Abstract
Interleukin-6 (IL-6) is involved in lung cancer tumorigenesis, tumor progression, metastasis, and drug resistance. Previous studies show that blockade of IL-6 signaling can inhibit tumor growth and increase drug sensitivity in mouse models. Clinical trials in non-small cell lung cancer (NSCLC) reveal that IL-6 targeted therapy relieves NSCLC-related anemia and cachexia, although other clinical effects require further study. We crossed IL-6 -/- mice with Kras G12D mutant mice, which develop lung tumors after activation of mutant Kras G12D, to investigate whether IL-6 inhibition contributes to tumor progression and survival time in vivo. Kras G12D; IL-6 -/- mice exhibited increased tumorigenesis, but slower tumor growth and longer survival, than Kras G12D mice. Further, in order to investigate whether IL-6 deletion contributes to suppression of lung cancer metastasis, we generated Kras G12D; p53 flox/flox; IL-6 -/- mice, which developed lung cancer with a trend for reduced metastases and longer survival than Kras G12D; p53 flox/flox mice. Tumors from Kras G12D; IL-6 -/- mice showed increased expression of TNFα and decreased expression of CCL-19, CCL-20 and phosphorylated STAT3 (pSTAT3) than Kras G12D mice; however, these changes were not present between tumors from Kras G12D; p53 flox/flox; IL-6 -/- and Kras G12D; p53 flox/flox mice. Upregulation of pSTAT3 and phosphorylated AKT (pAKT) were observed in Kras G12D tumors with p53 deletion. Taken together, these results indicate that IL-6 deletion accelerates tumorigenesis but delays tumor progression and prolongs survival time in a Kras-driven mouse model of lung cancer. However, these effects can be attenuated by p53 deletion.
Introduction
Accumulating evidence indicates that inflammation contributes to tumorigenesis, tumor progression, and metastasis [1,2]. Oncogene-associated inflammation leads to production of inflammatory cytokines such as interleukin-6 (IL-6) [3,4], a pleiotropic cytokine involved in inflammation, immunity, bone metabolism, neural development, reproduction, and hematopoiesis [5]. However, IL-6 is also associated with increased risk of lung cancer [6-8]. IL-6 can be detected in breath condensate of patients with non-small cell lung cancer (NSCLC) [9], and in serum of some lung cancer patients, but is not detectable in patients with benign lung disease [10]. Elevated IL-6 levels contribute to malignant pleural effusion [11,12], postoperative complications [13], and postoperative recurrence [14] of lung cancer. Several studies have correlated high circulating IL-6 levels with poor survival of lung cancer patients [15-23]. IL-6-mediated inflammation correlates with debilitating cancer-related symptoms such as fatigue, thromboembolism, cachexia, and anemia [3], and IL-6 signaling activation correlates with lung cancer chemotherapy resistance [16,24]. These studies suggest an important role for IL-6 in several aspects of lung cancer.
IL-6 expression can be detected in lung tumors [25] and in 53% of lung cancer cell lines [26], and IL-6 pathways are activated in a human lung cancer stem cell line [27-29]. Functional assays suggest that IL-6 influences the ability of cancer cells to metastasize to distant sites [30,31] and that IL-6 promotes tumor growth in a paracrine fashion in vivo [4,26,32]. Therefore, it is perhaps not surprising that IL-6 knockdown, genetic ablation, or treatment with a neutralizing IL-6 antibody inhibits tumor growth in vivo [4,33]. Conversely, activation of IL-6 signaling contributes to resistance to epidermal growth factor receptor (EGFR) inhibitors in a mouse model of NSCLC [34,35], while blockade increases drug sensitivity in xenograft models [34].
An IL-6 monoclonal antibody therapy would be predicted to inhibit the inflammatory microenvironment in lung cancer. One such therapy, ALD518, has undergone preclinical and Phase I and II clinical trials. It appears to be well tolerated and ameliorates NSCLC-related anemia and cachexia [3], although the totality of clinical outcomes needs further study.
To assess the contribution of IL-6 signaling inhibition on tumor progression and survival time in vivo, we crossed IL-6 -/- mice with mutant Kras G12D mice because IL-6 is a downstream effector of oncogenic Ras to promote tumorigenesis[4]. NSCLC is often diagnosed with metastasis and has a poor prognosis. The treatment and prevention of lung cancer metastases are major unmet needs [36]. Inactivating mutations in p53 are found in at least 50% of NSCLC cases [36], and Kras G12D activation accompanied by p53 deletion can cause lung tumor metastasis [37]. To study the function of IL-6 in metastasis, we also generated Kras G12D; p53 flox/flox; IL-6 -/- mice..
Materials and Methods
Mice
IL-6 -/- mice were purchased from The Jackson Laboratory and maintained in sterile housing [38]. Conditional Lox–Stop–Lox Kras G12D (hereafter referred to as Kras G12D) mice [39] and p53 flox/flox mice [40] were described previously. Kras G12D and Kras G12D; lL-6 -/- mice were inoculated with 5 × 106 PFU of adenoviral Cre (adeno-Cre) by intranasal inhalation to activate oncogenic Kras G12D in the lungs. Kras G12D; p53 flox/flox and Kras G12D; p53 flox/flox;IL-6 -/- mice were inoculated with 5 × 105 PFU of adeno-Cre. All experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Use Committee at Dana-Farber Cancer Institute (permit number 04-094). All surgeries were performed under Avertin anesthesia to minimize suffering. After euthanasia, organs, including heart, liver, spleen, kidney, stomach, intestine, spine, brain, breast, skin, and testis or ovary, were undergone gross inspection for metastases. Lung tumors adhered to the pleura were considered parietal pleural metastases. Suspected metastases were harvested and confirmed by histological features.
Histology and immunohistochemistry
After euthanasia, the lungs were removed and fixed in 10% neutral buffered formalin overnight before embedding in paraffin. Five-micrometer sections of mouse lung tissues were cut. Some sections were stained with H&E. For immunohistochemistry, heat treatment with citrate solution (Beijing ZhongShan Golden Bridge Biotechnology Co., China) in a decloaking chamber (Biocare Medical) unmasked antigens for phosphorylated ERK (pERK), BrdU, Ki67, Endomucin and Caspase-3 staining. Whole lung tissue sections were incubated overnight at 4°C with primary antibodies: pERK (4370, Cell Signaling) at 1:100; BrdU (ab6326, Abcam) at 1:200; Ki67 (ab15580, Abcam) at 1:200; Endomucin (14-5851, eBioscience) at 1:100; cleaved Caspase-3 (9661, Cell Signaling) at 1:300. Digest-All 2B Trypsin (Invitrogen) was used to retrieve the antigen for MAC2 (CL8942AP, Cedarlane) staining at 1:5000. At 400X magnification, all MAC2-positive macrophages in tumors were counted within 3 microscope fields with the most MAC2-positive macrophages after review of the whole lung section. Three mice per genotype were analyzed.
Proliferation analysis
At 20 weeks post-infection, mice were injected intraperitoneally with 10 μL of 10 mM BrdU in PBS per gram of body weight and euthanized after 2 hours. Whole lungs were harvested and processed as described above. At 400X magnification, all BrdU-positive tumor cell nuclei were counted within 3 microscope fields with the most BrdU-positive nuclei after review of the whole lung section. Four mice per genotype were analyzed. Same method was used to calculate Ki67-labeled tumor cells on sections from mice 28 weeks post-infection with adeno-Cre.
Western blotting
Lung tumors were harvested from Kras G12D and Kras G12D; lL-6 -/- mice 32 weeks post-infection and from Kras G12D; p53 flox/flox and Kras G12D; p53 flox/flox; IL-6 -/- mice 15 weeks post-infection for Western blot analysis. Tumors were lysed with a homogenizer in RIPA buffer (50 mM Tris pH 7.4, 150 mM sodium chloride, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM EDTA) containing complete mini protease inhibitors (Roche) and phosphatase inhibitors (5870, Cell Signaling). Nuclear and Cytoplasmic Extraction Kit (CW199B, CoWin Biotech Co., Ltd. China) was used to extract cytoplasmic (C) and nuclear (N) fractions from tumors. Lysates (20 μg per lane) were separated on 10% polyacrylamide gels, transferred to PVDF filters, and incubated overnight at 4°C with antibodies to β-actin (sc-1615, Santa Cruz), pERK (4376, Cell Signaling), total-ERK (9102, Cell Signaling), pAKT (4060, Cell Signaling), total-AKT (4685, Cell Signaling), pSTAT3 (9145, Cell Signaling), STAT3 (sc-7179, Santa Cruz), p65 (sc-372, Santa Cruz), PARP (9532, Cell Signaling), GAPDH (TA-8, Beijing ZhongShan Golden Bridge Biotechnology Co., China), or β-catenin (ab32572, Abcam). Western blots were exposed to X-ray films or scanned with an ImageQuant LAS 4000mini (GE healthcare).
Quantitative real-time PCR
mRNA was extracted from tumors of Kras G12D and Kras G12D; lL-6 -/- mice 32 weeks post-infection and Kras G12D; p53 flox/flox and Kras G12D; p53 flox/flox; IL-6 -/- mice around 16 weeks post-infection for analysis. 2μg total RNA was reverse transcribed to cDNA using SuperRT cDNA synthesis kit (Beijing CoWin Biosciences Co., Ltd. China). Real-time PCR was performed using the BioRad iQ5 Realtime PCR system and StepOnePlus Realtime PCR system (ABI) with Realtime PCR Master Mix containing SYBR Green (QPK-201 ,TOYOBO, Japan) and unique primers (Table S1). Three to four samples for each group were detected.Gene expression results were normalized to β-actin mRNA.
Statistical analysis
The Student’s t-test was used to evaluate lesion number and number of BrdU or Ki67- positive cells. Fisher’s exact test evaluated metastatic rate. Kaplan–Meier analysis evaluated survival time. Expression differences among four groups were analyzed by ANOVA. P<0.05 was considered statistically significant.
Results
IL-6 deletion accelerates oncogenic Kras G12D-induced lung tumorigenesis
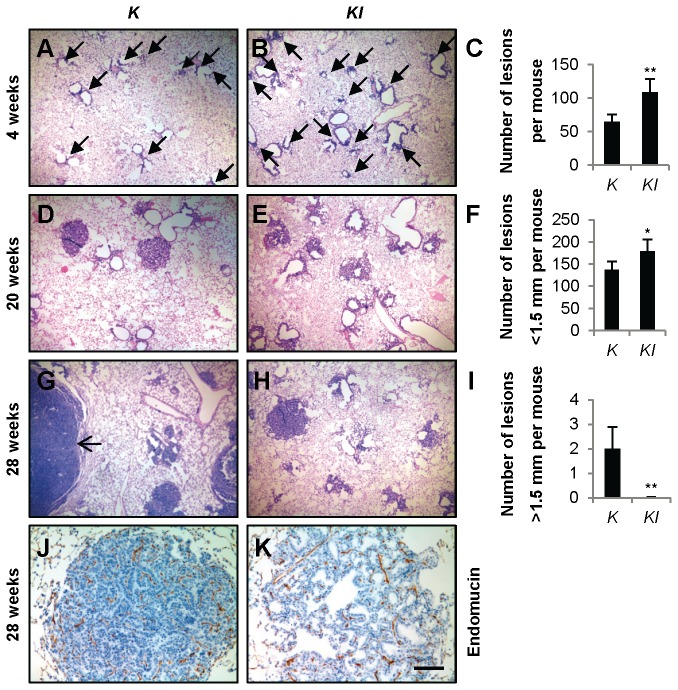
As previously described, Kras G12D mice developed lung tumors following a long latency [39]. IL-6 -/- mice developed normally [38], and did not show lung tumors through 54 weeks of age (data not shown). Following adeno-Cre inhalation, PCR analysis confirmed recombination of the conditional Kras G12D allele (Figure S1). Kras G12D ; IL-6 -/- mice had a median survival of 37 weeks after adeno-Cre inoculation, significantly longer than Kras G12D mice (P<0.0001) (Table 1). Mice were euthanized at 2, 4, 20, 28, and 32 weeks post-infection, and lung lesions in H&E-stained sections were analyzed at each time point. At 2 weeks post-infection (n = 3), both Kras G12D and Kras G12D ; IL-6 -/- mice had early lung lesions. At 4 weeks post-infection, Kras G12D ; IL-6 -/- mice had more early lung lesions than Kras G12D mice (Figure 1A-C). These lesions were atypical adenomatous hyperplasia (AAH) and epithelial hyperplasia (EH) of the bronchioles, as reported previously [39].
Table 1. Comparison of lung cancer cohorts.
| Genotype | Number treated | Median survival (weeks)* | Survival range (weeks) |
|---|---|---|---|
| IL-6-/- | 13 | >54 | |
| KrasG12D | 14 | 34.6 | 27.9 ~ 39.0 |
| KrasG12D; IL-6-/- | 38 | 37.0a | 29.3 ~ 46.7 |
| p53flox/flox | 8 | >52 | |
| p53flox/flox; IL-6-/- | 9 | >52 | |
| KrasG12D; p53flox/flox | 43 | 16.3 | 11.1 ~ 19.7 |
| KrasG12D; p53flox/flox; IL-6-/- | 44 | 17.4b | 12.7 ~ 23.4 |
a Kras G12D ; IL-6 -/- mice had significantly longer survival than Kras G12D mice (P<0.0001). b Kras G12D ; p53 flox/flox ; IL-6 -/- mice had significantly longer survival than Kras G12D ; p53 flox/flox mice (P<0.01). * Median latency shown is after adeno-Cre treatment at 6-10 weeks of age, estimated by Kaplan–Meier analysis.
Figure 1. IL-6 deletion promotes tumorigenesis but retards tumor progression of Kras G12D-driven lung cancer.
(A and B) Representative images of H&E-stained lung tissue sections from (A) K and (B) KI mice 4 weeks post-infection with adeno-Cre. Arrows indicate early lesions. (C) Quantification of lesions in K (n=3) and KI (n=5) mice 4 weeks post-infection with adeno-Cre. Data are shown as mean + s.e.m. **P<0.01. (D and E) Representative images of H&E-stained lung tissue sections from (D) K and (E) KI mice 20 weeks post-infection with adeno-Cre. (F) Quantification of small lesions (<1.5 mm) in K and KI mice (n=6) 28 weeks post-infection with adeno-Cre. Data shown are mean + s.e.m. *P<0.05. (G and H) Representative images of H&E-stained lung tissue sections from (G) K and (H) KI mice28 weeks post-infection with adeno-Cre. Arrow indicates a large tumor. (I) Quantification of large tumors (>1.5 mm) in K and KI mice (n=6) 28 weeks post-infection with adeno-Cre. Data shown are mean + s.e.m. **P<0.01. (J and K) Representative images of Endomucin-stained lung tissue sections from (J) K and (K) KI mice 28 weeks post-infection with adeno-Cre. Scale bar indicates 500 μm in (A, B, D, E, G and H), or 100 μm in (J and K). Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-.
IL-6 deletion retards oncogenic Kras G12D-induced lung tumor progression
At 20 weeks post-infection, lung tumors in Kras G12D ; IL-6 -/- mice were modestly smaller and less dense than those in Kras G12D mice (Figure 1D and E). At 28 weeks post-infection, in comparison with Kras G12D mice, more lesions were observed in Kras G12D ; IL-6 -/- mice with the majority of lesions in early stages of tumor development (Figure 1F). However, tumors 3-10 mm in diameter were observed in lungs of Kras G12D mice, while the majority of Kras G12D ; IL-6 -/- lung tumors were less than 1.5 mm (Figure 1G-I). Further, although IL-6 signaling promotes skin tumor growth and angiogenesis in a paracrine fashion [4], we did not detect any difference between Kras G12D and Kras G12D ; IL-6 -/- mice after immunohistochemical staining with Endomucin, a microvessel density marker to measure angiogenesis index (Figure 1J and K).
IL-6 deletion attenuates lung tumor proliferation
To determine whether tumor proliferation is affected by IL-6 deletion in vivo, we measured BrdU-labeling cells in lung tumors. Significantly fewer labeled nuclei were observed in lung sections from Kras G12D ; IL-6 -/- mice 20 weeks post-infection with adeno-Cre compared with those derived from control Kras G12D mice (Figure 2A-C). Similar results were observed from Ki67 staining in lung sections from Kras G12D and Kras G12D ; IL-6 -/- mice 28 weeks post-infection with adeno-Cre (Figure S2). Expression of pERK, which acts downstream of Kras and is associated with cancer cell proliferation, was reduced in tumors from Kras G12D ; IL-6 -/- mice 20 weeks post-infection with adeno-Cre compared to Kras G12D mice (Figure 2D and E). Caspase-3 staining revealed no differences in tumor cell apoptosis between Kras G12D and Kras G12D ; IL-6 -/- mice (Figure 2F and G).
Figure 2. IL-6 deletion attenuates proliferation but not apoptosis of tumor cells.
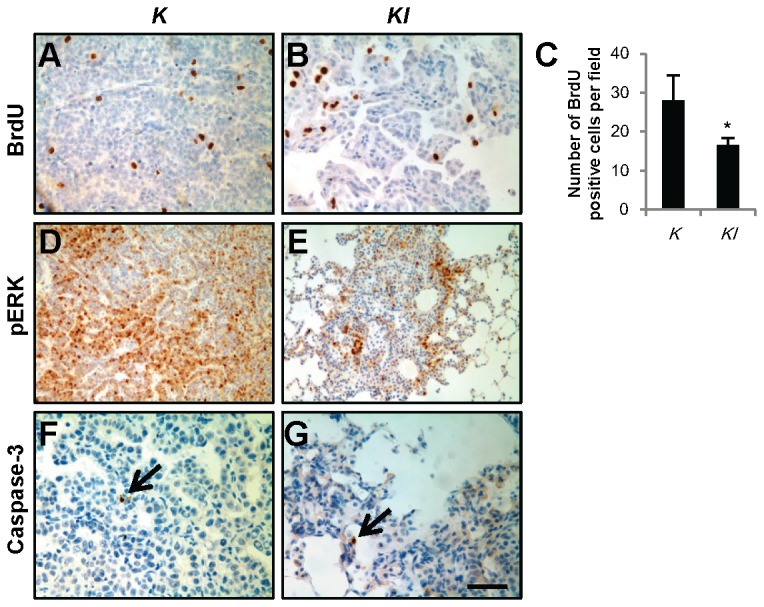
(A and B) Representative images of BrdU-stained lung tissue sections from (A) K and (B) KI mice 20 weeks post-infection with adeno-Cre. (C) Quantification of BrdU-positive tumor cells in lung tissue sections of K and KI mice (n=4) 20 weeks post-infection with adeno-Cre. *P<0.05. (D and E) Representative images of pERK stained lung tissue sections from (D) K and (E) KI mice 20 weeks post-infection with adeno-Cre. (F and G) Representative images of cleaved Caspase-3-stained lung tissue sections from (F) K and (G) KI mice 28 weeks post-infection with adeno-Cre. Arrows indicate Caspase-3 positive tumor cells. Scale bar indicates 50 μm in (A, B, F and G), or 100 μm in (D and E) . Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-.
IL-6 deletion extends survival of Kras G12D; p53 flox/flox mice
As previously reported, no metastases or local invasions were detected in Kras G12D mice [39], and similar results were observed in Kras G12D ; IL-6 -/- mice. Kras G12D activation accompanied by p53 deletion can cause lung tumor metastasis [37], therefore, Kras G12D ; p53 flox/flox ; IL-6 -/- mice were generated to investigate the influence of IL-6 deletion on lung cancer metastasis.
p53 allelic recombination was confirmed by PCR (Figure S3). IL-6 deletion increased median survival of Kras G12D ; p53 flox/flox mice (P<0.01) (Table 1) despite substantial lung tumor burden in both Kras G12D ; p53 flox/flox and Kras G12D ; p53 flox/flox ; IL-6 -/- mice 12 weeks post-infection (Figure 3A and B). BrdU staining indicated both groups of lung tumors were highly proliferative (Figure 3C and D), and pERK expression was high in both groups (Figure 3E and F); no statistical differences were observed.
Figure 3. KP and KPI mice have high tumor burden, tumor cell proliferation and metastases.
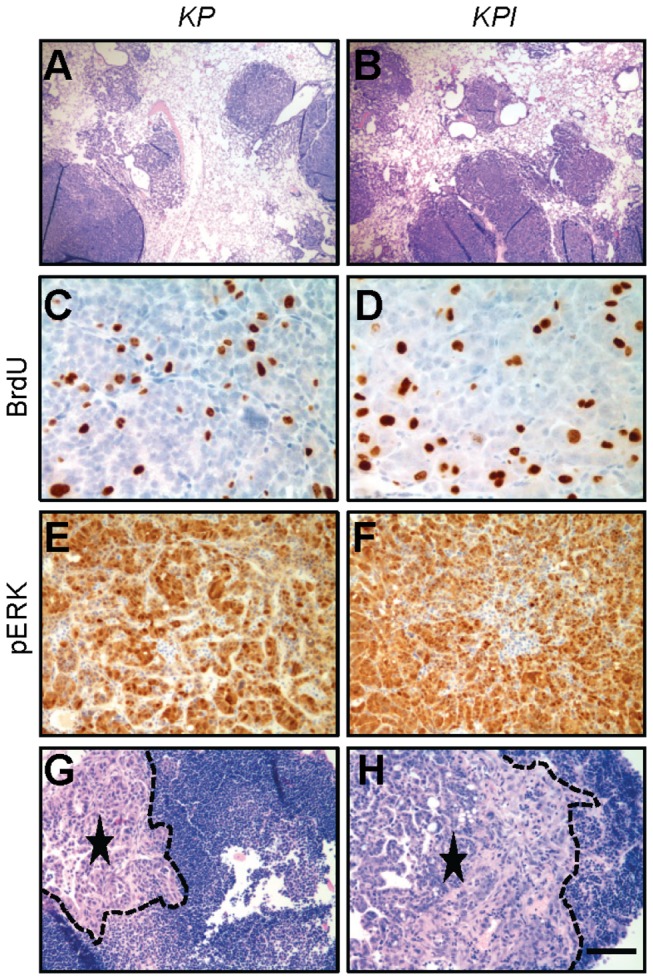
Representative images of lungs (A and B), BrdU staining (C and D), pERK staining (E and F) and tumor metastases (G and H) from KP (A, C, E, and G) and KPI (B, D, F, and H) mice 12 weeks post-infection with adeno-Cre. Dotted lines (G and H) show metastastic tumor edges. Asterisks indicate center of metastatic tumors. Scale bar indicates 500 μm (A, B, G, and H), 50 μm (C and D) or 100 μm (E and F). Abbreviations: KP=Kras G12D ; p53 flox/flox. KPI=KrasG12D; p53flox/flox;IL-6-/-.
For comparison, 17 Kras G12D ; p53 flox/flox ; IL-6 -/- mice and 19 Kras G12D ; p53 flox/flox mice were analyzed for metastases around 16 weeks post-infection with adeno-Cre (Table 2). Histologically, metastases were found in 5 of 17 Kras G12D ; p53 flox/flox ; IL-6 -/- mice (29.4%) and 10 of 19 Kras G12D ; p53 flox/flox mice (52.6%), although this difference was not significant (P=0.19). Metastatic lesions to the parietal pleura, thymus (Figure S4A and B), and lymph nodes were observed in both Kras G12D ; p53 flox/flox and Kras G12D ; p53 flox/flox ; IL-6 -/- mice (Figure 3G and H). Heart metastases (Figure S4C) were observed in 2 of 19 Kras G12D ; p53 flox/flox mice (Table 2).
Table 2. Site and frequency of metastases from primary lung tumors.
| Sites of metastases | KrasG12D; p53flox/flox | KrasG12D; p53flox/flox; IL-6-/- |
|---|---|---|
| Lymph node | 10 of 19 (52.6%) | 5 of 17 (29.4%) |
| Thymus | 1 of 19 (5.3%) | 1 of 17 (5.9%) |
| Heart | 2 of 19 (10.5%) | 0 of 17 |
IL-6 deletion alters, but p53 deletion attenuates, some inflammatory cytokines
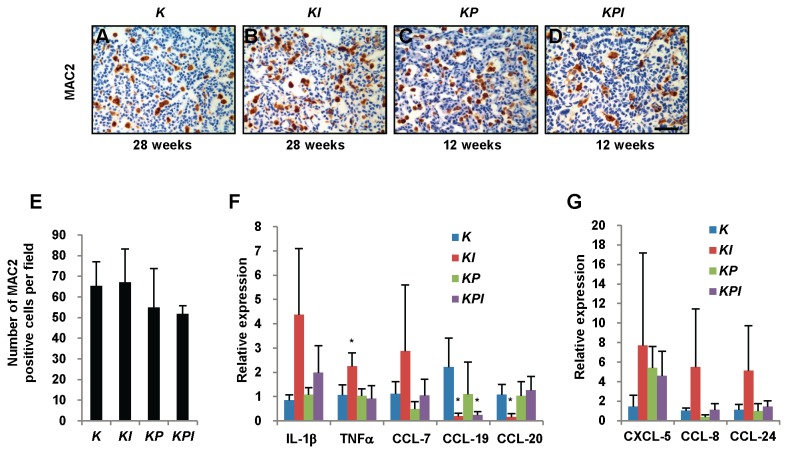
To investigate whether IL-6 deletion affected inflammation, we measured macrophage density using MAC2 staining [41]. No significant changes in macrophage number were observed among tumors from Kras G12D , Kras G12D ; IL-6 -/- , Kras G12D ; p53 flox/flox, and Kras G12D ; p53 flox/flox ; IL-6 -/- mice (Figure 4A-E). We also measured no change in CD3 expression, a T cell marker, in any of the four tumor groups (Figure S5B).
Figure 4. IL-6 deletion upregulates TNFα and downregulates CCL-19 and CCL-20 in tumors.
(A-D) Representative images of MAC2-stained lung tissue sections from (A) K and (B) KI mice 28 weeks post-infection and from (C) KP and (D) KPI mice 12 weeks post-infection with adeno-Cre. (E) Quantification of MAC2-positive macrophages in lung tumors from K, KI, KP, and KPI mice (n=3). No statistical difference was observed. (F and G) Gene expression of IL-1β, TNFα, CCL-7, CCL-19, CCL-20, CXCL-5, CCL-8 and CCL-24 in tumors from K, KI, KP, and KPI mice were determined by real-time PCR. Three to four tumors for each group were detected and triplicate PCRs were performed. Gene expression was normalized to β-actin mRNA. * P<0.05 vs. K tumors. Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-. KP=Kras G12D ; p53 flox/flox. KPI=KrasG12D; p53flox/flox;IL-6 -/-.
Several cytokines play important roles in the inflammatory process. The list includes IL-1, TNFα, and IL-6. Chemokines represent the largest family of cytokines and are classified into polypeptide groups by the location of cysteine residues near the amino terminus (e.g., C-C, C-X-C, or CX3C) [42]. Oncogenic Ras induces the secretion of the ELR1 + CXC chemokine family to promote tumorigenesis [43]. Some chemokines and growth factors are involved in tumor progression [42], so we screened inflammatory cytokine changes in tumors with real-time PCR. Three samples each tumor group were used to perform real-time PCR without replicate. There were no significant differences found for IL-1α, CXCL-1, CXCL-5, CXCL-9, CXCL-12, CXCL-16, TGF-β2, BMP2, BMP4, CCL-2, CCL-7, CCL-8, CCL-9, CCL-22, CCL-28 and CX3CL-1 expression among four groups of tumors (Figure S5). The screening results showed some changing trends in some inflammatory cytokines (Figure S5). We confirmed the changes using triplicate real-time PCR reactions with 3 to 4 samples in each group. Elevated expression of TNFα and reduced expression of CCL-19 and CCL-20 were detected in tumors from Kras G12D ; IL-6 -/- mice compared to Kras G12D mice. However, these changes were absent between tumors from Kras G12D ; p53 flox/flox and Kras G12D ; p53 flox/flox ; IL-6 -/- mice. While no statistical differences in IL-1β, CCL-7, CCL-8, CCL-24 and CXCL-5 gene expression were confirmed among four tumor groups (Figure 4F and G).
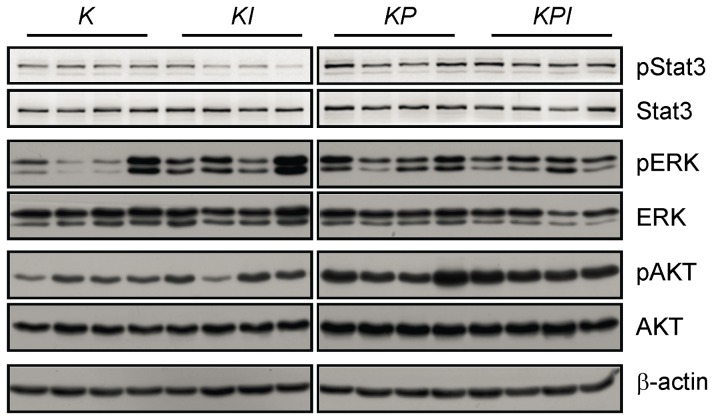
We also examined the nuclear localization of NF-κB subunit p65, which is important in cancer-related inflammation and malignant progression [44,45]. However, no significant localization change was observed among tumors from the four genotypes (Figure S6). And no dramatic change was observed in β-catenin expression in nucleus (Figure S6), which is related to lung cancer development [46]. Expression of pSTAT3, which is the main downstream target of IL-6, was reduced in some Kras G12D ; IL-6 -/- tumors (Figure 5) but increased in p53-deleted tumors. These data indicated that IL-6 deletion altered tumor expression of some inflammatory cytokines, although these changes were weakened by p53 deletion.
Figure 5. p53 deletion Increases pSTAT3 and pAKT expression in Kras G12D tumors.
Tumor lysates were extracted from K and KI mice 32 weeks post-infection and from KP and KPI mice 15 weeks post-infection for Western blot analysis. Western blot results of pSTAT3 and total-STAT3 were scanned by an ImageQuant LAS 4000mini (GE healthcare). Other results were exposed to X-ray films. Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-. KP=Kras G12D ; p53 flox/flox. KPI=KrasG12D; p53flox/flox;IL-6 -/-.
Discussion
Previous studies have shown that carcinogen-induced tumorigenesis in IL-6 −/− mice is delayed by 1-2 weeks [4,47]; however, we found no difference in Kras G12D-induced tumor onset regardless of IL-6 deficiency. One possible explanation is that Kras G12D activation may induce lung tumorigenesis more robustly than other carcinogens.
Some inflammatory cytokines are associated with tumor progression [42]. TNFα may act as a tumor promoter by regulating a cascade of cytokines, chemokines, adhesions, matrix metalloproteinases (MMPs) and pro-angiogenic activities [2,48]. In this study, IL-6 deletion in Kras G12D tumors upregulated TNFα expression. Elevated expression of TNFα may compensate for the loss of IL-6 and thus increase tumorigenesis. However, tumor progression is delayed in Kras G12D ; IL-6 -/- mice, consistent with previous results [4,47]. These data indicate that IL-6 is important for tumor progression in vivo and suggest that IL-6 inhibition may have biphasic stage-specific effects in lung cancer, enhancing tumorigenesis early while suppressing tumor progression later. Consequently, this may pose a risk to lung cancer patients treated with IL-6-targeted therapy.
CCL-20 (or macrophage pro-inflammatory chemokine-3α, MIP-3α), a C-C motif chemokine, is overexpressed in pancreatic carcinoma cells and stimulates growth of tumor cells [49]. CCL-19 (or macrophage inflammatory protein-3 beta, MIP-3β), plays an important role in the migration of mature dendritic cells and T-cells [50]. Both dendritic cells and T-cells are double-edged swords in the tumor microenvironment, in addition to initiating potent anti-tumor immune responses, these cells may also stimulate cancerous cell growth and spreading [51,52]. Persistently activated or tyrosine-phosphorylated STAT3 (pSTAT3) is found in 50% of lung adenocarcinomas [53,54]. pSTAT3 can enhance tumor proliferation and loss of pSTAT3 arrests growth of premalignant lesions, almost abrogating the development of advanced tumors [55]. In this study, IL-6 deletion in Kras G12D tumors resulted in downregulation of pSTAT3, CCL-19 and CCL-20. pERK expression was reduced in Kras G12D ; IL-6 -/- tumors 20 weeks post-infection (Figure 2), but increased in most Kras G12D ; IL-6 -/- tumors 32 weeks post-infection (Figure 5). These data suggest that early stage, tumor growth may be delayed by low expression of pERK, pSTAT3 and CCL-20. During later stages, tumor growth may be induced by upregulation of pERK and TNFα, although these mechanisms need further study.
Our data show that p53 deletion more dramatically affected Kras G12D-induced lung cancer than IL-6 deletion. To a large extent, p53 deletion attenuated the effects of IL-6 deletion on delayed tumor growth and prolonged survival. p53 deletion enhanced pSTAT3 expression (Figure 5) and abrogated the change in CCL-20 expression in Kras G12D ; p53 flox/flox ; IL-6 -/- tumors (Figure 4). p53 deletion also increased expression of pAKT and total-AKT expression, which are associated with high proliferation, in Kras G12D ; p53 flox/flox and Kras G12D ; p53 flox/flox ; IL-6 -/- tumors(Figure 5). p53 deletion may attenuate the effects of IL-6 deletion through these pathways.
We observed a trend for reduced metastases with IL-6 deletion (Table 2), although additional samples are required to confirm this result. Separately, we have observed dramatically increased IL-6 expression in primary and metastatic tumors from mice with high metastatic rates (unpublished data), similar to the report that IL-6 promotes cancer cells to metastasize to distant sites [30,31]. Furthermore, survival time of Kras G12D ; p53 flox/flox ; IL-6 -/- mice was significantly extended (P< 0.01) (Table 1). These results indicate that IL-6 deletion may reduce lung cancer metastases and prolong survival time in vivo although p53 deletion dominantly impacts the evolution of Kras G12D lung cancer.
The involvement of inflammation in tumorigenesis, progression, and metastasis is widely accepted; however, whether IL-6-targeted therapies will prolong the survival time of lung cancer patients remains uncertain. Our results indicate anti-IL-6 therapies may have some success in clinical trials. For example, when NSCLC has not metastasized, IL-6 inhibition may prolong survival but increase the risk of further tumorigenesis; if metastasized, IL-6 inhibition may only moderately impact metastasis but may lengthen survival time. Further studies are needed to elucidate these possibilities. In summary, our results provide evidence that IL-6 deficiency promotes lung tumorigenesis, but suppresses tumor progression and elongates survival in vivo. However, these effects can be attenuated by p53 deletion.
Supporting Information
PCR analysis of Kras allelic recombination. A 500 bp PCR product represents the floxed, unrecombined Kras G12D allele; a 622 bp fragment represents the wildtype Kras allele; and a 650 bp fragment represents a recombined Kras G12D allele after removal of floxed stop cassette by adeno-Cre. K, KI, KP, and KPI mice were treated with adeno-Cre and the 650 bp recombined band revealed. Abbreviations: WT= wildtype lungs. Floxed=floxed Kras G12D, without adeno-Cre treatment. K=Kras G12D. KI=Kras G12D; IL-6-/-. KP=Kras G12D ; p53 flox/flox. KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
IL-6 deletion attenuates tumor proliferation determined by Ki67 staining. (A and B) Representative images of Ki67-stained lung tissue sections from (A) K and (B) KI mice 28 weeks post-infection with adeno-Cre. (C) Quantification of Ki67-positive tumor cells in lung tissue sections of K and KI mice (n=3). **P<0.01. Scale bar indicates 50 μm. Abbreviations: K=Kras G12D . KI=Kras G12D; IL-6 -/-.
(TIF)
PCR analysis of p53 allelic recombination. A 212 bp PCR product represents the floxed, unrecombined p53 allele; a 168 bp fragment represents the recombined allele after inoculation with adeno-Cre; and a 130 bp fragment represents the wildtype p53 allele. K, KI, KP, and KPI mice were treated with adeno-Cre. The 168 bp recombined band was showed in KP and KPI mice and 212 bp fragment remained due to tumor stromal cells. Abbreviations: WT=wildtype lungs. Floxed=floxed p53, without adeno-Cre treatment. K=Kras G12D . KI=Kras G12D; IL-6 -/- . KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIFF)
KP and KPI mice develop metastatic lesions. (A and B) Representative images of metastatic lesions to the (A) pleura, (B) thymus in KPI mice 15 weeks post-infection with adeno-Cre. Dotted lines in the images indicate metastatic tumor edges. Asterisks indicate center of metastatic tumors. (C) Representative image of heart metastases in KP mice 14 weeks post-infection with adeno-Cre. Metastatic lesions in the heart are left of the dotted line. Scale bar indicates 200 μm (A) or 100 μm (B and C). Abbreviations: KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
Real-time PCR screen of changes in inflammatory cytokines levels. .
Three tumors from each genotype were analyzed by real-time PCR without replicate for expression of the indicated cytokine. Gene expression was normalized to β-actin mRNA. *P<0.05 vs. K tumors. # P<0.05 vs. KP tumors. Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-. KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
Nuclear localization of p65 and β-catenin are unchanged. Tumors from each mouse genotype were lysed to obtain cytoplasmic (C) and nuclear (N) fractions. Lysates were analyzed for the presence of nuclear p65 and β-catenin by Western blot. Fraction purity was determined by GAPDH (cytoplasmic) and PARP (nuclear) blots. Abbreviations: K=Kras G12D . KI=Kras G12D; IL-6 -/- . KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIFF)
Primers for real-time PCR analysis of gene expression.
(DOCX)
Acknowledgments
The authors thank Ms. Mei Zheng for technical assistance.
Funding Statement
This work is supported by the NIH (CA122794, CA140594, CA163896, CA166480, CA154303, and Lung SPORE P50CA090578), United against Lung Cancer, American Lung Association, Susan Spooner Research Fund (KKW), Chinese National Key Program on Basic Research (2012CB945100, 2011CB504202) and National Natural Science Foundation of China (31030040). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ballaz S, Mulshine JL (2003) The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer 5: 46-62. doi: 10.3816/CLC.2003.n.021. PubMed: 14596704. [DOI] [PubMed] [Google Scholar]
- 2. Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357: 539-545. doi: 10.1016/S0140-6736(00)04046-0. PubMed: 11229684. [DOI] [PubMed] [Google Scholar]
- 3. Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR (2011) A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther 11: 1663-1668. doi: 10.1517/14712598.2011.627850. PubMed: 21995322. [DOI] [PubMed] [Google Scholar]
- 4. Ancrile B, Lim KH, Counter CM (2007) Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev 21: 1714-1719. doi: 10.1101/gad.1549407. PubMed: 17639077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keller ET, Wanagat J, Ershler WB (1996) Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci 1: d340-d357. PubMed: 9159238. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Liu RY, Yang L, Zhao J, Zhao X et al. (2013) A two-SNP IL-6 promoter haplotype is associated with increased lung cancer risk. J Cancer Res Clin Oncol 139: 231-242. doi: 10.1007/s00432-012-1314-z. PubMed: 23052692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heikkilä K, Harris R, Lowe G, Rumley A, Yarnell J et al. (2009) Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control 20: 15-26. doi: 10.1007/s10552-008-9212-z. PubMed: 18704713. [DOI] [PubMed] [Google Scholar]
- 8. Bai L, Yu H, Wang H, Su H, Zhao J et al. (2013) Genetic single-nucleotide polymorphisms of inflammation-related factors associated with risk of lung cancer. Med Oncol 30: 414. doi: 10.1007/s12032-012-0414-6. PubMed: 23292870. [DOI] [PubMed] [Google Scholar]
- 9. Carpagnano GE, Resta O, Foschino-Barbaro MP, Gramiccioni E, Carpagnano F (2002) Interleukin-6 is increased in breath condensate of patients with non-small cell lung cancer. Int J Biol Markers 17: 141-145. PubMed: 12113582. [DOI] [PubMed] [Google Scholar]
- 10. Yanagawa H, Sone S, Takahashi Y, Haku T, Yano S et al. (1995) Serum levels of interleukin 6 in patients with lung cancer. Br J Cancer 71: 1095-1098. doi: 10.1038/bjc.1995.212. PubMed: 7734307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh HH, Lai WW, Chen HH, Liu HS, Su WC (2006) Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 25: 4300-4309. doi: 10.1038/sj.onc.1209464. PubMed: 16518408. [DOI] [PubMed] [Google Scholar]
- 12. Yanagawa H, Sone S, Munekata M, Atagi S, Nii A et al. (1992) IL-6 in malignant pleural effusions and its augmentation by intrapleural instillation of IL-2. Clin Exp Immunol 88: 207-212. PubMed: 1315227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Szczesny TJ, Slotwinski R, Stankiewicz A, Szczygiel B, Zaleska M et al. (2007) Interleukin 6 and interleukin 1 receptor antagonist as early markers of complications after lung cancer surgery. Eur J Cardiothorac Surg 31: 719-724. doi: 10.1016/j.ejcts.2007.01.027. PubMed: 17317198. [DOI] [PubMed] [Google Scholar]
- 14. Kita H, Shiraishi Y, Watanabe K, Suda K, Ohtsuka K et al. (2011) Does postoperative serum interleukin-6 influence early recurrence after curative pulmonary resection of lung cancer? Ann Thorac Cardiovasc Surg 17: 454-460. doi: 10.5761/atcs.oa.10.01627. PubMed: 21881374. [DOI] [PubMed] [Google Scholar]
- 15. Songür N, Kuru B, Kalkan F, Ozdilekcan C, Cakmak H et al. (2004) Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 90: 196-200. PubMed: 15237582. [DOI] [PubMed] [Google Scholar]
- 16. Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH et al. (2013) Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer 132: 1977-1985. doi: 10.1002/ijc.27892. PubMed: 23034889. [DOI] [PubMed] [Google Scholar]
- 17. Koh E, Iizasa T, Yamaji H, Sekine Y, Hiroshima K et al. (2012) Significance of the correlation between the expression of interleukin 6 and clinical features in patients with non-small cell lung cancer. Int J Surg Pathol 20: 233-239. doi: 10.1177/1066896911436274. PubMed: 22334615. [DOI] [PubMed] [Google Scholar]
- 18. Ujiie H, Tomida M, Akiyama H, Nakajima Y, Okada D et al. (2012) Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res 32: 3251-3258. PubMed: 22843899. [PubMed] [Google Scholar]
- 19. Enewold L, Mechanic LE, Bowman ED, Zheng YL, Yu Z et al. (2009) Serum concentrations of cytokines and lung cancer survival in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 18: 215-222. doi: 10.1158/1055-9965.EPI-08-0705. PubMed: 19124500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tas F, Duranyildiz D, Argon A, Oğuz H, Camlica H et al. (2005) Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol 22: 353-358. doi: 10.1385/MO:22:4:353. PubMed: 16260852. 10.1385/MO:22:4:353 PubMed: 16260852 [DOI] [PubMed] [Google Scholar]
- 21. Martín F, Santolaria F, Batista N, Milena A, González-Reimers E et al. (1999) Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 11: 80-86. doi: 10.1006/cyto.1998.0398. PubMed: 10080883. [DOI] [PubMed] [Google Scholar]
- 22. Wojciechowska-Lacka A, Adamiak E, Stryczynska G, Lacki JK (1997) Prognostic value of serial serum interleukin-6 level estimation in patients with lung cancer: a preliminary report. Yale J Biol Med 70: 139-148. PubMed: 9493846. [PMC free article] [PubMed] [Google Scholar]
- 23. Wójcik E, Jakubowicz J, Skotnicki P, Sas-Korczyńska B, Kulpa JK (2010) IL-6 and VEGF in small cell lung cancer patients. Anticancer Res 30: 1773-1778. PubMed: 20592377. [PubMed] [Google Scholar]
- 24. De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A et al. (1998) Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep 5: 649-652. PubMed: 9538169. [PubMed] [Google Scholar]
- 25. Takeuchi E, Ito M, Mori M, Yamaguchi T, Nakagawa M et al. (1996) Lung cancer producing interleukin-6. Intern Med 35: 212-214. doi: 10.2169/internalmedicine.35.212. PubMed: 8785456. [DOI] [PubMed] [Google Scholar]
- 26. Yamaji H, Iizasa T, Koh E, Suzuki M, Otsuji M et al. (2004) Correlation between interleukin 6 production and tumor proliferation in non-small cell lung cancer. Cancer Immunol Immunother 53: 786-792. PubMed: 15185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi H, Cho HJ, Cho SM, Jo K, Park JA et al. (2012) Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol 41: 310-316. PubMed: 22552503. [DOI] [PubMed] [Google Scholar]
- 28. Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE (2008) Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLOS ONE 3: e3077. doi: 10.1371/journal.pone.0003077. PubMed: 18728788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsu HS, Lin JH, Hsu TW, Su K, Wang CW et al. (2012) Mesenchymal stem cells enhance lung cancer initiation through activation of IL-6/JAK2/STAT3 pathway. Lung Cancer 75: 167-177. doi: 10.1016/j.lungcan.2011.07.001. PubMed: 21802163. [DOI] [PubMed] [Google Scholar]
- 30. Tawara K, Oxford JT, Jorcyk CL (2011) Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Manag Res 3: 177-189. PubMed: 21625400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang CL, Liu YY, Ma YG, Xue YX, Liu DG et al. (2012) Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLOS ONE 7: e37960. doi: 10.1371/journal.pone.0037960. PubMed: 22662257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M et al. (2011) Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer. Prev Res (Phila) 4: 51-64. doi: 10.1158/1940-6207.PREV-11-B51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song L, Rawal B, Nemeth JA, Haura EB (2011) JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther 10: 481-494. doi: 10.1158/1535-7163.MCT-10-0502. PubMed: 21216930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F et al. (2012) Activation of IL-6R/JAK1/STAT3 signaling induces De Novo resistance to irreversible EGFR inhibitors in non-small cell lung cancer with T790M resistance mutation. Mol Cancer Ther 11: 2254-2264. doi: 10.1158/1535-7163.MCT-12-0311. PubMed: 22891040. [DOI] [PubMed] [Google Scholar]
- 35. Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B et al. (2010) TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci U S A 107: 15535-15540. doi: 10.1073/pnas.1009472107. PubMed: 20713723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359: 1367-1380. doi: 10.1056/NEJMra0802714. PubMed: 18815398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K et al. (2007) LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807-810. doi: 10.1038/nature06030. PubMed: 17676035. [DOI] [PubMed] [Google Scholar]
- 38. Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M et al. (1994) Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368: 339-342. doi: 10.1038/368339a0. PubMed: 8127368. [DOI] [PubMed] [Google Scholar]
- 39. Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D et al. (2001) Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15: 3243-3248. doi: 10.1101/gad.943001. PubMed: 11751630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M et al. (2001) Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 29: 418-425. doi: 10.1038/ng747. PubMed: 11694875. [DOI] [PubMed] [Google Scholar]
- 41. Fukuda K, Kobayashi A, Watabe K (2012) The role of tumor-associated macrophage in tumor progression. Front Biosci (Schol Ed) 4: 787-798. PubMed: 22202090. [DOI] [PubMed] [Google Scholar]
- 42. Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420: 860-867. doi: 10.1038/nature01322. PubMed: 12490959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Hayer KM, Brady DC, Counter CM (2009) ELR+ CXC chemokines and oncogenic Ras-mediated tumorigenesis. Carcinogenesis 30: 1841-1847. doi: 10.1093/carcin/bgp198. PubMed: 19805574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H et al. (2008) "Re-educating" tumor-associated macrophages by targeting NF-kappaB. J Exp Med 205: 1261-1268. doi: 10.1084/jem.20080108. PubMed: 18490490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meylan E, Dooley AL, Feldser DM, Shen L, Turk E et al. (2009) Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462: 104-107. doi: 10.1038/nature08462. PubMed: 19847165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mazieres J, He B, You L, Xu Z, Jablons DM (2005) Wnt signaling in lung cancer. Cancer Lett 222: 1-10. doi: 10.1016/j.canlet.2004.08.040. PubMed: 15837535. [DOI] [PubMed] [Google Scholar]
- 47. Suganuma M, Okabe S, Kurusu M, Iida N, Ohshima S et al. (2002) Discrete roles of cytokines, TNF-alpha, IL-1, IL-6 in tumor promotion and cell transformation. Int J Oncol 20: 131-136. PubMed: 11743653. [PubMed] [Google Scholar]
- 48. Balkwill F (2002) Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev 13: 135-141. doi: 10.1016/S1359-6101(01)00020-X. PubMed: 11900989. [DOI] [PubMed] [Google Scholar]
- 49. Kleeff J, Kusama T, Rossi DL, Ishiwata T, Maruyama H et al. (1999) Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. Int J Cancer 81: 650-657. doi: 10.1002/(SICI)1097-0215(19990517)81:4. PubMed: 10225458. [DOI] [PubMed] [Google Scholar]
- 50. Ott TR, Lio FM, Olshefski D, Liu XJ, Struthers RS et al. (2004) Determinants of high-affinity binding and receptor activation in the N-terminus of CCL-19 (MIP-3 beta). Biochemistry 43: 3670-3678. doi: 10.1021/bi035895h. PubMed: 15035637. [DOI] [PubMed] [Google Scholar]
- 51. Ma Y, Shurin GV, Gutkin DW, Shurin MR (2012) Tumor associated regulatory dendritic cells. Semin Cancer Biol 22: 298-306. doi: 10.1016/j.semcancer.2012.02.010. PubMed: 22414911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Amedei A, Della Bella C, Silvestri E, Prisco D, D'Elios MM (2012) T cells in gastric cancer: friends or foes. Clin Dev Immunol 2012:690571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N et al. (2003) Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC). Lung Cancer 41: 123-130. doi: 10.1016/S0169-5002(03)00156-9. PubMed: 12871775. [DOI] [PubMed] [Google Scholar]
- 54. Seki Y, Suzuki N, Imaizumi M, Iwamoto T, Usami N et al. (2004) STAT3 and MAPK in human lung cancer tissues and suppression of oncogenic growth by JAB and dominant negative STAT3. Int J Oncol 24: 931-934. PubMed: 15010832. [PubMed] [Google Scholar]
- 55. Bollrath J, Greten FR (2009) IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep 10: 1314-1319. doi: 10.1038/embor.2009.243. PubMed: 19893576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR analysis of Kras allelic recombination. A 500 bp PCR product represents the floxed, unrecombined Kras G12D allele; a 622 bp fragment represents the wildtype Kras allele; and a 650 bp fragment represents a recombined Kras G12D allele after removal of floxed stop cassette by adeno-Cre. K, KI, KP, and KPI mice were treated with adeno-Cre and the 650 bp recombined band revealed. Abbreviations: WT= wildtype lungs. Floxed=floxed Kras G12D, without adeno-Cre treatment. K=Kras G12D. KI=Kras G12D; IL-6-/-. KP=Kras G12D ; p53 flox/flox. KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
IL-6 deletion attenuates tumor proliferation determined by Ki67 staining. (A and B) Representative images of Ki67-stained lung tissue sections from (A) K and (B) KI mice 28 weeks post-infection with adeno-Cre. (C) Quantification of Ki67-positive tumor cells in lung tissue sections of K and KI mice (n=3). **P<0.01. Scale bar indicates 50 μm. Abbreviations: K=Kras G12D . KI=Kras G12D; IL-6 -/-.
(TIF)
PCR analysis of p53 allelic recombination. A 212 bp PCR product represents the floxed, unrecombined p53 allele; a 168 bp fragment represents the recombined allele after inoculation with adeno-Cre; and a 130 bp fragment represents the wildtype p53 allele. K, KI, KP, and KPI mice were treated with adeno-Cre. The 168 bp recombined band was showed in KP and KPI mice and 212 bp fragment remained due to tumor stromal cells. Abbreviations: WT=wildtype lungs. Floxed=floxed p53, without adeno-Cre treatment. K=Kras G12D . KI=Kras G12D; IL-6 -/- . KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIFF)
KP and KPI mice develop metastatic lesions. (A and B) Representative images of metastatic lesions to the (A) pleura, (B) thymus in KPI mice 15 weeks post-infection with adeno-Cre. Dotted lines in the images indicate metastatic tumor edges. Asterisks indicate center of metastatic tumors. (C) Representative image of heart metastases in KP mice 14 weeks post-infection with adeno-Cre. Metastatic lesions in the heart are left of the dotted line. Scale bar indicates 200 μm (A) or 100 μm (B and C). Abbreviations: KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
Real-time PCR screen of changes in inflammatory cytokines levels. .
Three tumors from each genotype were analyzed by real-time PCR without replicate for expression of the indicated cytokine. Gene expression was normalized to β-actin mRNA. *P<0.05 vs. K tumors. # P<0.05 vs. KP tumors. Abbreviations: K=Kras G12D. KI=Kras G12D; IL-6 -/-. KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIF)
Nuclear localization of p65 and β-catenin are unchanged. Tumors from each mouse genotype were lysed to obtain cytoplasmic (C) and nuclear (N) fractions. Lysates were analyzed for the presence of nuclear p65 and β-catenin by Western blot. Fraction purity was determined by GAPDH (cytoplasmic) and PARP (nuclear) blots. Abbreviations: K=Kras G12D . KI=Kras G12D; IL-6 -/- . KP=Kras G12D ; p53 flox/flox . KPI=KrasG12D; p53flox/flox;IL-6 -/-.
(TIFF)
Primers for real-time PCR analysis of gene expression.
(DOCX)