Abstract
Neurocognitive deficits arising from anesthetic exposure have recently been debated, while studies have shown that the phosphorylation of cyclic AMP response element-binding protein (CREB) in the hippocampus is critical for long-term memory. To better understand the neural effects of inhalational anesthetics, we studied the behavioral and biochemical changes in aged rats that were exposed to sevoflurane (Sev) and nitrous oxide (N2O) for 4 h. Eighteen-month-old rats were randomly assigned to receive 1.3% sevoflurane and 50% nitrous oxide/50% oxygen or 50% oxygen for 4 h. Spatial learning and memory were tested with the Morris water maze 48 h after exposure, and the results showed that sevoflurane–nitrous oxide exposure induced a significant deficit in spatial learning acquisition and memory retention. Experiments revealed that the cAMP and pCREB levels in the dorsal hippocampus were decreased in rats with anesthetic exposure in comparison with control rats 48 h after anesthesia as well as 15 min after the probe trial, but there were no significant differences in CREB expression. Besides these, the current study also found the DG neurogenesis significantly decreased as well as neuronal loss and neuronal apoptosis increased in the hippocampus of rats exposed to Sev+N2O. The current study demonstrated that down-regulation of cAMP/CREB signaling, decrease of CREB-dependent neurogenesis and neuronal survival in the hippocampus contributed to the neurotoxicity and cognitive dysfunction induced by general anesthesia with sevoflurane–nitrous oxide.
Introduction
The combination of sevoflurane with nitrous oxide is widely used in clinical anesthesia practice. However, recent studies have raised concerns about the neurotoxicity of inhalational anesthetics and their contribution to postoperative cognitive dysfunction (POCD) [1], [2], [3]. Studies indicated that general anesthesia with a combination of nitrous oxide (N2O) and isoflurane (ISO) or sedation with 70% N2O produced lasting impairment in spatial working memory in rats [4], [5], [6], [7], [8]. Exposure of neonatal mice to inhaled sevoflurane not only caused persistent learning deficits in fear conditioning later in adulthood, but also abnormal social behaviors resembling autism spectrum disorder [9]. In addition, such exposure induced apoptosis, increased beta-amyloid protein levels [1] and tau phosphorylation through activation of specific kinases, which is considered a potential mechanism of cognitive dysfunction caused by anesthesia [10]. Although detrimental effects of anesthetics on cognitive function have been reported, to our knowledge, no study has investigated the effects of the anesthetic sevoflurane combined with N2O on spatial learning and memory in aged rats.
A current “hot spot” of memory research involves the cyclic AMP response element-binding protein (CREB), which has been extensively implicated in learning and memory [11], long term potentiation(LTP) [12], and neuroprotection [13]. It is fairly well established that hippocampus-mediated memory consolidation involves signaling cascades leading to gene transcription of the transcription factor CREB [14]. Phosphorylation/activation of CREB (pCREB) on Ser 133 by cyclic AMP- or Ca2+-dependent protein kinase is critical for long-term memory consolidation [15], [16], [17]. Inhibition of phosphodiesterase-4 (PDE4), an enzyme that catalyzes cAMP hydrolysis, increases cAMP and phosphorylation of CREB [18], [19], facilitates induction of hippocampal LTP [20] and enhances memory [21], [22]. Consistent with this, several studies have shown that pCREB is also involved in hippocampal neurogenesis, influences the neurotrophic factor-dependent survival of culture neurons and regulates several steps of neurogenesis including proliferation, differentiation, and survival [23], [24], [25]. To our knowledge, adult neurogenesis in the hippocampus plays a key role in spatial memory function, regulating acquisition of a spatial memory and the subsequent flexible use of spatially precise learning strategies [26], [27], [28]. Besides the neurogenesis, CREB phosphorylation has also been found to be important in the neurotrophin-mediated neuronal survival [29], [30]. Studies showed that ablation of neuronal CREB during development resulted in a massive neuronal apoptosis, and a full CREB-KO mice showed a significant increase in neuronal cell death in dorsal root ganglion neurons [31], [32]. Based on these, inhibition of cAMP/CREB induced by anesthetics would lead to the decrease of neurogenesis but increase of neuronal cell death, and further aggravated cognitive dysfunctions.
The aim of the present study is to determine whether anesthesia with sevoflurane combined with N2O in aged rats could induce spatial learning and memory deficit. We also evaluated the cAMP/CREB signaling, neurogenesis levels and cell survival in the hippocampus in an effort to test the hypothesis that general anesthesia by Sev+N2O down-regulates cAMP/CREB pathway, and then suppresses neuronal survival and hippocampal DG neurogenesis, subsequently aggravating learning and memory deficit.
Materials and Methods
Animals
The experimental protocol was approved by the Shanghai Medical Experimental Animal Care Commission. Male Sprague Dawley rats were obtained from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. Aged rats (18 months old) were housed one or two per cage in a climate- and humidity-controlled room in the animal facilities on a 12-h light–dark artificial cycle (lights on at 7∶00 AM) with free access to food and water. All experiments were performed during the light phase between 7∶00 AM and 7∶00 PM.
Anesthesia Procedure
Animals (n = 40) were randomized into four groups (10 in each group): Sev+N2O-MWM, in which rats received sevoflurane-N2O and behavioral training(Morris Water Maze, MWM); Sev+N2O, in which rats received anesthesia without behavioral training; Con-MWM, in which rats received control gas and behavioral training; and Con, in which rats received control gas without behavioral training. Rats that were randomly assigned to the anesthesia groups received 1.3% sevoflurane (USP, Baxter, Deerfield, IL, USA) in 50% N2O/50% oxygen for 4 h at a flow rate of approximately 3 L/min in a Plexiglas anesthetizing chamber, which was adjusted to maintain minimum alveolar concentration, oxygen, and carbon dioxide at constant levels. Gases within the anesthetic chamber were monitored continuously, and arterial oxygen saturation was measured noninvasively using a pulse oximeter during anesthesia. Control groups received 50% oxygen in their home cage at identical flow rates as anesthetized animals for 4 h, but arterial oxygen saturation was not measured to prevent the introduction of stress as a confounding variable. All anesthetized rats were breathing spontaneously, and the temperature of the anesthetizing chamber was controlled to maintain rat temperature at 37°C ±0.5°C using a heating pad. Anesthesia was terminated by discontinuing the anesthetics. Rats were allowed to recover for 48 h to avoid the confounding influence of residual anesthetic. Forty-eight hours after anesthesia, rats in the groups without behavioral training were sacrificed, and the remaining rats were tested in the Morris water maze from day 1 to day 6.
Morris Water Maze Task
Spatial memory ability was examined in a water maze that consisted of a swimming pool as described by Morris [33] and adapted for rats. It consisted of a circular tank (160-cm diameter, 50 cm high), filled to a depth of 30 cm with water maintained at 22°C and rendered opaque by the addition of white nontoxic paint. The pool was located in a room uniformly illuminated by a halogen lamp and equipped with various distal cues. Located inside the pool was a removable, circular (12-cm diameter) platform (PF) made of transparent Plexiglas, positioned such that its top surface was 1.0 cm below the surface of the water. The platform, which served as a refuge from the water, was generally located in the center of an arbitrarily defined quadrant of the maze. The quadrant where the PF was located was defined as the target quadrant (E quadrant), and the other three were defined as N, S, W quandrant. The swim paths of the animals were captured by a video camera mounted above the water maze, and the corresponding tracks were recorded and analyzed by a computer tracking system (VideoTrack, Viewpoint).
Behavioral Procedures
During the learning procedure, rats were tested during the light phase between 7∶00 AM and 7∶00 PM. Each rat was given a daily four-trial session (30-min intertrial interval) for six consecutive days. Each trial consisted of releasing the rat into the water facing the outer edge of the pool at one of the quadrants (in a random sequence) and letting the animal escape to the submerged platform. Rats were allowed to swim for a maximum of 60 s in each trial, and the time they spent before reaching the platform (i.e., escape latency) was a measure of acquisition of spatial navigation. If the rat failed to find the platform within 60 s, it was manually guided to the platform, and the escape latency was accepted as 60 s. After climbing onto the platform, the rat was left on it for 15 s and then removed from the pool and returned to its cage beneath a heat lamp to reduce the drop in core temperature. The release point differed in each trial, and different sequences of release points were used from day to day.
Twenty-four hours following the acquisition phase, rats were subjected to a probe trial in which the platform was removed. Starting from the quadrant opposite from the target quadrant where the platform had been located, each rat was allowed to swim for 60 s. Time spent and distances covered in the four round probe zones were measured by the tracking system. Data were collected using the VideoTrack system.
Protein Extraction from Hippocampal Tissue
Five rats in Sev+N2O-MWM group and six rats in Con-MWM group were deeply anesthetized at 15 min after the probe trial, the brains were immediately removed, and both hippocampi from each rat were dissected out. Hippocampi were homogenized by brief sonication in an extract buffer (400 µl) containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 µg/ml aprotinin, 1% Triton X-100, and phosphatase inhibitor cocktail 1 and 2 (Sigma, St. Louis, MO). The homogenate was centrifuged at 12,000 g for 10 min at 4°C. The supernatant was removed and stored at −80°C until use.
For the two groups without Morris water maze training, 48 h after Sev+N2O anesthesia or control, hippocampi from five rats per group were extracted and tissues were processed as already described.
Western Blot
Samples (30 µg of protein) were subjected to SDS-PAGE (10% gels) and then transferred from the gel to nitrocellulose membranes using a tank transfer apparatus with buffer containing 25 mM bicine, 25 mM Bis-Tris, and 20% methanol. Membranes were incubated in blocking buffer (TBS/0.1% Tween 20 with 5% nonfat dried milk) for 2 h at room temperature. To control for protein loading, an antibody against β-actin (Santa Cruz Technologies, Santa Cruz, CA) was used. Anti-CREB antibody (1∶1000; Cell Signaling Technologies, Danvers, MA), anti-pCREB antibody (1∶1000; Cell Signaling Technologies, Danvers, MA) and anti-Bax (1∶1000; Santa Cruz Technologies, CA) were added to the buffer solution, and the membranes were incubated overnight at 4°C. The membrane was then washed for 10 min three times in 1× TBS/0.1% Tween 20, and then incubated in HRP-conjugated anti-rabbit IgG (secondary antibody, 1∶3000; Cell Signaling) in blocking buffer for 1 h. Subsequently, membranes were washed with 1× TBS/0.1% Tween 20 several times. Blots were visualized with an ECL detection kit (Pierce, IL) and analyzed using Quantity One 1-D Analysis Software (Bio-Rad, San Francisco, CA).
Cyclic AMP Analysis
Cyclic AMP (cAMP) levels were determined using a cAMP Complete ELISA kit (Enzo Life Sciences, USA) according to the manufacturer’s instructions. The level of cAMP in the sample was determined based on a standard curve and expressed as pmol/mg per each sample.
Immunohistochemical Procedures
The remaining rats in each group were deeply anesthetized and perfused transcardially with ice-cold phosphate-buffered solution (PBS) and 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4). The brains were then dissected out, and the meninges were carefully removed and then post-fixed in the same fixative overnight, cryoprotected by first sinking in 10% and then in 30% sucrose (in 0.1 M phosphate buffer) at 4°C. Coronal sections (20 µm) were cut on a microtome.
Immunofluorescence
After blocking of nonspecific epitopes with PBS containing 10% donkey serum and 0.5% Triton-100 for 1 h at room temperature and then in the primary antibody : Phospho-CREB Rabbit mAb (1∶100, Cell Signaling), NeuN (1∶200, Millipore), five sections (20 µm thickness, 80 µm space) of each rat were incubated for 48 h at 4°C. Sections were then washed in PBS and incubated for 4 h with a 1∶1000 dilution of anti-rabbit or anti-mouse IgG secondary antibody (Invitrogen, Carlsbad, CA, USA). Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) for 30 min at room temperature before three washes with dH2O for 10 min each time and coverslipped. Quantitative analysis was performed using an imaging analysis system (Leica Qwin). Assessment of pCREB staining was performed in CA1 and CA3 area of the dorsal hippocampus, while assessment of NeuN-positive neurons was performed in the CA1 area of the dorsal hippocampus.
Nissl Staining
Sections rinse in tap water and then in distilled water. Stain in 0.1% cresyl violet solution for 3–10 min. Rinse quickly in distilled water. Then differentiate and dehydrate in alcohol then clearing and finally mount with permanent mounting medium. The Nissl body will be stained purple-blue.
After Nissl staining, neuronal cells in the hippocampal were identified from five sections (20 µm thickness, 80 µm apart) per rat (5 rats in each of Sev+N2O group, Con group, and Sev+N2O-MWM group, and 4 rats in Con-MWM group). In each section, the number of neurons was averaged from three random different vision fields in the hippocampal CA1 in each hemisphere (six vision fields per section) under Leica microscope (400× magnification). Only intact neurons with a clearly defined cell body and nucleus were counted.
Double Labeling with NeuN and Annexin V
Apoptosis in hippocampus was detected using an Annexin V- FITC/neuronal marker NeuN doubled staining according to the protocol by Bian Z et al [34]. Brains were sectioned at 12 um thickness and mounted on cover slides followed by air-drying before labeling with Annexin V-FITC. Five Sections (60 µm space) per animal were stained first for Annexin V-FITC using an Annexin V staining Kit (BD Biosciences, CA) according to the manufacturer’s instructions, then blocked with blocking solution as described above, incubated with primary anti-NeuN antibody (1∶100, cell signaling) at RT for 2 h and secondary antibody for 1 h. Nuclear countstaining was performed with Hochest (Invitrogen, CA). Assessment of the doubled NeuN and Annexin V- positive neurons was performed in the CA1 subfield of the hippocampus.
Nestin and DCX Immunohistochemistry
Nestin, a type VI intermediate filament protein, is predominantly expressed in neurogenic and myogenic stem cells, and has been known as a marker of neuroepithelial/progenitor cells. Doublecortin (DXC) is a microtubule-associated protein and has been known as a marker of immature neurons.
In this experiment, five sections (12 µm thickness, 60 µm space) per rat (n = 5 in each of Sev+N2O group, Con group, Sev+N2O-MWM group, and n = 4 in Con-MWM group) were analyzed. The protocol for Nestin and DCX staining was essentially the same as for pCREB staining, expect the primary antibody: anti-Nestin (1∶100, Boster, Wuhan, China) and anti-DCX (1∶100, Epitomics, Burlingame, CA). Analysis of the Nestin and DCX positive neurons was performed in the DG subfield of the hippocampus.
Statistical Analysis
All analyses were performed using SPSS 18.0 for Windows (SPSS, Inc.). Data were submitted to repeated-measures ANOVA (the escape latencies and the swimming speed) and Tukey honestly significant difference test was used for post hoc testing in the Morris water maze. All the other analyses were conducted using Student unpaired two-tailed t test. A value of p<0.05 was considered significant.
Results
Aged Rats Exhibit Impaired Performance on Spatial Learning and Memory After Sevoflurane–N2O Anesthesia
No rat had an episode of hypoxia (defined as SpO2<90%) during the sevoflurane–N2O exposure, and rats did not exhibit floating behavior in our study.
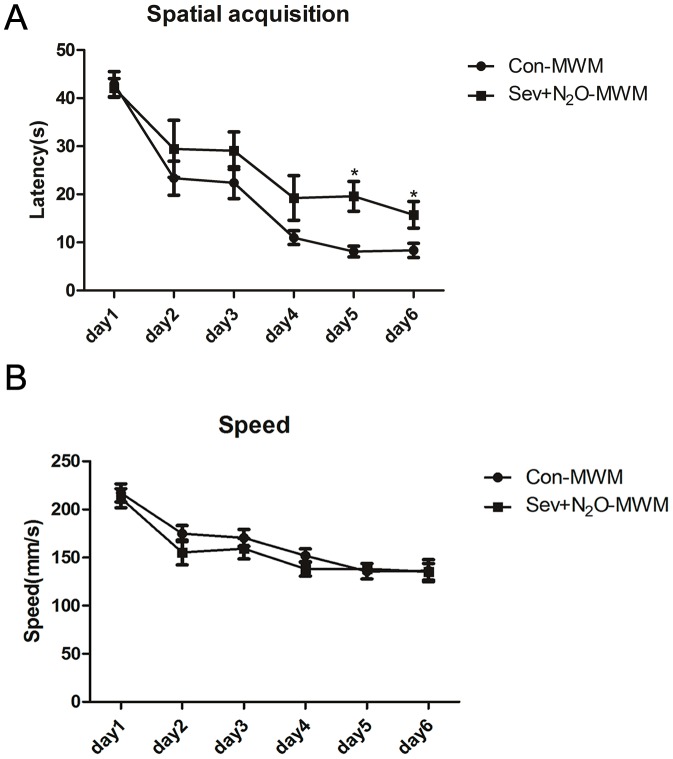
Spatial acquisition for learning in the water maze is presented in Figure 1, which depicts the escape latency to reach the platform. In the first day of spatial acquisition trials, all rats tended to swim around the edges looking for an escape, but after being placed on the platform at the end of each trial, rats gradually learned that there was an escape platform and would venture into the center of the pool to locate it. By the second day’s session, all rats could rapidly locate the platform and the latency was significant less than the first day. A two-way repeated measure ANOVA on the escape latency revealed a significant effect of days for testing the water maze (p<0.001) and a main effect of group (p = 0.016), but no day × group interaction (p = 0.406; Figure 1A) and no effect of speed (p = 0.699; Figure 1B). In the Sev+N2O-MWM group, the impairment was found on day 5 (p = 0.02) and day 6 (p = 0.024), postanesthesia days 7 and 8, respectively, compared to the control rats, which represented the learning deficit after sevoflurane-N2O anesthesia. These results indicated that both the Con-MWM group and Sev+N2O-MWM group exhibited improvement in spatial learning and memory over time during the acquisition phase, but the latter had a longer latency to reach the target quadrant. The lack of effect of speed suggested that the poorer performance of the Sev+N2O-MWM group did not result from lack of motivation or reduced motor ability.
Figure 1. Swimming data for six consecutive training days in the Morris water maze.
(A) The mean escape latency (in seconds) to find the hidden platform across training days. The significant difference were found on day 5 (p = 0.02) and day 6 (p = 0.024). (B) There was no difference in the swimming speed of the two groups during training days (p = 0.699). The data were analyzed by a repeated measure analysis of variance (ANOVA) and results are represented as mean ± SEM. *p<0.05.
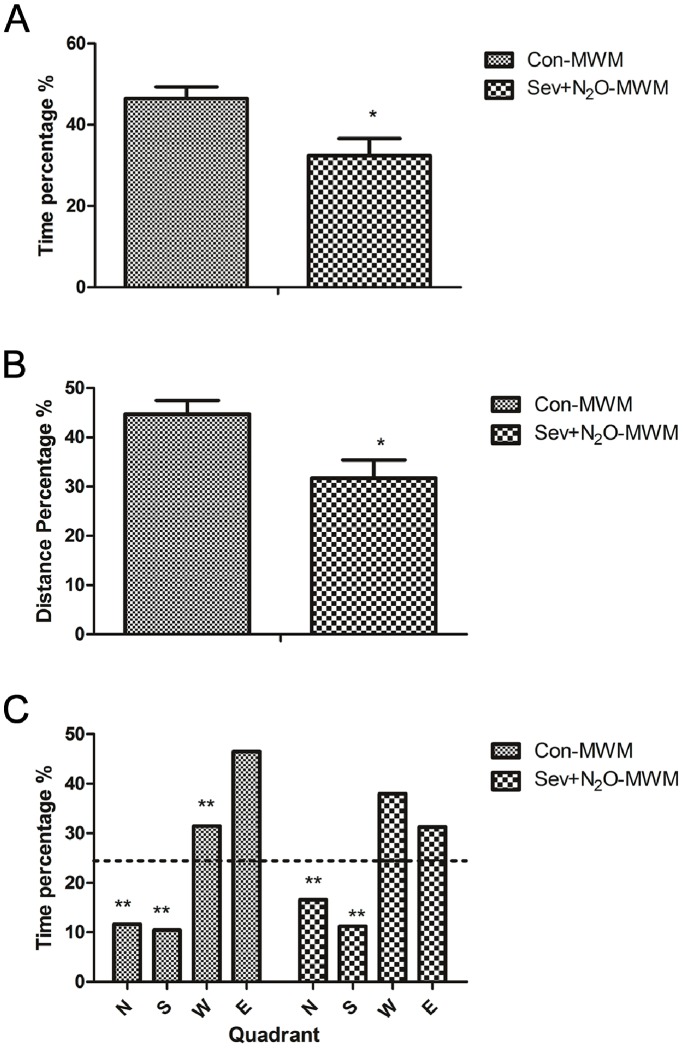
The probe trial conducted 24 h after the last water maze acquisition session was used to assess the spatial memory based on the percentage of time spent in the target quadrant (E quadrant) where platform had been located as well as the distance covered in the E quadrant (Figure 2). Figure 2A shows that Sev+N2O-MWM rats spent significantly less time in the E quadrant compared with Con-MWM rats (p<0.05), and Figure 2B shows that the distances travelled by Con-MWM rats in the E quadrant were more than those by Sev+N2O-MWM rats (p<0.05). Figure 2C shows that Con-MWM rats spent much more time in the E quadrant than the other three ones (all p<0.01), as revealed by a significant effect of quadrant (p<0.01). But unlike the Con-MWM group, the Sev+N2O-MWM rats spent no more time in the E quadrant than in the W quadrant (p = 0.381). Furthermore, t-test comparison confirmed that animals spent significantly more time in the target quadrant than would be expected by chance (p<0.01).
Figure 2. Probe trial conducted after the last water maze acquisition.
(A) The percentage of time spent in the target quadrant. (B) The percentage of distance traveled in the target quadrant. (C) The percentage of time spent in each quadrant. Results are represented as mean ± SEM. *p<0.05, **p<0.01.
The Expression of pCREB was Decreased in the Hippocampus of Aged Rats 48 h after Sevoflurane-N2O Anesthesia
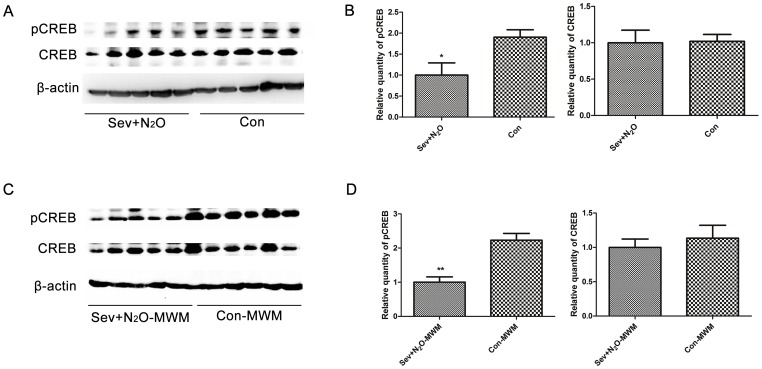
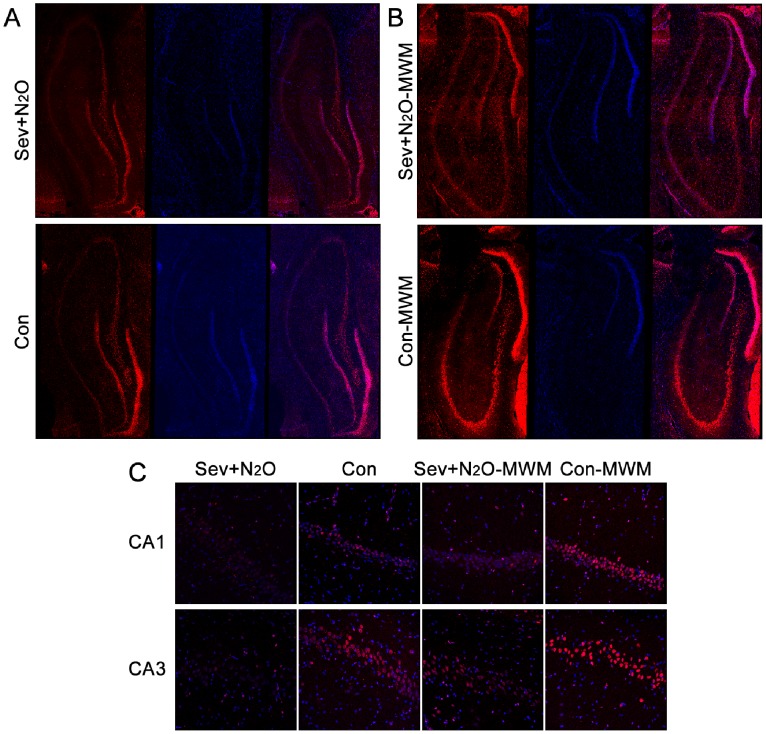
We killed the rats 48 h after anesthesia and measured the pCREB and total CREB levels in the Sev+N2O and Con rats. The Western blot results showed that the immunoreactive bands of pCREB as well as CREB appeared at 43 kDa, and the pCREB levels in the Sev+N2O group were significantly decreased (p = 0.029), but no significant difference in the levels of total CREB was observed (p = 0.923, Figure 3A, 3B). Figure 4A shows the pCREB immunoreactivity (pCREB-ir) distribution in all the subfields of the hippocampus, while Figure 4C shows the pCREB-ir in all the layers of CA1 and CA3 sections. The immunofluorescence revealed that pCREB was exclusively located in neuronal nuclei and there was a significant reduction of pCREB-ir in Sev+N2O rats compared with the Con group. These results implied that sevoflurane-N2O anesthesia would inhibit the activation of CREB 48 h after exposure in aged rats.
Figure 3. Phosphorylated CREB decreased 48-N2O anesthesia as well as 15 min after probe test.
(A) The levels of pCREB in the Sev+N2O group were lower than the Con group, but no differences of CREB levels were detected. (B) Relative levels of pCREB and CREB were quantified (p = 0.029 for pCREB, p = 0.923 for CREB). (C) The pCREB levels in the hippocampus of Sev+N2O-MWM rats were significantly reduced compared to the Con-MWM rats, but CREB expression had no significant difference. (D) Relative levels of pCREB and CREB were quantified (p = 0.01 for pCREB, p = 0.58 for CREB). *p<0.05, **p<0.01.
Figure 4. pCREB immunoreactivity (pCREB-ir) distribution in all the subfields of hippocampus and exclusively located in the neuronal nuclei. The pCREB-ir decreased both 48 h after sevoflurane-N2O anesthesia and 15 min after probe test.
(A) There was a derease of pCREB-ir in all the subfields of the hippocampus in Sev+N2O rats compared with the Con group. (B) The pCREB-ir significantly decreased in the Sev+N2O-MWM group compared with Con-MWM group. (C) pCREB-ir both in CA1 and CA3 areas decreased in the exposed rats compared with controls. Magnification: ×200 for (A), ×400 for (B).
Expression of pCREB in the Hippocampus of Aged Rats after Morris Water Maze
Both the Sev+N2O-MWM group and the control group were sacrificed 15 min after the probe trial on day7, and we compared the levels of pCREB between groups by Western blot. The results showed that the pCREB levels in the Sev+N2O-MWM rats were significant lower than those in the Con-MWM rats, but no significant difference of total CREB was detected (Figure 3C, 3D).
The immunofluorescence results showed that pCREB was higher in the Con-MWM group than in the Con group (Figure 4A, 4C), which was in accordance with our previous study showing that the retrieval of spatial memory could activate the phosphorylation of CREB [35]. However, the levels of pCREB in the Sev+N2O-MWM rats were significantly lower than those in the Con-MWM rats, suggesting that the decrease of pCREB expression induced by general anesthesia with Sev+N2O was sustained until at least the end of the behavioral procedures (Figure 4B, 4C).
cAMP Levels
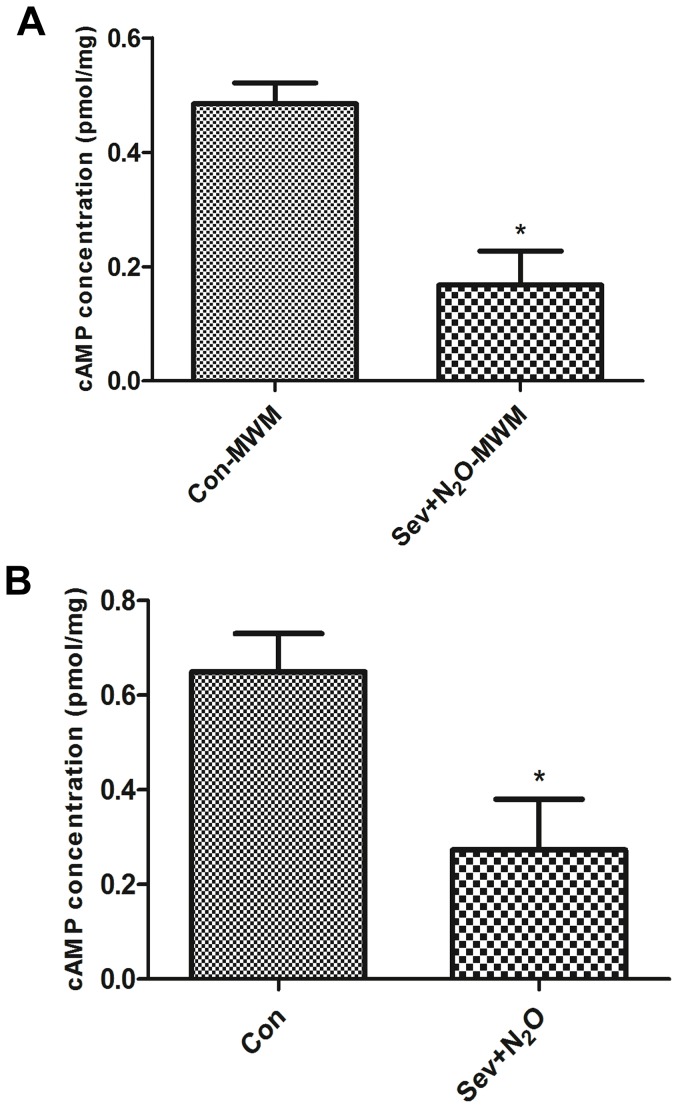
CREB phosphorylation can be achieved by a number of up-stream signaling cascades, among which a pathway is triggered by cAMP accumulation to cause liberation of catalytic subunits of cAMP – dependent protein kinase A (PKA) [36]. Because general anesthesia induced the decrease of pCREB expression, we assessed whether it was related to the cAMP pathway. The ELISA results revealed that the cAMP levels decreased by 65% in hippocampus of Sev+N2O rats compared to the Con rats (p = 0.02, Figure 5A). Similarly, in the Sev+N2O-MWM group, there was a 58% decrease of cAMP levels compared to Con-MWM (p = 0.048, Figure 5B). Thus, the results suggested that general anesthesia with Sev+N2O for 4 hours decreased the cAMP levels and in turn suppressed the cAMP/CREB signaling.
Figure 5. cAMP concentration in the hippocampus of all the groups.
(A) cAMP levels decreased by 65% in hippocampus of Sev+N2O rats compared to the Con rats (p = 0.02). (B) cAMP levels decreased by 58% in Sev+N2O-MWM compared to Con-MWM (p = 0.048).
Neuronal Cell Death Increased after Sev+N2O General Anesthesia
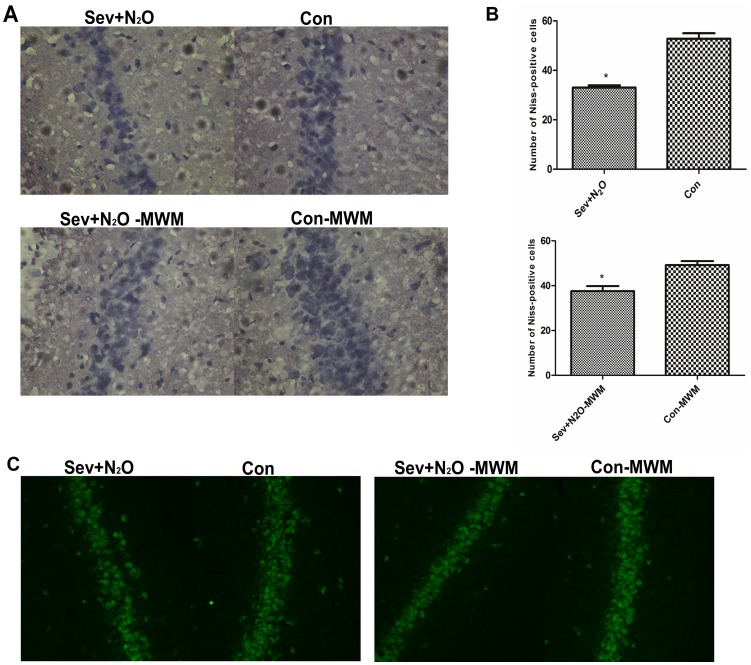
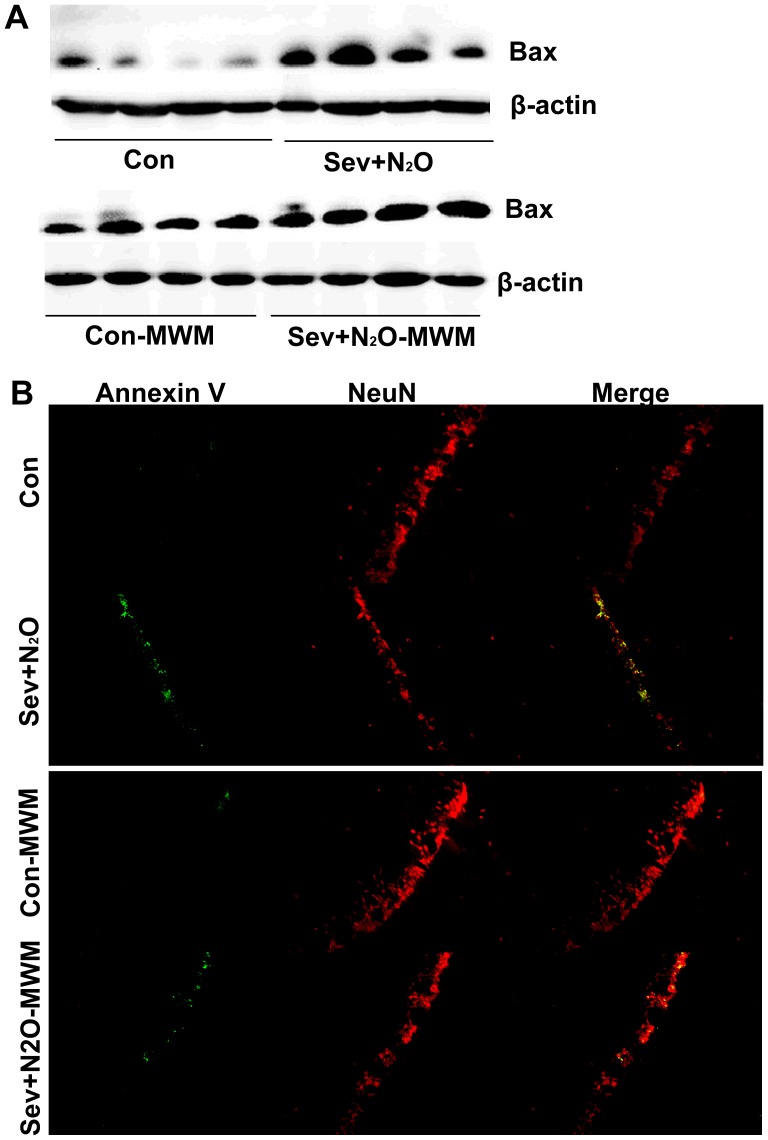
To understand whether cAMP/pCREB down-regulation affect the neuronal survival after Sev+N2O anesthesia, we evaluated the neuronal survival by Nissl staining and NeuN staining. Nissl staining is used for the detection of Nissl Body in the cytoplasm of neurons and identifying the basic neuronal structure. Quantitative analysis of Nissl-positive cells in hippocampal CA1 showed a neuronal loss in exposed rats compared with their controls (p<0.05, Figure 6A, B). Otherwise, expression of NeuN, a neuronal marker, also decreased in the Sev+N2O group as well as Sev+N2O-MWM group (Figure 6C). To confirm the neuronal loss is due to the neuronal apoptosis, double labeled of Annexin V-FITC and NeuN was performed. Figure 7B shows that the total number of Annexin V-positive cells in the hippocampal CA1 significantly increased in the rats exposed to anesthetics compared with the controls. However, most of the Annexin V-positive cells also stained positive for the neuronal marker NeuN. As loss of CREB triggered Bax-dependent apoptosis [31], it is possible that inhibition of CREB signaling by general anesthesia induces the Bax overexpression and contributes to the neuronal apoptosis. To verify it, we tested the Bax protein levels by Western Blot and found that the Bax expression significantly increased in the Sev+N2O group compared with Con group (Figure 7A). However, the Bax expression was only slightly higher in Sev+N2O-MWM rats than in Con-MWM rats (Figure 7A).
Figure 6. More neuronal loss in the CA1 subfield of hippocampus in exposed rats than control rats by Nissl staining and NeuN staing.
(A) Nissl-positve cells in CA1 subfield decreased in the Sev+N2O group and Sev+N2O-MWM group compared with their respective controls. (B) The number of Nissl-postive cells was quantified. The number of neurons in each section was averaged from three random different vision fields in the CA1 area of hippocampus per hemisphere (six vision fields per section) under 40×objective Leica microscope. 5 sections per rat were analyzed (n = 5 in each of Sev+N2O, Con and Sev+N2O-MWM group, and n = 4 in Con-MWM group. p = 0.0286, 0.016, respectively). (C) Expression of the mature neuronal marker NeuN in CA1 subfield decreased in exposed rats compared with controls. Magnification: ×400 for (A), ×200 for (C).
Figure 7. Expression of Bax was detected by Western Blot and neuronal apoptosis was evaluated by doubled staining with Annexin V-FITC and NeuN.
(A) Bax expression was significantly increased in the Sev+N2O group compared with Con group, but only slightly increased in the Sev+N2O-MWM compared with Con-MWM group. (B) Doubled staining with Annexin V-FITC and NeuN showed that there were more neurons underwent apoptosis in the CA1 subfield of hippocampus in Sev+N2O and Sev+N2O-MWM rats. Magnification: ×200 for (B).
Neurogenesis in the Hippocampal DG
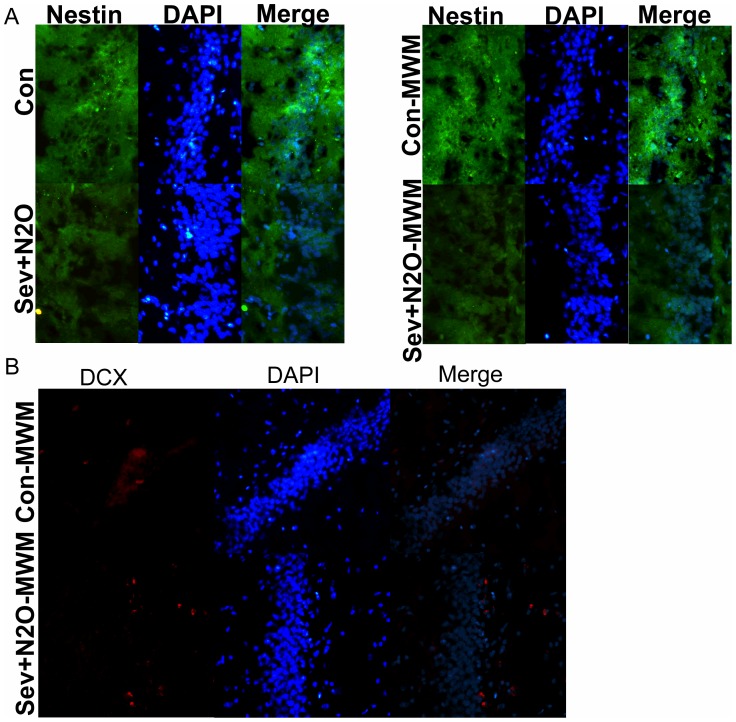
As training on hippocampus-dependent tasks, such as the spatial Morris water maze, increases hippocampal neurogenesis [37]. Decreasing hippocampal neurogenesis has been demonstrated to impair long-term spatial memory [38]. We examined the possibility that the spatial learning and memory impairments in aged rats after general anesthesia with Sev+N2O might be related to a deficiency in DG neurogenesis due to the downregulation of cAMP/pCREB. We analyzed the differences in expression of various neuronspecific markers related to different neuronal stages, Nestin used as a marker of neuroepithelial/progenitor cells and DCX as immature neuronal marker. The immunofluorescence staining results showed that less neurons expressed Nestin in the Sev+N2O group as well as Sev+N2O-MWM group (Figure 8A). Moreover, we found less DCX-positive cells in the Sev+N2O-MWM group compared with Con-MWM group (Figure 8B). These results indicated that neuronal progenitor proliferation and differentiation was inhibited.
Figure 8. Expression of Nestin and DCX in DG area of hippocampus was detected by Immunofluorescence.
(A) Nestin-postive cells were less in the exposed rats than controls. (B) DCX-positive cells decreased in the Sev+N2O-MWM group compared with Con-MWM group. Magnification: ×200 for (A) and (B).
Discussion
With the coming of the aging society, more and more surgical procedures currently performed are in elderly patients. Patients with an age≥60 years have an increased incidence of POCD, which is associated with an increased mortality [39], [40], [41]. The aged brain might be more susceptible to anesthetic-mediated changes, as the aged brain is different from the younger in several respects, including size, distribution and type of neurotransmitters, metabolic function, and capacity for plasticity [42]. As sevoflurane and N2O are commonly used anesthetic agents in clinical practice, the current study focused on the behavioral and biochemical effects to aged rats by general anesthesia with sevoflurane and N2O. We first found that 18-month-old rats developed cognitive deficits after exposure to 1.3% sevoflurane combined with 50% N2O for 4 h without surgery. It was unlikely that this impairment was caused by residual anesthetic because we did not perform the MWM training until 48 h after anesthesia. Culley et al [8] showed that aged rats subjected to ISO+N2O anesthesia exhibited poor performance on a spatial memory task for at least 2 weeks after general anesthesia, and the induced impairment may have been worse than ISO alone. Moreover, a 4-h exposure of aged rats to N2O alone has been reported to cause learning impairment up to 2 weeks [4]. Recent studies also showed that exposure to sevoflurane increased β-amyloid protein levels, which could induce further apoptosis and contribute to or cause POCD [1], [3], [43], [44], [45]. Sevoflurane at doses from 0.5% to 2.6% administered either during or immediately after a learning task has been shown to inhibit memory retention [46], [47], [48], [49], [50]. Although the inhalation anesthesia–POCD model has been well documented, it is debated. Rammes et al [51] reported ISO enhanced long-term potentiation (LTP) in CA1 hippocampal neurons and improved hippocampus-dependent cognitive performance, while a recent study demonstrated that 4 h of sevoflurane exposure did not impair acquisition learning or retention memory and might even improve learning in young adult or aged rat [52]. Different methodologies, including different anesthetic, dosage, rat strain, duration of exposure, outcome measurements, and anesthetic carrier gas may have contributed to these contradictory results. In the current study, we used 1.3% sevoflurane and 50% N2O as the anesthetics and 50% O2 for the carrier gas, which could be one of the factors that contributed to the differences.
The precise mechanism of molecular biology of POCD is still not very clear so far. Our current study tried to demonstrate the effects of anesthetics (i.e., sevoflurane, N2O) on biochemical changes associated with cognitive dysfunction. Current hypotheses attribute the neurocognitive deficits produced by anesthetics to neurotoxic effects, endogenous neurodegenerative, neuroinflammatory mechanisms, or age-sensitive suppression of neuronal stem cell proliferation and differentiation [53], [54], [55]. Moreover, brain-derived neurotrophic factor has been implicated in the neurotoxicity of N2O, midazolam, and ISO [56]. As mentioned previously, pCREB promotes the transcription of immediate-early gene mRNA, which is then translated into proteins. These proteins are necessary for the maintenance of LTP and long-term memory, while spatial learning has been shown to increase CREB phosphorylation in the dorsal hippocampus [12], [57], [58], [59], [60], [61]. In the current study, we measured the pCREB and CREB levels in the hippocampus 48 h after exposure but before water maze test, as well as 15 min after the probe trial. The results found that pCREB levels in the Sev+N2O and Sev+N2O-MWM group were significantly lower than their respective controls. Our results suggested that sevoflurane-N2O anesthesia decreased pCREB levels and lasted until at least the probe trial finished, which would affect the capacity of pCREB’s maintenance of LTP and long-term memory. However, we found the decrease of pCREB could be due to the phosphorylation of preexisting CREB, because there was no difference in total CREB levels among groups.
To identity the upstream regulators of CREB-signaling, studies have demonstrated that NMDA-receptor activation, BDNF (brain derived neurotrophic factor) signaling or growth factor signaling can trigger intracellular signaling cascades that phosphorylate CREB at Ser133, which is a rate-limiting step in the CREB-signaling [62], [63]. Among various signaling pathways, the most thoroughly pathway is to stimulate adenylyl cyclase and accumulate second messenger cAMP to activate PKA and lead to the release of the catalytical subunit of PKA which then shuttles to the nucleus and phosphorylates CREB [64]. In the current study, we found the cAMP levels decreased in the hippocampus of Sev+N2O group and Sev+N2O-MWM group compared to their respective control group. The above evidence indicated that general anesthesia with Sev+N2O down-regulated the cAMP/CREB signaling and further affected its downstream targets.
New neurons are produced each day through the process of neurogenesis in the hippocampus, while physical training can modify the process by increasing the number of new cells that mature into functional neurons in the adult brain and improve the cognitive ability including learning and memory [65]. In the past years, researchers have found that rats exposure to isoflurane on P7 showed decreased neuronal progenitor proliferation with deficits in fear conditioning and spatial reference task, and P14 rats exposed to isoflurane showed a decreased neurogenesis in hippocampus and impaired cognitive function compared with controls [66]. It has previously been noted that CREB signaling is essential for survival and morphological development of newborn neurons, and for maintenance of expression of proteins involved in neuronal development and the control of neurogenic transcriptional programs, while activation of cAMP signaling promotes the proliferation and morphological maturation of newborn cells [23], [24]. The in vivo function of CREB-signaling in adult neurogenesis has been examined using pharmacological, genetic and retrovirus-mediated gene transfer [67]. Thus, examination of DG neurogenesis may provide some information about how anesthetics cause learning and memory deficits via downregulation of CREB signaling. We found that the down-expression of pCREB in the hippocampus of rats exposed to Sev+N2O anesthesia disrupted the DG neurogenesis and exacerbated the cognitive dysfunction. This was underscored, at least in part, by our observation that the number of Nestin-positve cells and Dcx-positive cells was less in the exposed rats compared to the controls, which indicated the decrease of neuronal progenitor proliferation, differentiation and maturation.
Researchers have shown that CREB DNA binding activity and phosphorylation of CREB are necessary for nerve growth factor (NGF)-dependent survival of sympathetic neurons. Bonni et al [29] indicated that CREB may also have a function in the regulation of neuronal survival in the developing central nervous system. Furthermore, experiments using genetic transfer have demonstrated that CREB is a key executor of neurotrophin-mediated cell survival and loss of CREB triggers Bax-dependent apoptosis in vivo [31]. These findings demonstrated a critical role for CREB and CREB-dependent gene expression in supporting neuronal survival. In our work, we found an increase of neuronal loss in the exposed rats compared with controls. Annexin V-FITC by immunofluorescence and Bax expression by Westren blot revealed the neuronal loss was due to the trigger of neuronal apoptosis. Importantly, double staining of Annexin V and the neuronal marker NeuN indicated that most of the apoptotic cells in these structures were indeed neurons. In conjunction with the role of CREB in DG neurogenesis, these findings partly support our prediction that downregulation of cAMP/CREB induced by general anesthesia leads to a decrease of the neurogenesis in hippocampus as well as an increase of neuronal apoptosis, both of which play critical roles in the deficit of learning and memory after Sev+N2O exposure.
In summary, we found that general anesthesia with 1.3% sevoflurane and 50% N2O impaired hippocampus-dependent learning and memory in aged rats. To explore the mechanism of neurotoxicity induced by Sev+N2O, we found the downregulation of cAMP/CREB signaling which was implicated in the learning and memory, long term potentiation, and neuroprection. Furthermore, we observed the decrease of DG neurogenesis and neuronal survival in the hippocampus, both of which highly depended on the normal activation of CREB signaling. Thus, the pathway of cAMP/CREB-neurogensis/neuronal apoptosis contributed to the neurotoxicity and impairment of learning and memory induced by Sev+N2O. The study provided some theoretical basis for the further study of neurotrophin factors, which could prevent POCD in elderly patients and decrease the postoperative morbidity and mortality.
Funding Statement
This project was supported by Shanghai Natural Science Fund (10ZR1406500). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1. Dong Y, Zhang G, Zhang B, Moir RD, Xia W, et al. (2009) The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol 66: 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, et al. (2008) The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol 64: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, et al. (2006) The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 104: 988–994. [DOI] [PubMed] [Google Scholar]
- 4. Culley DJ, Raghavan SV, Waly M, Baxter MG, Yukhananov R, et al. (2007) Nitrous oxide decreases cortical methionine synthase transiently but produces lasting memory impairment in aged rats. Anesth Analg 105: 83–88. [DOI] [PubMed] [Google Scholar]
- 5. Mellon RD, Simone AF, Rappaport BA (2007) Use of anesthetic agents in neonates and young children. Anesth Analg 104: 509–520. [DOI] [PubMed] [Google Scholar]
- 6. Culley DJ, Baxter MG, Yukhananov R, Crosby G (2004) Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology 100: 309–314. [DOI] [PubMed] [Google Scholar]
- 7. Culley DJ, Baxter M, Yukhananov R, Crosby G (2003) The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg 96: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 8. Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G (2004) Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg 99: 1393–1397. [DOI] [PubMed] [Google Scholar]
- 9. Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, et al. (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110: 628–637. [DOI] [PubMed] [Google Scholar]
- 10. Le Freche H, Brouillette J, Fernandez-Gomez FJ, Patin P, Caillierez R, et al. (2012) Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology 116: 779–787. [DOI] [PubMed] [Google Scholar]
- 11. Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, et al. (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79: 59–68. [DOI] [PubMed] [Google Scholar]
- 12. Impey S, Mark M, Villacres EC, Poser S, Chavkin C, et al. (1996) Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron 16: 973–982. [DOI] [PubMed] [Google Scholar]
- 13. Ao H, Ko SW, Zhuo M (2006) CREB activity maintains the survival of cingulate cortical pyramidal neurons in the adult mouse brain. Mol Pain 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlezon WJ, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436–445. [DOI] [PubMed] [Google Scholar]
- 15. Rosenegger D, Parvez K, Lukowiak K (2008) Enhancing memory formation by altering protein phosphorylation balance. Neurobiol Learn Mem 90: 544–552. [DOI] [PubMed] [Google Scholar]
- 16. Brightwell JJ, Smith CA, Neve RL, Colombo PJ (2007) Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus. Learn Mem 14: 195–199. [DOI] [PubMed] [Google Scholar]
- 17. Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, et al. (2006) Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem 13: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schneider HH (1984) Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochem Pharmacol 33: 1690–1693. [DOI] [PubMed] [Google Scholar]
- 19. Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, et al. (2009) Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34: 2404–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E (1998) Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A 95: 15020–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Romano A, Delorenzi A, Pedreira ME, Tomsic D, Maldonado H (1996) Acute administration of a permeant analog of cAMP and a phosphodiesterase inhibitor improve long-term habituation in the crab Chasmagnathus. Behav Brain Res 75: 119–125. [DOI] [PubMed] [Google Scholar]
- 22. Otmakhov N, Khibnik L, Otmakhova N, Carpenter S, Riahi S, et al. (2004) Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol 91: 1955–1962. [DOI] [PubMed] [Google Scholar]
- 23. Fujioka T, Fujioka A, Duman RS (2004) Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci 24: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, et al. (2002) Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci 22: 9868–9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, et al. (2002) Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci 22: 3673–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, et al. (2011) Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci 31: 13469–13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, et al. (2007) Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol 5: e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garthe A, Behr J, Kempermann G (2009) Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4: e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, et al. (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286: 1358–1362. [DOI] [PubMed] [Google Scholar]
- 30. Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD (1999) Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286: 2358–2361. [DOI] [PubMed] [Google Scholar]
- 31. Lonze BE, Riccio A, Cohen S, Ginty DD (2002) Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 34: 371–385. [DOI] [PubMed] [Google Scholar]
- 32. Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, et al. (2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31: 47–54. [DOI] [PubMed] [Google Scholar]
- 33. Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60. [DOI] [PubMed] [Google Scholar]
- 34. Bian Z, Li L, Cui J, Zhang H, Liu Y, et al. (2011) Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol 225: 544–553. [DOI] [PubMed] [Google Scholar]
- 35. Zhou G, Xiong W, Zhang X, Ge S (2013) Retrieval of Consolidated Spatial Memory in the Water Maze Is Correlated with Expression of pCREB and Egr1 in the Hippocampus of Aged Mice. Dement Geriatr Cogn Dis Extra 3: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamashima T (2012) ‘PUFA-GPR40-CREB signaling’ hypothesis for the adult primate neurogenesis. Prog Lipid Res 51: 221–231. [DOI] [PubMed] [Google Scholar]
- 37. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260–265. [DOI] [PubMed] [Google Scholar]
- 38. Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM (2005) A role for adult neurogenesis in spatial long-term memory. Neuroscience 130: 843–852. [DOI] [PubMed] [Google Scholar]
- 39. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, et al. (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351: 857–861. [DOI] [PubMed] [Google Scholar]
- 40. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, et al. (2008) Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108: 18–30. [DOI] [PubMed] [Google Scholar]
- 41. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS (2009) Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110: 548–555. [DOI] [PubMed] [Google Scholar]
- 42. Mrak RE, Griffin ST, Graham DI (1997) Aging-associated changes in human brain. J Neuropathol Exp Neurol 56: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 43. Xie Z, Dong Y, Maeda U, Moir RD, Xia W, et al. (2007) The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci 27: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang B, Dong Y, Zhang G, Moir RD, Xia W, et al. (2008) The inhalation anesthetic desflurane induces caspase activation and increases amyloid beta-protein levels under hypoxic conditions. J Biol Chem 283: 11866–11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, et al. (2004) Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 101: 703–709. [DOI] [PubMed] [Google Scholar]
- 46. Wiklund A, Granon S, Faure P, Sundman E, Changeux JP, et al. (2009) Object memory in young and aged mice after sevoflurane anaesthesia. Neuroreport 20: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 47. Liu XS, Xue QS, Zeng QW, Li Q, Liu J, et al. (2010) Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus. Neurobiol Learn Mem 94: 461–467. [DOI] [PubMed] [Google Scholar]
- 48. Alkire MT, Gorski LA (2004) Relative amnesic potency of five inhalational anesthetics follows the Meyer-Overton rule. Anesthesiology 101: 417–429. [DOI] [PubMed] [Google Scholar]
- 49. Alkire MT, Nathan SV, McReynolds JR (2005) Memory enhancing effect of low-dose sevoflurane does not occur in basolateral amygdala-lesioned rats. Anesthesiology 103: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 50. Alkire MT, Nathan SV (2005) Does the amygdala mediate anesthetic-induced amnesia? Basolateral amygdala lesions block sevoflurane-induced amnesia. Anesthesiology 102: 754–760. [DOI] [PubMed] [Google Scholar]
- 51. Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, et al. (2009) Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology 56: 626–636. [DOI] [PubMed] [Google Scholar]
- 52. Callaway JK, Jones NC, Royse AG, Royse CF (2012) Sevoflurane anesthesia does not impair acquisition learning or memory in the morris water maze in young adult and aged rats. Anesthesiology 117: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 53. Wei H, Liang G, Yang H, Wang Q, Hawkins B, et al. (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108: 251–260. [DOI] [PubMed] [Google Scholar]
- 54. Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, et al. (2007) Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 106: 436–443. [DOI] [PubMed] [Google Scholar]
- 55. Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, et al. (2009) Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology 110: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11: 1603–1615. [DOI] [PubMed] [Google Scholar]
- 57. Barco A, Alarcon JM, Kandel ER (2002) Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108: 689–703. [DOI] [PubMed] [Google Scholar]
- 58. Bito H, Deisseroth K, Tsien RW (1996) CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203–1214. [DOI] [PubMed] [Google Scholar]
- 59. Josselyn SA, Nguyen PV (2005) CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord 4: 481–497. [DOI] [PubMed] [Google Scholar]
- 60. Segal M, Murphy DD (1998) CREB activation mediates plasticity in cultured hippocampal neurons. Neural Plast 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jones MW, Errington ML, French PJ, Fine A, Bliss TV, et al. (2001) A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci 4: 289–296. [DOI] [PubMed] [Google Scholar]
- 62. Yamashima T (2012) ‘PUFA-GPR40-CREB signaling’ hypothesis for the adult primate neurogenesis. Prog Lipid Res 51: 221–231. [DOI] [PubMed] [Google Scholar]
- 63. Merz K, Herold S, Lie DC (2011) CREB in adult neurogenesis–master and partner in the development of adult-born neurons? Eur J Neurosci 33: 1078–1086. [DOI] [PubMed] [Google Scholar]
- 64. Carlezon WJ, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436–445. [DOI] [PubMed] [Google Scholar]
- 65. Curlik DN, Shors TJ (2013) Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology 64: 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, et al. (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110: 834–848. [DOI] [PubMed] [Google Scholar]
- 67. Dworkin S, Malaterre J, Hollande F, Darcy PK, Ramsay RG, et al. (2009) cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 27: 1347–1357. [DOI] [PubMed] [Google Scholar]