Abstract
Beside a cardinal role in coordination of many developmental processes in the plant, the phytohormone auxin has been recognized as a regulator of plant defense. The molecular mechanisms involved are still largely unknown. Using a sensitive chemiluminescence assay, which measures the oxidation of luminol in the presence of H2O2 by horseradish peroxidase (HRP), we report here on the ability of exogenously added indole-3-acetic acid (IAA) to enhance the suppressive effect of the root endophyte Piriformospora indica on the chitin-elicited oxidative burst in barley roots. Thus, the potential of P. indica to produce free IAA during the early colonization phase in barley might provide the symbiont with a means to interfere with the microbe-associated molecular patterns (MAMP)-triggered immunity.
Keywords: chitin-elicited oxidative burst, IAA, Piriformospora indica, MAMP-triggered immunity, Hordeum vulgare
Introduction
The beneficial root endophyte Piriformospora indica induces growth in many different hosts and is able to produce auxin.1,2 In order to clarify the role played by fungal-derived auxin in the mutualistic interaction with barley, we recently analyzed the P. indica biochemical pathways involved in IAA production.1 We showed elevated levels of free IAA in barley roots during the early biotrophic colonization and that exogenous IAA induced susceptibility to P. indica in this host. Silencing of the piTam1 gene, encoding a tryptophan aminotransferase, via an RNA interference (RNAi) approach resulted in P. indica strains compromised in IAA production and in reduced colonization of barley roots in the biotrophic phase, further supporting the role of IAA in the establishment of P. indica-barley symbiosis. Attenuated IAA production by the P. indica RNAi strains did not hamper growth promotion. The implication of IAA in biotrophic colonization but not in the elicitation of growth promotion prompted us to test the role of IAA in the generation of reactive oxygen species (ROS) and plant defense. ROS are important signaling molecules that are rapidly generated in response to abiotic and biotic stimuli and which regulate diverse physiological processes such as stomatal aperture, root gravitropism and cell death.3-6 Plant peroxidases (POXs) are thought to be one of the mediator of ROS production in plants and beside their important role in plant defense, POXs bound to the cell wall were shown to be involved in cell wall loosening via the production of hydroxyl radicals (OH.) together with NAD(P)H-oxidases which catalyze the formation of superoxide radicals (O2.-) at the plasma membrane.7-11 The ability to produce hydroxyl radicals by exogenous IAA was shown for several plant systems, including the cell suspension culture of Chenopodium rubrum and maize coleoptiles.6,12,13 There is growing evidence that POXs are the mediators of the IAA-induced cell wall loosening.8,10,12,14,15 However, the potential of IAA-elicited ROS production in root defense is unclear. Plants recognize microbial invaders (pathogens or mutualistic symbionts) by detecting conserved microbial structures, so-called microbe-associated molecular patterns (MAMPs) or pathogen-associated molecular patterns (PAMPs), such as fungal chitin. The elicited defense reaction is defined as MAMP/PAMP-triggered immunity (MTI or PTI, respectively). Perception of MAMPs induces an extracellular oxidative burst with production of ROS, which requires the function of the membrane bound NADPH oxidase in Arabidopsis.16 In this report we provide first evidence that IAA potentiates the suppressive effect of P. indica on the oxidative burst leading to the speculation that exogenous application of auxin, e.g., produced by microbes, increases susceptibility via manipulation of the root defense.
Results and Discussion
Combined treatment of barley roots with IAA and P. indica suppresses the chitin-elicited oxidative burst
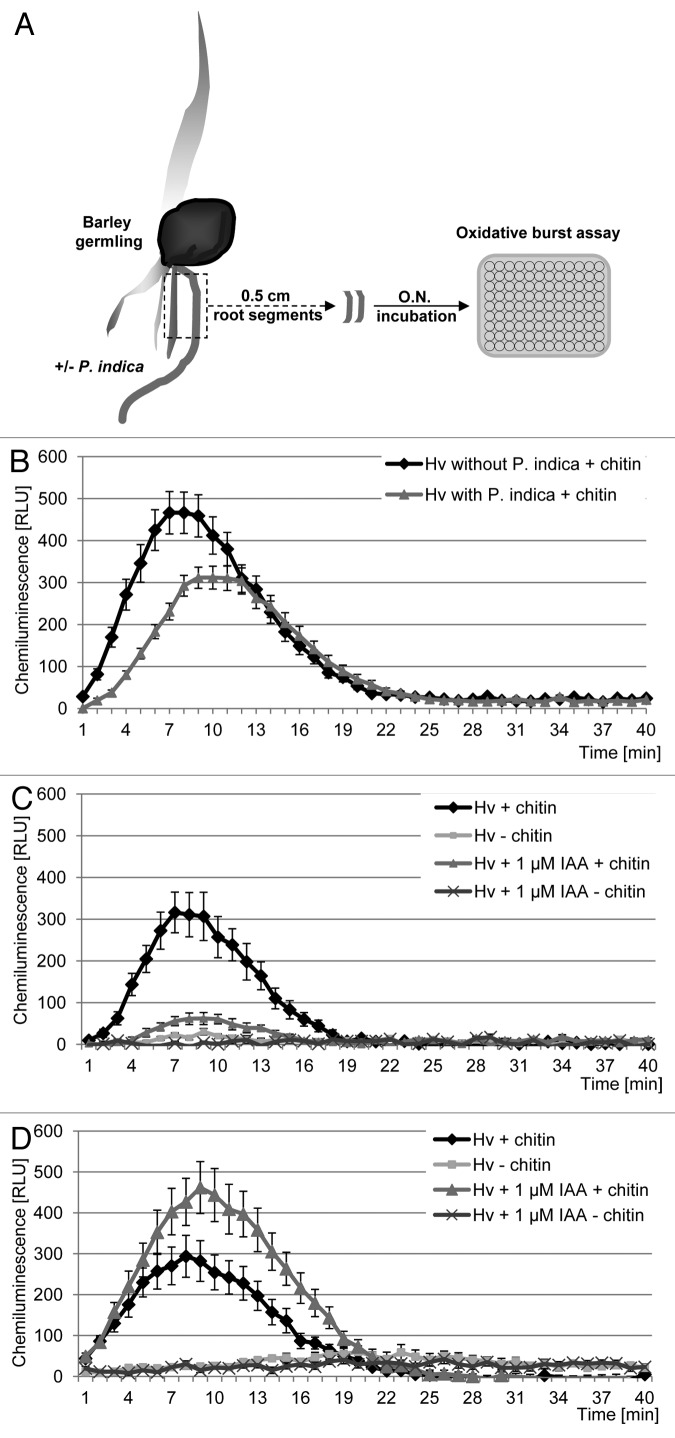
ROS can be easily measured by chemiluminescence with a luminol assay in the presence of a catalyst, such as a peroxidase (e.g., HRP from the roots of horseradish, Fig. 1A). The horseradish peroxidase uses H2O2 in a first reaction step which converts the enzyme into the two-electron oxidized species known as compound I. Compound I reacts with a substrate, e.g., with luminol anion to form a luminol radical and the half-reduced compound II which returns to the native state by oxidizing a second molecule of luminol.10,17
Figure 1. Analyses of chitin-elicited oxidative burst using the luminol-horseradish peroxidase chemiluminescence reaction. (A) Preparation of barley root segments. (B) Effect of P. indica colonization on the chitin-elicited oxidative burst. Error bars represent SE of the mean from 12 biological repetitions. Significant differences in chitin-elicited oxidative burst between P. indica-colonized and mock treated roots are visible (t-testt(0–20) p < 0.05). Three independent replicate experiments were performed with similar results. (C) Effect of IAA on the chitin-elicited oxidative burst in P. indica colonized roots. ROS production was measured after 10 min pre-incubation with or without 1 µM IAA and subsequent elicitation with a chitin solution or with water (control). Error bars represent SE of the mean from 12 biological repetitions. Significant suppression of chitin-elicited oxidative burst in P. indica-colonized roots pretreated with 1 µM IAA is visible (t-testt(0–20) p < 0.01). Two independent replicate experiments were performed with similar results. (D) Effect of IAA on the chitin-elicited oxidative burst in mock treated barley roots. ROS production was measured after 10 min pre-incubation with or without 1 µM IAA and subsequent elicitation with a chitin solution or with water (control). Error bars represent SE of the mean from 12 biological repetitions. Significant enhancement of chitin-elicited oxidative burst in roots pretreated with 1 µM IAA is visible (t-testt(0–20) p < 0.05). Two independent replicate experiments were performed with similar results.
HRP + H2O2 → HRP-I + H2O
HRP-I + LH- → HRP-II + L.- + H2O
HRP-II + LH- → HRP + L.-
Where LH- is the substrate, e.g., luminol, and HRP-I and HRP-II are the intermediates formed from HRP by electron donation from the substrate to form the luminol radical L.-.
It was reported that P. indica can suppress the MAMP-elicited oxidative burst reaction in Arabidopsis and in barley.18,19 In this study we confirm this data and additionally we show that exogenously added IAA enhances P. indica’s ability to suppress the chitin-induced oxidative burst (Fig. 1B and C). IAA alone did not suppress the chitin-induced oxidative burst and pretreatment of the non-inoculated roots with 1 µM IAA in the presence of HRP resulted in a stronger chemiluminescence upon addition of chitin compared with non-IAA treated roots, showing a potentiating effect of the IAA on the luminol-roots-horseradish peroxidase reaction (Fig. 1D). It was reported that IAA can be oxidized by HRP in the presence of O2 yielding IAA cation radicals and O2.- superoxide radicals that rapidly undergoes dismutation in aqueous solution to H2O2.10,14
HRPFe3+ + IAA ↔ [HRP-IAA]
[HRP-IAA] + O2 ↔ [HRP-IAA-O2]
[HRP-IAA-O2] → HRP + IAA.+ + O2.-
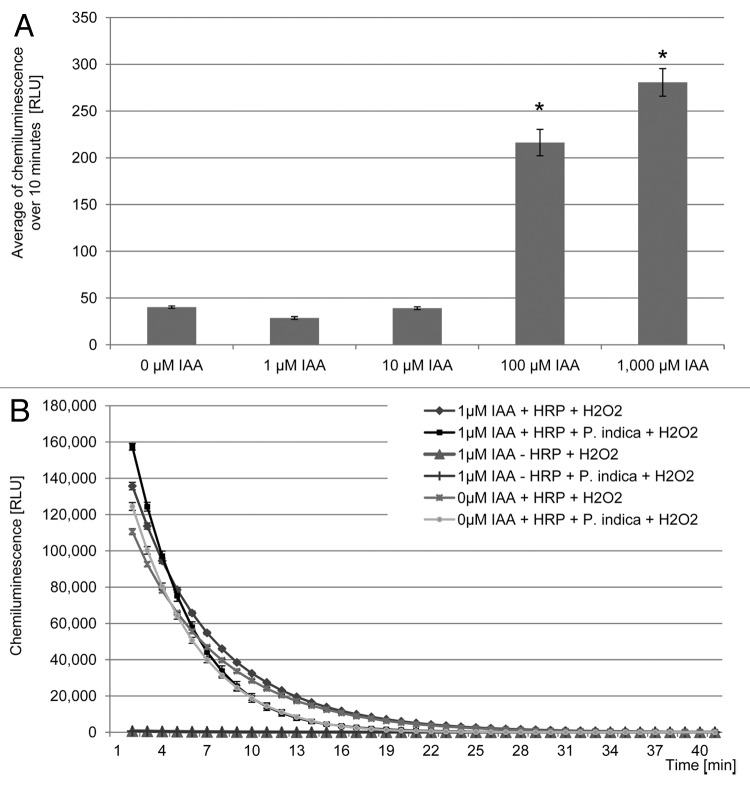
In our assay addition of IAA at the concentration of 1 and 10 µM did not induce an oxidative burst reaction in the roots of barley neither these concentrations of IAA produced chemiluminescence in the luminol-HRP system in the absence of barley roots (Fig. 2A and data not shown). Addition of 100 and 1,000 µM IAA induced the production of a detectable amount of H2O2 in the presence and absence of barley roots, confirming that IAA can act as a substrate for the HRP in an O2-dependent oxidative reaction (Fig. 2A and data not shown). HRP can oxidize IAA via a second known mechanism that requires H2O2 but does not display strict substrate specificity:10,14
Figure 2. Effect of exogenously applied IAA on luminol-horseradish peroxidase chemiluminescence with and without barley roots. (A) Auxin-dependent induction of H2O2 production in barley roots. The values are given as the average of the chemiluminescence measured over 10 min after application of different concentrations of IAA. Error bars represent SE of the mean from 12 biological repetitions. Asterisks represent significant differences (ANOVA *p < 0.01). Two independent replicate experiments were performed with similar results. (B) Effect of P. indica and IAA on HRP activity after H2O2 treatment. Working solutions with and without P. indica germinated chlamydospores were pre-incubated with or without 1 µM IAA for 10 min. Subsequently, 1 µM H2O2 was applied in order to start the HRP-dependent luminol oxidation reaction. Working solutions without HRP were used as controls. Error bars represent SE of the mean from 12 biological repetitions. Two independent replicate experiments were performed with similar results.
HRP + H2O2 → HRP-I + H2O
HRP-I + IAA → HRP-II + P
HRP-II + IAA + H+ → HRP + H2O + P
Where P is the product of one-electron oxidation of IAA. Additionally, it was shown that compounds with radicals with a greater or lower reduction potential than luminol can act as enhancers or inhibitors of chemiluminescence in the luminol-H2O2-HRP system. In particular, phenol derivatives can function as enhancers or inhibitors of the luminol-H2O2-HRP chemiluminescence in a conventional H2O2-dependent HRP cycle:17
HRP + H2O2 → HRP-I + H2O
HRP-I + EH → HRP-II + E· + H2O
HRP-II + EH → HRP + E·
LH- + E· ↔ L.- + EH
Where EH represent the chemiluminescence enhancer and E· is the enhancer radical that convert luminol into the luminol radicals favoring the step of light emission. This leads to the speculation that the indolic compound IAA might also accelerate or retard the formation of luminol radicals. In our study IAA was not able to enhance or suppress the luminol-H2O2-HRP chemiluminescence in the absence of barley roots (Fig. 2B). This suggests that the higher elicited levels of H2O2 in the IAA-pretreated roots following addition of chitin is not due to the H2O2-dependent oxidation of IAA by HRP neither to the production of IAA radicals with higher reduction potential than luminol, but it may represent an auxin priming effect on the roots. The presence of the endophyte in this system overturned the situation and led to the suppression of the chitin-elicited burst in the presence of IAA. In the absence of host roots, P. indica was not able to suppress the luminol-H2O2-HRP chemiluminescence upon pre-incubation with IAA, showing that the IAA-P. indica induced suppression of the oxidative burst reaction in barley roots is not a direct effect of P. indica on IAA, HRP or H2O2 but rather a combined effect of P. indica and barley roots (Fig. 2B). Further analyses are required to clarify if the suppression of the chitin-elicited oxidative reaction by IAA in P. indica colonized roots is a consequence of an increased H2O2 scavenging activity or it resides upstream of H2O2 production in the root. A possible explanation for this might be that upon colonization of barley roots by P. indica a considerable level of fungal-derived peroxidases that can oxidize IAA in the presence of H2O2 but not in the presence of O214 accumulate in the extracellular milieu. These enzymes possibly function as scavenger and prevent the accumulation of the signaling molecule H2O2 upon chitin-elicitation. Under this condition low amount of exogenous IAA would serve as antioxidant and hence provide the symbiont with a means to interfere with barley MAMP-triggered immunity.
Conclusions
Detection of H2O2 via luminol-horseradish peroxidase chemiluminescence in the supernatant of plant leaves and roots is often used to study the effect of microbes and elicitors/effectors on the MAMP-induced oxidative burst reaction. The sensitivity and simplicity of this assay make it attractive for a wide range of biological questions, but it should not be forgotten that in this detection system there are several players, such as HRP, luminol, H2O2, plant cells and the microbes and therefore the effects detected might be multilayered making the interpretation of the data difficult.
Figure 3 summarizes the results of the luminol-HRP assay in the presence of barley roots colonized and non-colonized by P. indica. Although the mechanism of ROS suppression by exogenous IAA on P. indica colonized roots of barley remains unclear, our results indicate a potential role of IAA in chitin-elicited oxidative burst. The success of microbes to invade plants reflects their ability to evade and/or to manipulate the immune response and reprogram host metabolism. Many microbes are known to produce auxin during colonization of the host plant were it interferes with plant developmental processes and defense. In this report we provided some hints on the ability of free IAA to possibly interfere with MAMP-triggered immunity. The characterization of the IAA effects on POXs and on roots will be an important step in the understanding of the mechanism by which microbial-derived IAA influence defense in host plants.
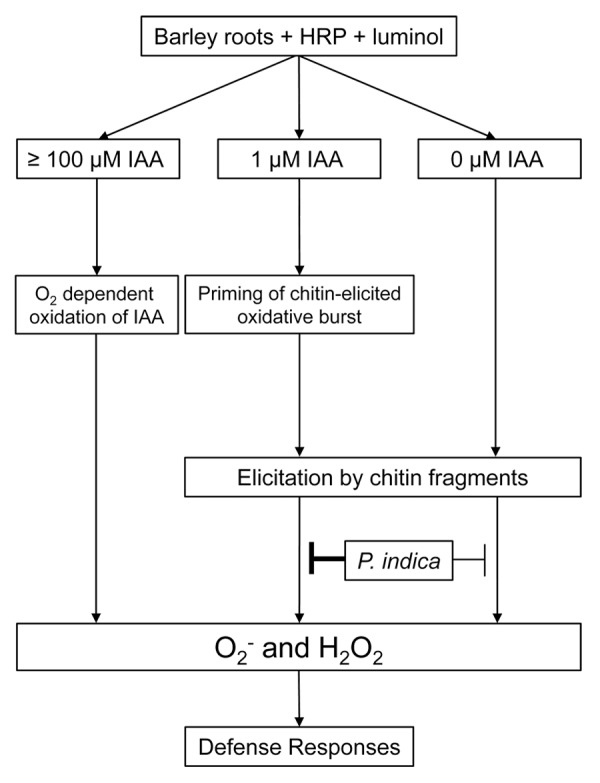
Figure 3. Schematic overview of the influence of IAA and P. indica on the chitin-elicited ROS production in barley roots. Chitin-elicited oxidative burst is suppressed in the presence of P. indica. This effect is potentiated by exogenous application of 1 µM IAA.
Materials and Methods
Oxidative burst assay
Three-day-old barley germlings (Hordeum vulgare L. cv Golden Promise) were inoculated with P. indica (5 × 105 chlamydospores/ml) or mock treated and cultivated on 1/10 PNM medium in sterile jars for 3 d in a growth chamber (Persival AR-95XL) with a day/night cycle of 16/8 h (light intensity: approx. 390 μmol m2/s) and temperature of 22/18°C. For determination of the oxidative burst, the part of the root corresponding to the elongation zone (first 4 cm below the seed) was subjected to a luminol-based assay. The 0.5-cm-long root segments were incubated overnight in H2O and then transferred to a 96-well plate (2 pieces per well, corresponding to approximately 10 mg fresh weight) with 195 µl of working solution [3.4 mg/l Luminol (Sigma); 0.4 mM NaOH and 10 µg/ml horseradish peroxidase (Sigma) in sterile ddH2O] and 5 µl of 0.2 M sodium phosphate buffer pH 8. Luminescence was measured over 40 min after elicitation with 50 µl of chitin solution (approximately 10 mg/ml, Sigma) with Tecan Infinite® PRO 200 microplate multimode reader (Tecan). Effect of exogenous auxin on oxidation of luminol in the presence of barley roots was assessed by application of 1, 10, 100 or 1,000 µM IAA in the working solution. Additionally, influence of 1 µM IAA and/or P. indica germinated chlamydospores on luminol oxidation was examined in the absence of roots. 200 µl of working solution with or without HRP buffered with sodium phosphate was mixed with approximately 3 × 105 P. indica chlamydospores/ml or 50 µl sterile water and pre-incubated 10 min with or without 1 µM IAA. Oxidation of luminol was started by application of 1 µM H2O2. For each assay 12 biological replicates and two to three independent replicate experiments were performed.
Glossary
Abbreviations:
- HRP
horseradish peroxidase
- IAA
indole-3-acetic acid
- MAMP
microbe-associated molecular patterns
- POXs
plant peroxidases
- OH.
hydroxyl radicals
- O2.-
superoxide radicals
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/23572
References
- 1.Hilbert M, Voll LM, Ding Y, Hofmann J, Sharma M, Zuccaro A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012;196:520–34. doi: 10.1111/j.1469-8137.2012.04275.x. [DOI] [PubMed] [Google Scholar]
- 2.Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, et al. Piriformospora indica affects plant growth by auxin production. Physiol Plant. 2007;131:581–9. doi: 10.1111/j.1399-3054.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–90. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang M, Zhang J. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta. 2002;215:1022–30. doi: 10.1007/s00425-002-0829-y. [DOI] [PubMed] [Google Scholar]
- 5.Murata Y, Pei ZM, Mori IC, Schroeder J. Abscisic acid activation of plasma membrane Ca(2+) channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell. 2001;13:2513–23. doi: 10.1105/tpc.010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–60. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O(2)(.-), H(2)O(2), and (.)OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136:3114–23, discussion 3001. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28:679–88. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen SX, Schopfer P. Hydroxyl-radical production in physiological reactions. A novel function of peroxidase. Eur J Biochem. 1999;260:726–35. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–37. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- 11.Kukavica B, Mojovic M, Vuccinic Z, Maksimovic V, Takahama U, Jovanovic SV. Generation of hydroxyl radical in isolated pea root cell wall, and the role of cell wall-bound peroxidase, Mn-SOD and phenolics in their production. Plant Cell Physiol. 2009;50:304–17. doi: 10.1093/pcp/pcn199. [DOI] [PubMed] [Google Scholar]
- 12.Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–8. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer W, Hoftberger M. Oxidative burst in Chenopodium rubrum suspension cells: Induction by auxin and osmotic changes. Physiol Plant. 2001;111:144–50. doi: 10.1034/j.1399-3054.2001.1110203.x. [DOI] [Google Scholar]
- 14.Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, Ruzgas T, Gorton L. Oxidation of indole-3-acetic acid by dioxygen catalysed by plant peroxidases: specificity for the enzyme structure. Biochem J. 1999;340:579–83. doi: 10.1042/0264-6021:3400579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai N, Nevins DJ, Masuda Y. Auxin-induced and hydrogen ion-induced cell-wall loosening and cell extension in avena-coleoptile segments. Plant Cell Physiol. 1977;18:371–80. [Google Scholar]
- 16.Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 17.Navas Díaz A, García Sánchez F, González Garcia JA. Phenol derivatives as enhancers and inhibitors of luminol-H2O2-horseradish peroxidase chemiluminescence. J Biolumin Chemilumin. 1998;13:75–84. doi: 10.1002/(SICI)1099-1271(199803/04)13:2<75::AID-BIO469>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, et al. Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol. 2011;156:726–40. doi: 10.1104/pp.111.176446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatabi B, Molitor A, Lindermayr C, Pfiffi S, Durner J, von Wettstein D, et al. Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica. PLoS ONE. 2012;7:e35502. doi: 10.1371/journal.pone.0035502. [DOI] [PMC free article] [PubMed] [Google Scholar]