Abstract
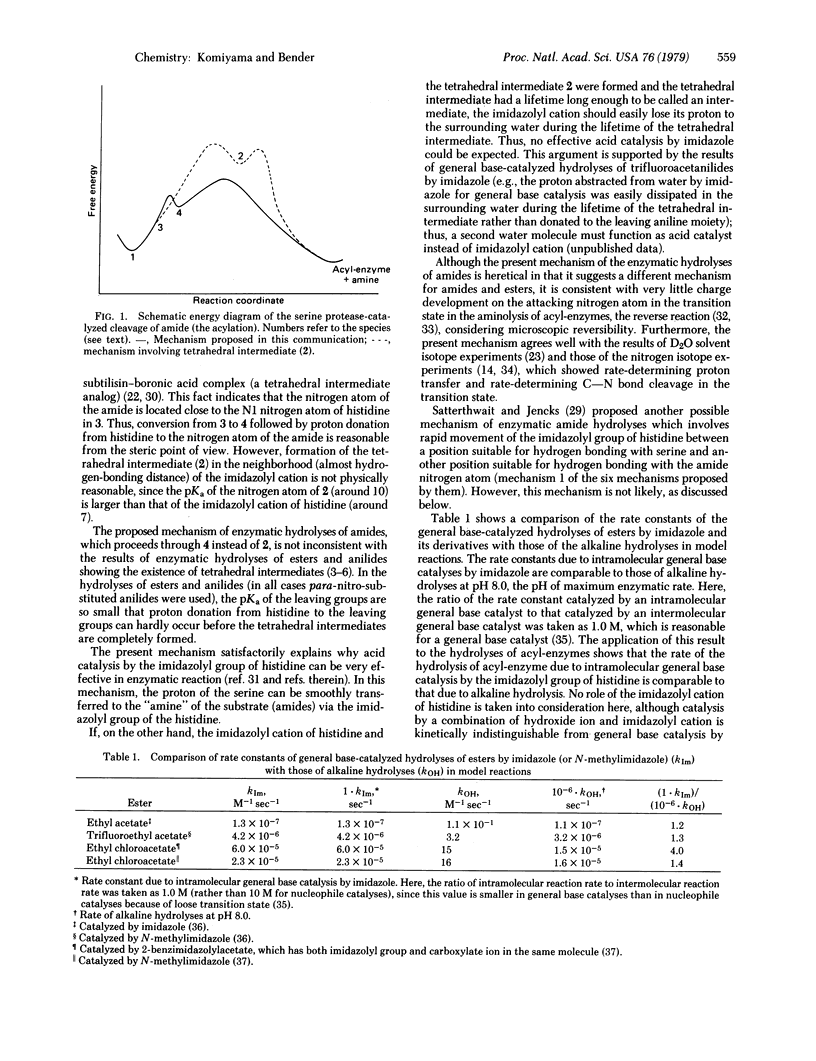
The mechanism of the serine protease-catalyzed cleavage of amides (acylation) was examined in terms of the basicity of the functional groups participating in the catalysis. It is proposed that the reaction does not proceed through a stepwise pathway, as opposed to the cleavage of esters and anilides, which start with general base-catalyzed formation of the tetrahedral intermediate followed by its general acid-catalyzed breakdown. Instead, the proton abstracted from the hydroxyl group of the serine by the imidazolyl group of the histidine is donated to the nitrogen atom of the leaving group of the amide before the bond between the carbonyl carbon atom of the amide and the attacking serine oxygen atom is completed. Reactions proceed by a SN2-like reaction through the cooperation of acid catalysis by the imidazolyl cation and nucleophilic attack by the serine. The mechanisms of the enzymatic hydrolyses of anilides and esters proceed through a discrete tetrahedral intermediate, but the enzymatic hydrolyses of amides probably do not.
Full text
PDF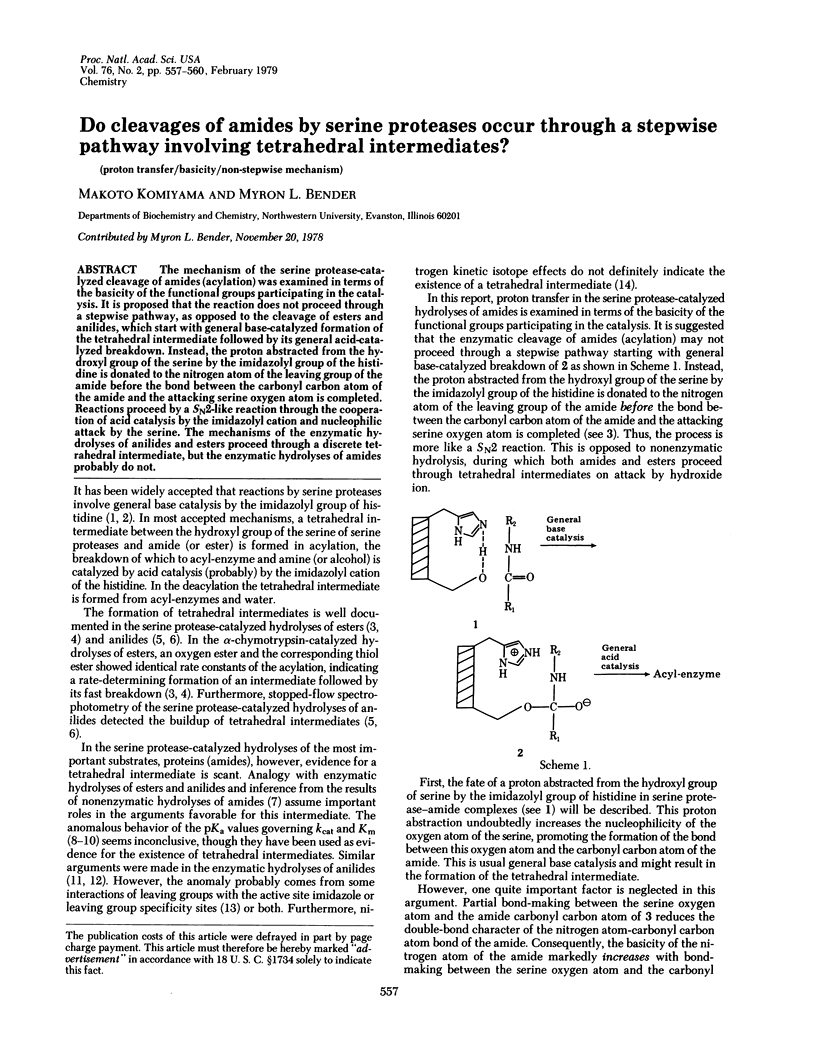


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. L., Killheffer J. V. Chymotrypsins. CRC Crit Rev Biochem. 1973 Apr;1(2):149–199. doi: 10.3109/10409237309102546. [DOI] [PubMed] [Google Scholar]
- Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1969 Jun 18;91(13):3639–3645. doi: 10.1021/ja01041a037. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Acyl-transfer reactions of amides and esters with alcohols and thiols. A reference system for the serine and cysteine proteinases. Concerning the N protonation of amides and amide-imidate equilibria. J Am Chem Soc. 1971 Jul 14;93(14):3504–3515. doi: 10.1021/ja00743a035. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Mechanism of the -chymotrypsin-catalyzed hydrolysis of specific amide substrates. J Am Chem Soc. 1972 Jan 12;94(1):293–295. doi: 10.1021/ja00756a061. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Requena Y. Mechanism of the -chymotrypsin-catalyzed hydrolysis of amides. pH dependence of k c and K m . Kinetic detection of an intermediate. J Am Chem Soc. 1971 Dec 15;93(25):7079–7087. doi: 10.1021/ja00754a066. [DOI] [PubMed] [Google Scholar]
- Frankfater A., Kézdy F. J. Kinetics of hydrolysis of p-nitrophenyl thiolacetate by chymotrypsin. J Am Chem Soc. 1971 Aug 11;93(16):4039–4043. doi: 10.1021/ja00745a036. [DOI] [PubMed] [Google Scholar]
- Hess G. P., McConn J., Ku E., McConkey G. Studies of the activity of chymotrypsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):89–104. doi: 10.1098/rstb.1970.0011. [DOI] [PubMed] [Google Scholar]
- Hiroara H., Bender M. L., Stark R. S. Acylation of alpha-chymotrypsin by oxygen and sulfur esters of specific substrates: kinetic evidence for a tetrahedral intermediate. Proc Natl Acad Sci U S A. 1974 May;71(5):1643–1647. doi: 10.1073/pnas.71.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Forgac M. D., Richards J. H. Mechanism of action of serine proteases: tetrahedral intermediate and concerted proton transfer. Biochemistry. 1976 Dec 14;15(25):5581–5588. doi: 10.1021/bi00670a024. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Smallcombe S. H., Whitaker D. R., Richards J. H. Carbon nuclear magnetic resonance studies of the histidine residue in alpha-lytic protease. Implications for the catalytic mechanism of serine proteases. Biochemistry. 1973 Nov 6;12(23):4732–4743. doi: 10.1021/bi00747a028. [DOI] [PubMed] [Google Scholar]
- INWARD P. W., JENCKS W. P. THE REACTIVITY OF NUCLEOPHILIC REAGENTS WITH FUROYL-CHYMOTRYPSIN. J Biol Chem. 1965 May;240:1986–1996. [PubMed] [Google Scholar]
- Inagami T., Patchornik A., York S. S. Participation of an acidic group in the chymotrypsin catalysis. J Biochem. 1969 May;65(5):809–819. doi: 10.1093/oxfordjournals.jbchem.a129081. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Stroud R. M. Mechanism of hydrolysis by serine proteases: direct determination of the pKa's of aspartyl-102 and aspartyl-194 in bovine trypsin using difference infrared spectroscopy. Biochemistry. 1976 Aug 10;15(16):3450–3458. doi: 10.1021/bi00661a009. [DOI] [PubMed] [Google Scholar]
- Lucas E. C., Caplow M., Bush K. J. Chymotrypsin catalysis. Evidence for a new intermediate. 3. J Am Chem Soc. 1973 Apr 18;95(8):2670–2673. doi: 10.1021/ja00789a043. [DOI] [PubMed] [Google Scholar]
- Lucas E. C., Caplow M. Chymotrypsin catalysis. Evidence for a new intermediate. J Am Chem Soc. 1972 Feb 9;94(3):960–963. doi: 10.1021/ja00758a039. [DOI] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer S. T., Kraut J. X-ray crystallographic study of boronic acid adducts with subtilisin BPN' (Novo). A model for the catalytic transition state. J Biol Chem. 1975 Sep 25;250(18):7120–7126. [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer T., Kraut J. Re-examination of the charge relay system in subtilisin comparison with other serine proteases. J Biol Chem. 1977 Dec 25;252(24):8875–8883. [PubMed] [Google Scholar]
- O'Leary M. H., Kluetz M. D. Identification of the rate-limiting step in the chymotrypsin-catalyzed hydrolysis of N-acetyl-L-tryptophanamide. J Am Chem Soc. 1970 Oct 7;92(20):6089–6090. doi: 10.1021/ja00723a062. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Kluetz M. D. Nitrogen isotope effects on the chymotrypsin-catalyzed hydrolysis of N-acetyl-L-tryptophanamide. J Am Chem Soc. 1972 May 17;94(10):3585–3589. doi: 10.1021/ja00765a055. [DOI] [PubMed] [Google Scholar]
- Petkov D. D. Detection of a tetrahedral intermediate in the trypsin-catalysed hydrolysis of specific ring-activated anilides. Biochim Biophys Acta. 1978 Apr 12;523(2):538–541. doi: 10.1016/0005-2744(78)90057-8. [DOI] [PubMed] [Google Scholar]
- Philipp M., Pollack R. M., Bender M. L. Influence of Leaving-Group Electronic Effect on alpha-Chymotrypsin: Catalytic Constants of Specific Substrates. Proc Natl Acad Sci U S A. 1973 Feb;70(2):517–520. doi: 10.1073/pnas.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwait A. C., Jencks W. P. The mechanism of the aminolysis of acetate esters. J Am Chem Soc. 1974 Oct 30;96(22):7018–7031. doi: 10.1021/ja00829a034. [DOI] [PubMed] [Google Scholar]
- Satterthwait A. C., Jencks W. P. The mechanism of the aminolysis of acetate esters. J Am Chem Soc. 1974 Oct 30;96(22):7018–7031. doi: 10.1021/ja00829a034. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Parker L. Pretransition-state protonation and the rate of chymotrypsin catalysis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2451–2454. doi: 10.1073/pnas.58.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. Chymotrypsin-catalyzed phenyl ester hydrolysis. Evidence for electrophilic assistance on carbonyl oxygen. Biochemistry. 1970 Aug 18;9(17):3383–3390. doi: 10.1021/bi00819a014. [DOI] [PubMed] [Google Scholar]
- Zeeberg B., Caplow M. Transition state charge distribution in reactions of an acetyltyrosylchymotrypsin intermediate. J Biol Chem. 1973 Aug 25;248(16):5887–5891. [PubMed] [Google Scholar]



