Abstract
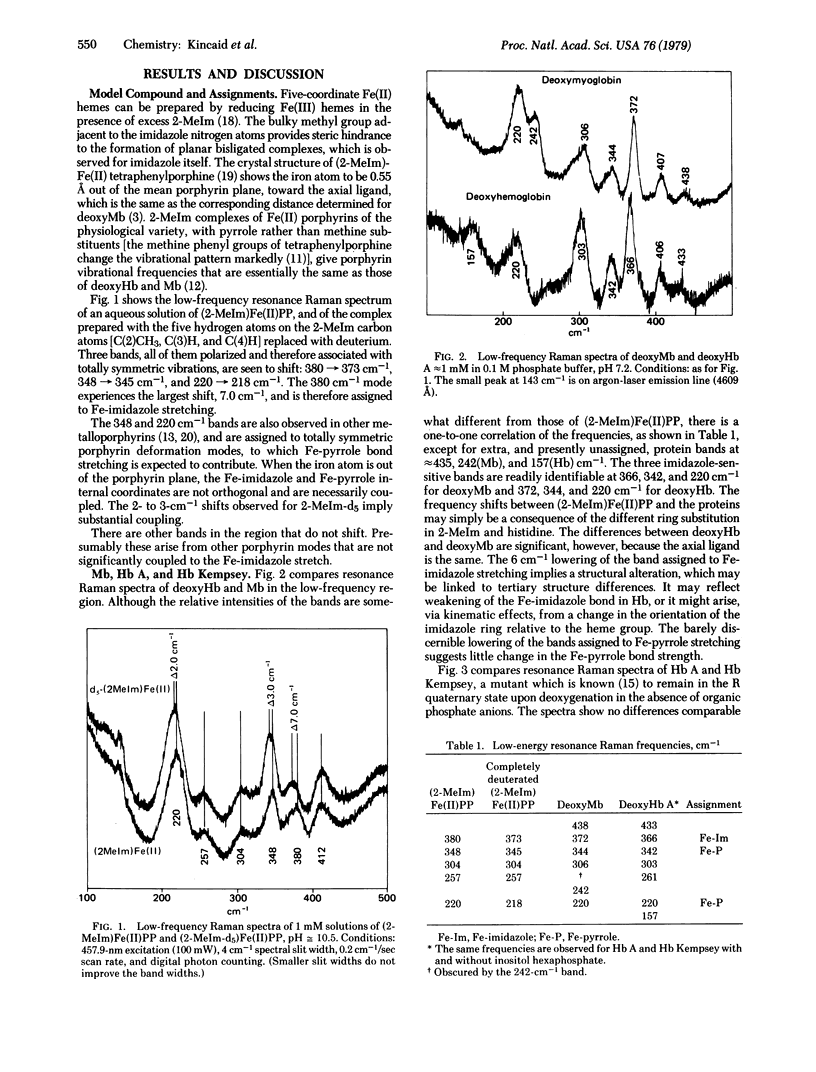
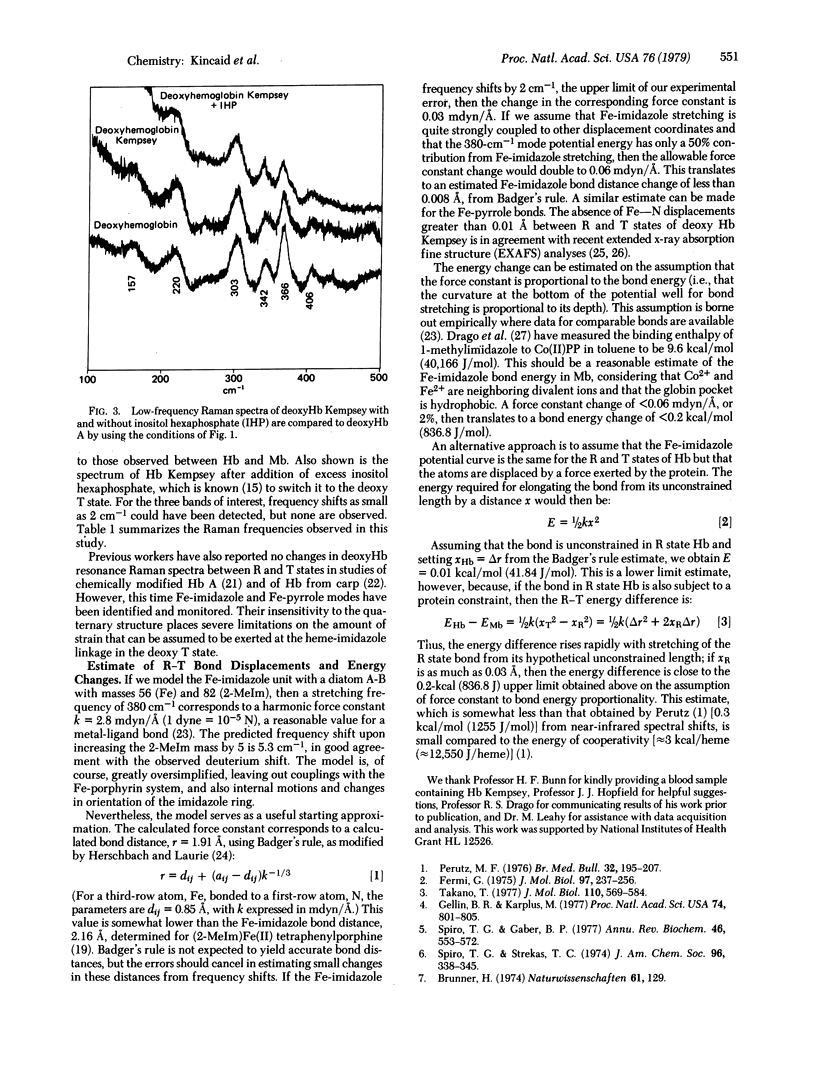
Substitution of pentadeuterated 2-methylimidazole in (2-methylimidazole)-Fe(II)-protoporphyrin IX, a model complex for deoxyHb, shifts three bands in the low-frequency resonance Raman spectrum 380 leads to 373 cm-1, 348 leads to 345 cm-1, and 220 leads to 218 cm-1. The first of these is assigned primarily to Fe-imidazole stretching, and the other two are assigned to porphyrin deformation modes with substantial Fe-pyrrole stretching contributions. The three bands are observed in deoxyHb and Mb. The Fe-pyrrole modes are at essentially the same frequencies in the two proteins, but the Fe-imidazole mode is 6 cm-1 lower in deoxyHb than Mb, implying a slight alteration in the heme-imidazole linkage. No change greater than 2 cm-1 is observed when Hb Kempsey is switched from the R to the T state. This observation places an upper limit on the energy stored in the Fe-imidazole bond of T state deoxyHb, which is estimated to be less than 0.2 kcal/mol (less than 836.8 J/mol).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher S. A., Vickery L. E., Schuster T. M., Sauer K. Resonance Raman spectra of methemoglobin derivatives. Selective enhancement of axial ligand vibrations and lack of an effect of inositol hexaphosphate. Biochemistry. 1977 Dec 27;16(26):5849–5856. doi: 10.1021/bi00645a032. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Wohl R. C., Bradley T. B., Cooley M., Gibson Q. H. Functional properties of hemoglobin Kempsey. J Biol Chem. 1974 Dec 10;249(23):7402–7409. [PubMed] [Google Scholar]
- Collman J. P., Reed C. A. Syntheses of ferrous-porphyrin complexes. A hypothetical model for deoxymyoglobin. J Am Chem Soc. 1973 Mar 21;95(6):2048–2049. doi: 10.1021/ja00787a075. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Shulman R. G., Brown G. S., Ogawa S. Structure-function relations in hemoglobin as determined by x-ray absorption spectroscopy. Proc Natl Acad Sci U S A. 1976 Feb;73(2):491–495. doi: 10.1073/pnas.73.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger P., Shulman R. G., Kincaid B. M., Brown G. S., Ogawa S. Extended X-ray absorption fine structure determination of iron nitrogen distances in haemoglobin. Nature. 1978 Jul 6;274(5666):30–34. doi: 10.1038/274030a0. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoard J. L., Scheidt W. R. Stereochemical trigger for initiating cooperative interaction of the subunits during the oxygenation of cobaltohemoglobin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3919–3922. doi: 10.1073/pnas.70.12.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Structure and mechanism of haemoglobin. Br Med Bull. 1976 Sep;32(3):195–208. doi: 10.1093/oxfordjournals.bmb.a071363. [DOI] [PubMed] [Google Scholar]
- Scholler D. M., Hoffman B. M., Shriver D. F. Resonance Raman spectra of liganded and unliganded carp hemoglobin in both R and T states. J Am Chem Soc. 1976 Nov 24;98(24):7866–7868. doi: 10.1021/ja00440a091. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Burke J. M. Protein control of porphyrin conformation. Comparison of resonance Raman spectra of heme proteins with mesoporphyrin IX analogues. J Am Chem Soc. 1976 Sep 1;98(18):5482–5489. doi: 10.1021/ja00434a013. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Gaber B. P. Laser Raman scattering as a probe of protein structure. Annu Rev Biochem. 1977;46:553–572. doi: 10.1146/annurev.bi.46.070177.003005. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Sussner H., Mayer A., Brunner H., Fasold H. Raman study on the two quaternary states of unligated hemoglobin. Eur J Biochem. 1974 Feb 1;41(3):465–469. doi: 10.1111/j.1432-1033.1974.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]



