Abstract
The purpose of this project was to determine whether bacteriophage can reduce bacterial colonization and biofilm formation on central venous catheter material. Twenty silicone discs were inoculated for 24 h with broth culture of Methicillin sensitive staphylococcus aureus (0.5 McFarland standard). The inoculate was aspirated and discs placed into two equal groups for 24 h: (1) untreated controls; (2) bacteriophage treatment (staphylococcal bacteriophage K, propagated titer > 108). At the completion of the experiment discs were processed for quantitative culture. Statistical testing was performed using the rank sum test. Mean colony forming units (CFU) were significantly decreased in experimental compared with controls (control 6.3 × 105 CFU, experimental 6.7 × 101, P ≤ 0.0001). Application of bacteriophage to biofilm infected central venous catheter material significantly reduced bacterial colonization and biofilm presence. Our data suggests that bacteriophage treatment may be a feasible strategy for addressing central venous catheter staph aureus biofilm infections.
Keywords: central venous catheters, biofilm, phage therapy, staph aureus, bacteriophage k
Introduction
Microorganisms that colonize indwelling catheters have the capability to form biofilms on the catheter surface, which are a microbially derived community of cells embedded in a matrix of extracellular polymeric substances that are irreversibly attached to a living or nonliving substratum. These biofilms form a microenvironment that confers increased antimicrobial resistance to the embedded microorganisms via a variety of mechanisms. Biofilm-associated organisms can also elicit disease processes by detachment of individual cells or aggregates of cells resulting in bloodstream infections, by production of endotoxin, or by providing a niche for the development of antibiotic-resistant organisms.1-3
Approximately 250000 cases of intravascular catheter-related bloodstream infections occur in the United States each year, resulting in a mortality of between 12–25% with an estimated cost of treatment per episode of approximately $25000.3-10 The standard management of catheter-related infection involves decisions regarding removal of the catheter and the administration of appropriate antibiotics. Catheter removal and eventual replacement, raises important practical problems in these patients requiring parenteral nutrition, chemotherapy, and hemodialysis, among others. To avoid catheter removal, strategies for treatment of catheter-related bacteremia with antibiotics administered through the catheter or locked within the catheter lumen have been previously studied. However, these antibiotic catheter salvage protocols are not recommended due to a high failure rate of well over 30% and the serious risk of contributing to the development of antibiotic resistant bacteria. It is widely believed that the high rate of therapy failure is explained in part by the inability of most antibiotics to kill bacteria growing in a biofilm.3,6,9-15
Certain infections now thought to be associated with biofilms, including otitis media, urinary tract infections, periodontitis, and burn infections, have been effectively treated with phage therapy.16-18 Phage therapy has also been proposed to be used against multidrug-resistant bacteria, and may reduce the use of antimicrobial drugs and the spread of antimicrobial resistance.9,15,17-21 Despite the potential advantages of phage therapy, only a few studies have concentrated on its direct application toward biofilm control and treatment. Prior investigations suggest that the application of bacteriophage to indwelling medical devices, such as intravascular catheters, could provide a strategy for the reduction in biofilm formation by clinically relevant bacteria such as Staphylococcus.19,20
The purpose of this study was to evaluate bacteriophage antimicrobial therapy application for eliminating Staphylococcus aureus biofilm on central venous catheter material.
Results
Mean colony forming units (CFU) were significantly decreased in experimental group compared with controls (control 6.3 × 105 CFU, experimental 6.7 × 101, P ≤ 0.0001) (Fig. 1).
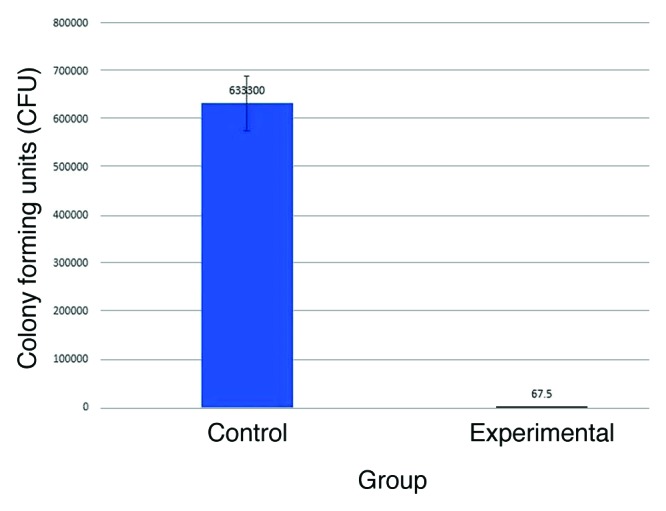
Figure 1. Mean CFU in the experimental group compared with controls.
Discussion
The broad objective of this research was to further investigate an alternative antimicrobial therapy for salvage treatment of long-term central venous catheter-related infection using bacteriophage. We demonstrated a significant decrease in Staphylococcus aureus biofilm formation on the surface of central venous catheter material treated with bacteriophage compared with untreated controls in an in-vitro model. Phage therapy involves the targeted application of bacteriophages that, upon encounter with specific pathogenic bacteria, can infect and kill them. In order to be successful, phage therapy must deliver sufficient phage density in the vicinity of the target bacteria in order to achieve bacterial clearance.21,22 For this reason, concentrations of phage utilized in the current study would be needed to be clinically effective as a treatment for indwelling central venous catheter infections; a catheter “lock” technique, whereby a volume of phage solution equal to that of the catheter lumen is placed into a catheter and the then closed, sealing the solution within the indwelling catehter, has a high likelihood of delivering satisfactorily high concentrations of phage used in this experiment to the target in vivo.
Bacteriophage K, used in the current study, is a polyvalent Staphylococcus phage and capable of lysing 10 different Staphylococcus epidermidis strains and nine different Staphylococcus species including a vancomycin-resistant S. aureus (VRSA) strain and several methicillin-resistant S. aureus (MRSA) strains.23-26 Our study utilized bacteriophage K titers in a bacteriophage lock in the lumen of colonized catheters with concentrations of greater than 108 PFU/ml. Further work is needed to delineate an ideal dwell time, concentration, and systemic response to therapy, and the effectiveness of this approach in-vivo; it is virtually unknown how phage will interact when exposed to the intravenous environment in humans, and research is needed to understand what is likely to be a complex biological interaction. We also acknowledge the additional challenge of bacterial resistance to phage K, which has yet to be shown by this work, may require additional phage treatment strategies. This study is a preliminary step toward the development of a new strategy for treating central venous catheter infections, as bacteriophage therapy has never been reported, to our knowledge, for an intravascular surface.
This study has additional limitations. The small sample size may limit a broad generalization of the findings, though statistical significance was easily achieved. The biofilm in this work was artificially composed entirely of a single strain of S. aureus. However, we chose bacteriophage K as it has a broad spectrum of lytic activity, and particularly against the organisms most commonly found in device associated infections. Further work will be needed to investigate effectiveness of phage therapy on polymicrobial biofilms.
In conclusion, treatment of central venous catheter material with a bacteriophage antimicrobial-lock technique significantly reduced bacterial colonization and biofilm presence in an in-vitro model. Though evidence supporting the use of phages for the treatment of device-associated biofilms in humans is lacking, this and other recent studies involving the interaction of phage and biofilms have shown promise as an alternative therapy for the treatment of biofilm-associated infection and suggests that further investigation is warranted.
Material and Methods
Microorganisms and culture conditions
Staphylococcus aureus 46106, a methicillin-susceptible isolate from an abdominal wound that was negative for toxic shock syndrome toxin and Panton-Valentine leucocidin, obtained from the CDC Clinical and Environmental Laboratory Branch culture collection, was used for growing biofilms. Cultures were stored at −71 °C and subcultured on trypticase soy agar containing 5% sheep’s blood (blood agar) (BD Diagnostics) overnight and grown in Brain Heart Infusion Broth (BD Diagnostics) at 37 °C with shaking to obtain a cell suspension equivalent to a 0.5 MacFarland standard (108 CFU/ml) on the day of use.
Phage strain selection
Staphylococcus aureus phage K and its host strain Staphylococcus aureus (ATCC 19685) were obtained from ATCC. Phage K was propagated using the soft agar overlay technique to titer levels of 108 PFU.27 Crude high titer phage broth cultures were prepared according to Adams28 using Brain Heart Infusion Broth supplemented with 3 mM MgCl2 and 4 mM CaCl2 (added as MgCl2 • 6 H2O and CaCl2 • 2 H2O). Phage broth cultures were filter sterilized (0.2 µ) prior to use.
Experimental approach
Twenty 1 cm2 silicone discs were arranged into separate 3 ml sterile wells filled with 2 mL of 108 CFU/ml log phase culture of S. aureus 46106. The inoculum remained in place for 24 h. After 24 h the inoculum was carefully withdrawn from all wells by aspiration. The 20 discs were randomized to treatment or control arms. Following 24 h of bacterial inoculation, 10 control-arm discs were bathed in sterile phosphate buffered saline (PBS) and 10 experimental-arm discs which were bathed in 2 mL of a 108 plaque forming units/ml (PFU/ml) solution of phage K, prepared as described above, for 24 h.
Silicone discs were removed for microbiological evaluation using aseptic technique, and processed to recover microorganisms using a modified previously published method.20 Briefly the discs were rinsed gently in sterile PBS and placed into a tube containing 10 ml of PBS and subjected to three alternating 30 s cycles of water bath sonication (45 kHZ, Branson Water Bath Sonicator) and vortexing. The resulting biofilm suspension was diluted in Butterfield buffer, spread plated on Blood agar, incubated for 48 h at 37 °C and counted.
Statistical analysis
All statistical tests were performed using the Statistical Package for Social Sciences version 19 (SPSS). Mean plate counts (as log10 CFU per cm2) for treated and untreated discs were compared using the Mann-Whitney U Test. A P value of < 0.05 was considered statistically significant.
Acknowledgments
This study was funded in part by grants from the Society of Interventional Radiology and the American Society for Parenteral and Enteral Nutrition (ASPEN).
Citation: Christensen D, Kankotia R, Falk I, Paxton B, Kim C, Lungren M. Bacteriophage K for reduction of Staphylococcus aureus biofilm on central venous catheter material. Bacteriophage 2013; 3:e26825; 10.4161/bact.26825
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/26825
References
- 1.Kay MK, Erwin TC, McLean RJ, Aron GM. Bacteriophage ecology in Escherichia coli and Pseudomonas aeruginosa mixed-biofilm communities. Appl Environ Microbiol. 2011;77:821–9. doi: 10.1128/AEM.01797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreira JA, Carr JH, Starling CE, de Resende MA, Donlan RM. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob Agents Chemother. 2009;53:4377–84. doi: 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicalini S, Palmieri F, Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter-related infections. Crit Care. 2004;8:157–62. doi: 10.1186/cc2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segarra-Newnham M, Martin-Cooper EM. Antibiotic lock technique: a review of the literature. Ann Pharmacother. 2005;39:311–8. doi: 10.1345/aph.1E316. [DOI] [PubMed] [Google Scholar]
- 5.Bestul MB, Vandenbussche HL. Antibiotic lock technique: review of the literature. Pharmacotherapy. 2005;25:211–27. doi: 10.1592/phco.25.2.211.56947. [DOI] [PubMed] [Google Scholar]
- 6.Snaterse M, Rüger W, Scholte Op Reimer WJ, Lucas C. Antibiotic-based catheter lock solutions for prevention of catheter-related bloodstream infection: a systematic review of randomised controlled trials. J Hosp Infect. 2010;75:1–11. doi: 10.1016/j.jhin.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Carratalà J. The antibiotic-lock technique for therapy of ‘highly needed’ infected catheters. Clin Microbiol Infect. 2002;8:282–9. doi: 10.1046/j.1469-0691.2002.00388.x. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J Antimicrob Chemother. 2009;64:88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 9.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002;61:1136–42. doi: 10.1046/j.1523-1755.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 10.Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dündar Y, Gamble C, McLeod C, Walley T, Dickson R. The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii–iv, xi-xii, 1-154. doi: 10.3310/hta12120. [DOI] [PubMed] [Google Scholar]
- 11.Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis. 2004;190:1498–505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 12.Capdevila JA, Gavaldà J, Fortea J, López P, Martin MT, Gomis X, Pahissa A. Lack of antimicrobial activity of sodium heparin for treating experimental catheter-related infection due to Staphylococcus aureus using the antibiotic-lock technique. Clin Microbiol Infect. 2001;7:206–12. doi: 10.1046/j.1469-0691.2001.00233.x. [DOI] [PubMed] [Google Scholar]
- 13.Bassetti S, Hu J, D’Agostino RB, Jr., Sherertz RJ. Prolonged antimicrobial activity of a catheter containing chlorhexidine-silver sulfadiazine extends protection against catheter infections in vivo. Antimicrob Agents Chemother. 2001;45:1535–8. doi: 10.1128/AAC.45.5.1535-1538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Wetering MD, van Woensel JB. Prophylactic antibiotics for preventing early central venous catheter Gram positive infections in oncology patients. Cochrane Database Syst Rev. 2007:CD003295. doi: 10.1002/14651858.CD003295.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Miedzybrodzki R, Fortuna W, Weber-Dabrowska B, Górski A. Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment. Postepy Hig Med Dosw (Online) 2007;61:461–5. [PubMed] [Google Scholar]
- 16.Weber-Dabrowska B, Mulczyk M, Górski A. Bacteriophage therapy of bacterial infections: an update of our institute’s experience. Arch Immunol Ther Exp (Warsz) 2000;48:547–51. [PubMed] [Google Scholar]
- 17.Weber-Dabrowska B, Dabrowski M, Slopek S. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch Immunol Ther Exp (Warsz) 1987;35:563–8. [PubMed] [Google Scholar]
- 18.Slopek S, Weber-Dabrowska B, Dabrowski M, Kucharewicz-Krukowska A. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch Immunol Ther Exp (Warsz) 1987;35:569–83. [PubMed] [Google Scholar]
- 19.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother. 2010;54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JJ, Donlan RM. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob Agents Chemother. 2006;50:1268–75. doi: 10.1128/AAC.50.4.1268-1275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1:66–85. doi: 10.4161/bact.1.2.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol. 2010;11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 23.Kutter E, Sulakvelidze A. Bacteriophages biology and applications, Boca Raton, FL, CRC Press, 2005. [Google Scholar]
- 24.O’Flaherty S, Ross RP, Meaney W, Fitzgerald GF, Elbreki MF, Coffey A. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl Environ Microbiol. 2005;71:1836–42. doi: 10.1128/AEM.71.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholl D, Rogers S, Adhya S, Merril CR. Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J Virol. 2001;75:2509–15. doi: 10.1128/JVI.75.6.2509-2515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerca N, Oliveira R, Azeredo J. Susceptibility of Staphylococcus epidermidis planktonic cells and biofilms to the lytic action of staphylococcus bacteriophage K. Lett Appl Microbiol. 2007;45:313–7. doi: 10.1111/j.1472-765X.2007.02190.x. [DOI] [PubMed] [Google Scholar]
- 27.Obuchowski M, Stopa M. Quick and efficient method for recovering of bacteriophages from soft agar after their propagation by the plate lysate technique. Acta Biochim Pol. 1993;40:98–9. [PubMed] [Google Scholar]
- 28.Adams MH. The stability of bacterial viruses in solutions of salts. J Gen Physiol. 1949;32:579–94. doi: 10.1085/jgp.32.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]


