean value in each cell of the heatmap corresponds to gene expression fold change relative to uninjected larvae control (basal expression). Gene expression was visualized using a color gradient. The color gradients correspond to 3 different levels of gene expression relative to PBS control. Light color corresponds to low gene expression i.e.0–25% of that expressed by the PBS control; intermediate color to moderate gene expression i.e. 25%–50% of PBS control; dark color to high gene expression, above 50% of PBS control. MYR = myriocin; AMPH = amphotericin; FLC = fluconazole, AMP = antimicrobial peptide; MP = metalloproteinase; TR = Toll receptor; GAL = gallerimycin; GLIO = galiomicin, 6tox; CER D = cecropin D; GLV = gloverin; TSF = transferrin; IMPI = Inducible metalloproteinase inhibitor; 18W = 18 wheeler; HS = heat shock protein 90; Contig codes for putative stress management genes Contig 17373 (C1); Contig 03093 (C2); Contig 15265 (C3); Contig 20595 (C4); Contig19101 (C8).
(EPS)
Gene expression comparison between C. albicans alone and drug-treated groups in Galleria mellonella . Expression of 13 gene transcripts in response to antifungal drugs in C. albicans infected larvae 48 h post treatment. The value in each heatmap cell corresponds to the mean gene expression fold change relative to the uninjected larvae control (basal expression). To visualize the effect of drug-treated groups on gene expression during Candida infection a color gradient was used. The color gradients correspond to 3 different levels of gene expression relative to CA group. A light color represents low gene expression (0–25% of CA group baseline), an intermediate color moderate gene expression (25%–50% of CA group baseline) and a dark color represents high gene expression, (above 50% of CA group baseline). Abbreviations are as listed for Figure S4.
(EPS)
Acknowledgments
The authors thank Professors Andrew Rowley and Venkateswarlu Kanamarlapudi for kindly reviewing the manuscript. Mr James Taylor for maintaining the Galleria mellonella colony and Dr. I.M. Dubovskiy for helpful discussions.
Funding Statement
KM and AV were funded by the DFG Priority Program 1399 “Host-Parasite-Coevolution – rapid reciprocal adaptation and its genetic basis” to AV (VI 219/3-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Strader MR, Pearce CJ, Oberlies NH (2011) Fingolimod (FTY720):A Recently approved Multiple Sclerosis drug based on fungal secondary metabolite. J Nat Prod 74: 900–907. [DOI] [PubMed] [Google Scholar]
- 2. Kluepfel D, Bagli J, Baker H, Charest MP, Kudelski A (1972) Myriocin, a new antifungal antibiotic from Myriococcum albomyces . J Antibiot 25: 109–115. [DOI] [PubMed] [Google Scholar]
- 3. Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9: 883–897. [DOI] [PubMed] [Google Scholar]
- 4. Chiba K, Hoshino Y, Suzuki C, Masubuchi Y, Yanagawa Y, et al. (1996) FTY720, a novel immunosuppressant possessing unique mechanisms. I. Prolongation of skin allograft survival and synergistic effect in combination with cyclosporine in rats. Transplant Proc 28: 1056–1059. [PubMed] [Google Scholar]
- 5. Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, et al. (2002) Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349. [DOI] [PubMed] [Google Scholar]
- 6. Taha TA, Hannun YA, Obeid LM (2006) Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol 39: 113–131. [DOI] [PubMed] [Google Scholar]
- 7. Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121: 4222–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang XC, Goldberg IJ, Park TS (2011) Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol 721: 19–39. [DOI] [PubMed] [Google Scholar]
- 9. Lee YS, Choi KM, Lee S, Sin DM, Lim Y, et al. (2012) Myriocin, a serine palmitoyltransferase inhibitor, suppresses tumor growth in a murine melanoma model by inhibiting de novo sphingolipid synthesis. Cancer Biol Ther 13: 92–100. [DOI] [PubMed] [Google Scholar]
- 10. Strettoi E, Gargini C, Novelli E, Sala G, Piano I, et al. (2010) Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci U S A 107: 18706–18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glaros EN, Kim WS, Quinn CM, Jessup W, Rye KA, et al. (2008) Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J Lipid Res 49: 324–331. [DOI] [PubMed] [Google Scholar]
- 12. Melo NR, Taguchi H, Culhari VP, Kamei K, Mikami Y, et al. (2009) Oral candidiasis of HIV-infected children undergoing sequential HIV therapies. Med Mycol 47: 149–156. [DOI] [PubMed] [Google Scholar]
- 13. Morgan J (2005) Global trends in candidemia: review of reports from 1995–2005. Curr Infect Dis Rep 7: 429–439. [DOI] [PubMed] [Google Scholar]
- 14. Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, et al. (2006) Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents 27: 359–366. [DOI] [PubMed] [Google Scholar]
- 15. Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, et al. (2011) Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol 7: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, et al. (2005) Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73: 3842–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desbois AP, Coote PJ (2011) Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J Antimicrob Chemother 66: 1785–1790. [DOI] [PubMed] [Google Scholar]
- 18. Lionakis MS (2011) Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence 2: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brennan M, Thomas DY, Whiteway M, Kavanagh K (2002) Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 34: 153–157. [DOI] [PubMed] [Google Scholar]
- 20. Jander G, Rahme LG, Ausubel FM (2000) Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J Bacteriol 182: 3843–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubovskiy IM, Martemyanov VV, Vorontsova YL, Rantala MJ, Gryzanova EV, et al. (2008) Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp Biochem Physiol C Toxicol Pharmacol 148: 1–5. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3nd ed. M27-A3 National Committee for Clinical Laboratory Standards, Wayne, Pa. 67: 2982–2992.
- 23. Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52: 1. [DOI] [PubMed] [Google Scholar]
- 24. Fuchs BB, O'Brien E, Khoury JB, Mylonakis E (2010) Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1: 475–482. [DOI] [PubMed] [Google Scholar]
- 25. Ratcliffe NA, Gagen SJ (1977) Studies on the in vivo cellular reactions of insects: an ultrastructural analysis of nodule formation in Galleria mellonella. Tissue Cell 9: 73–85. [DOI] [PubMed] [Google Scholar]
- 26. Ashida M, Brey PT (1995) Role of the integument in insect defense: pro-phenol oxidase cascade in the cuticular matrix. Proc Natl Acad Sci U S A 92: 10698–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCord JM, Fridovich I (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244: 6056–6063. [PubMed] [Google Scholar]
- 28. Rael LT, Thomas GW, Craun ML, Curtis CG, Bar-Or R, et al. (2004) Lipid peroxidation and the thiobarbituric acid assay: standardization of the assay when using saturated and unsaturated fatty acids. J Biochem Mol Biol 37: 749–752. [DOI] [PubMed] [Google Scholar]
- 29. Vogel H, Altincicek B, Glockner G, Vilcinskas A (2011) A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics 12: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu PJ, Li T, Xiao JH, Murphy RW, Huang DW (2011) Universal primers for amplifying the complete coding sequence of cytoplasmic heat shock protein 90 (HSP90) in Lepidoptera. European Journal of Entomology 108: 164–168. [Google Scholar]
- 31. Staheli JP, Boyce R, Kovarik D, Rose TM (2011) CODEHOP PCR and CODEHOP PCR primer design. Methods Mol Biol 687: 57–73. [DOI] [PubMed] [Google Scholar]
- 32. Ansari MA, Carpenter S, Butt TM (2010) Susceptibility of Culicoides biting midge larvae to the insect-pathogenic fungus, Metarhizium anisopliae: prospects for bluetongue vector control. Acta Trop 113: 1–6. [DOI] [PubMed] [Google Scholar]
- 33. Lilic D (2012) Unravelling fungal immunity through primary immune deficiencies. Curr Opin Microbiol 15: 420–426. [DOI] [PubMed] [Google Scholar]
- 34. Dunphy GB, Oberholzer U, Whiteway M, Zakarian RJ, Boomer I (2003) Virulence of Candida albicans mutants toward larval Galleria mellonella (Insecta, Lepidoptera, Galleridae). Can J Microbiol 49: 514–524. [DOI] [PubMed] [Google Scholar]
- 35. Contreras FX, Sot J, Alonso A, Goni FM (2006) Sphingosine increases the permeability of model and cell membranes. Biophys J 90: 4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hannich JT, Umebayashi K, Riezman H Distribution and functions of sterols and sphingolipids. Cold Spring Harb Perspect Biol 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, et al. (2009) Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 106: 2818–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filler SG Insights from human studies into the host defense against candidiasis. Cytokine 58: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Slater JL, Gregson L, Denning DW, Warn PA (2011) Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol 49 Suppl 1: S107–113. [DOI] [PubMed] [Google Scholar]
- 40. Mylonakis E, Aballay A (2005) Worms and flies as genetically tractable animal models to study host-pathogen interactions. Infect Immun 73: 3833–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vilcinskas A, Jegorov A, Landa Z, Gotz P, Matha V (1999) Effects of beauverolide L and cyclosporin A on humoral and cellular immune response of the greater wax moth, Galleria mellonella . Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 122: 83–92. [DOI] [PubMed] [Google Scholar]
- 42. Dubovskiy IM, Whitten MM, Yaroslavtseva ON, Greig C, Kryukov VY, et al. (2013) Can insects develop resistance to insect pathogenic fungi? PLoS One 8 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Franco MC, Ye Y, Refakis CA, Feldman JL, Stokes AL, et al. (2013) Nitration of Hsp90 induces cell death. Proc Natl Acad Sci U S A 110: E1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hamamoto H, Kurokawa K, Kaito C, Kamura K, Manitra Razanajatovo I, et al. (2004) Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob Agents Chemother 48: 774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rowan R, Moran C, McCann M, Kavanagh K (2009) Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. Biometals 22: 461–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Lysozyme activity. The lysozyme activity in the hemolymph of Galleria mellonella larvae was measured 24 h after injection with antifungal drugs with and without Candida albicans. All the treatments induced lysozyme activity to different levels. AMPH, FLC and MYR levels compared with PBS injected group were higher (* p<0.01, n = 20). CA and FLC+CA groups compared with PBS injected groups showed markedly increased lysozyme activity (**p<0.001, n = 20). MYR+AMPH, MYR+FLC, AMPH+CA and MYR+CA groups did not show statistical differences when compared with PBS inject group (***p>0.05, n = 20). PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was triplicated.
(EPS)
Superoxide dismutase (SOD) activity. SOD activity (antioxidant defense response) in the hemolymph of G. mellonella larvae was measured 24 h after injection with antifungal drugs with and without C. albicans. MYR+FLC treatment resulted in a slightly decreased SOD activity (Mean±SD 0.46±0.026, *p>0.05, n = 20) than the PBS control, although this was not statistically significant. A marked increase in SOD activity was detected in the AMPH+CA and MYR+FLC+CA groups (**p<0.01, n = 20) when compared with PBS control. PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was done in triplicate.
(EPS)
Malondialdehyde (MDA) activity. The MDA content in the hemolymph of G. mellonella larvae was measured 24 h after injection with antifungal drugs followed by C. albicans. The FLC+CA and MYR+FLC+CA groups showed the greatest MDA activity (*p<0.05, n = 20) when compared with the PBS control. PBS = injected buffer; CA = Candida; MYR = myriocin; AMPH = amphotericin; FLC = fluconazole. Each experiment was done triplicate.
(EPS)
Gene expression comparison between PBS control and treated groups in Galleria mellonella . Expression of 13 gene transcripts in response to C. albicans and antifungal drugs 48 h post treatment. Mean value in each cell of the heatmap corresponds to gene expression fold change relative to uninjected larvae control (basal expression). Gene expression was visualized using a color gradient. The color gradients correspond to 3 different levels of gene expression relative to PBS control. Light color corresponds to low gene expression i.e.0–25% of that expressed by the PBS control; intermediate color to moderate gene expression i.e. 25%–50% of PBS control; dark color to high gene expression, above 50% of PBS control. MYR = myriocin; AMPH = amphotericin; FLC = fluconazole, AMP = antimicrobial peptide; MP = metalloproteinase; TR = Toll receptor; GAL = gallerimycin; GLIO = galiomicin, 6tox; CER D = cecropin D; GLV = gloverin; TSF = transferrin; IMPI = Inducible metalloproteinase inhibitor; 18W = 18 wheeler; HS = heat shock protein 90; Contig codes for putative stress management genes Contig 17373 (C1); Contig 03093 (C2); Contig 15265 (C3); Contig 20595 (C4); Contig19101 (C8).
(EPS)
Gene expression comparison between C. albicans alone and drug-treated groups in Galleria mellonella . Expression of 13 gene transcripts in response to antifungal drugs in C. albicans infected larvae 48 h post treatment. The value in each heatmap cell corresponds to the mean gene expression fold change relative to the uninjected larvae control (basal expression). To visualize the effect of drug-treated groups on gene expression during Candida infection a color gradient was used. The color gradients correspond to 3 different levels of gene expression relative to CA group. A light color represents low gene expression (0–25% of CA group baseline), an intermediate color moderate gene expression (25%–50% of CA group baseline) and a dark color represents high gene expression, (above 50% of CA group baseline). Abbreviations are as listed for Figure S4.
(EPS)