Abstract
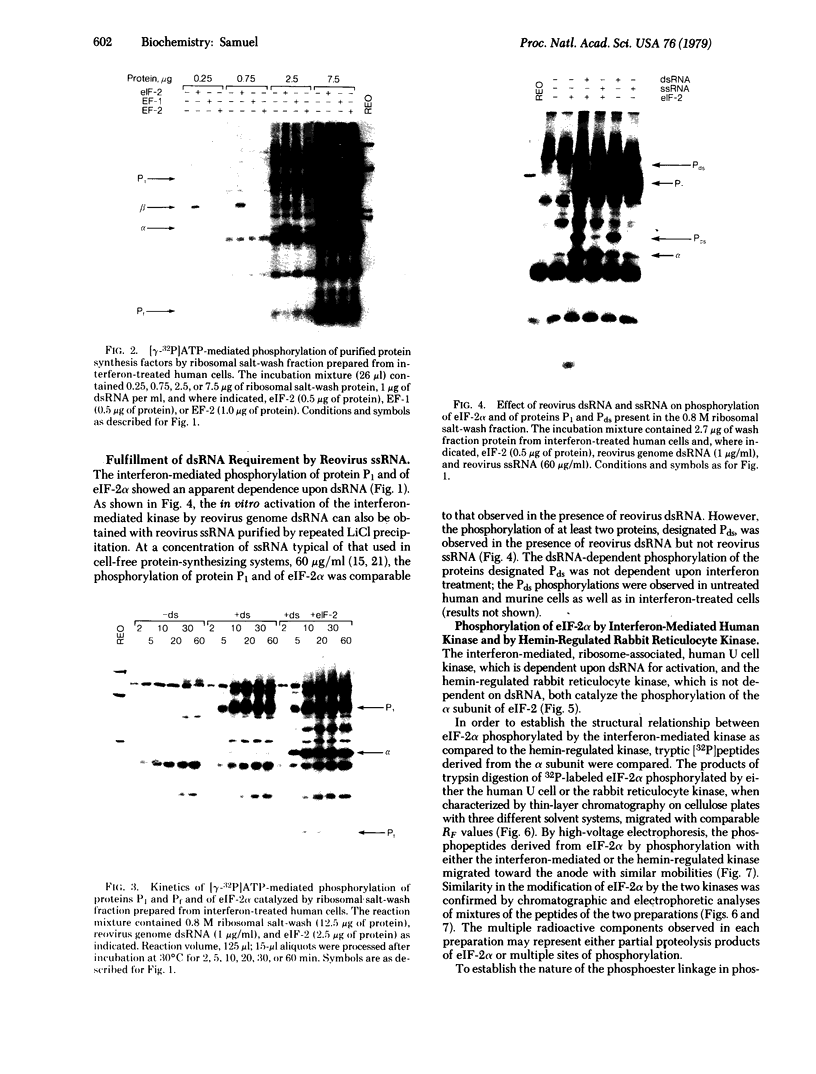
The phosphorylation of purified protein synthesis factors catalyzed by protein kinase preparations isolated from interferon-treated human amnion cells was examined. Ribosomal salt-wash fractions prepared from interferon-treated human cells contained a protein kinase that catalyzed the [γ-32P]ATP-mediated phosphorylation of the 38,000-dalton subunit of eukaryotic initiation factor 2 (eIF-2α); this kinase activity was significantly enhanced in interferon-treated as compared to untreated cells. The tryptic [32P]phosphopeptide pattern obtained for eIF-2α phosphorylated by the interferon-mediated human kinase was indistinguishable from the pattern obtained for eIF-2α phosphorylated by the hemin-regulated rabbit reticulocyte kinase when analyzed by thin-layer chromatography with three different solvent systems and by high-voltage electrophoresis. O-[32P]Phosphoserine was liberated by partial acid hydrolysis from eIF-2α phosphorylated by either the human or the rabbit kinase. In addition to the phosphorylation of eIF-2α, interferon treatment of human cells enhanced the phosphorylation of two additional ribosome-associated proteins designated P1 and Pf. The major phosphoester linkage observed for the human, as well as murine, phosphoprotein P1 was O-phosphoserine. The interferon-mediated phosphorylation of both eIF-2α and protein P1 was dependent upon the presence of RNA with double-stranded character; Pf phosphorylation was not affected by double-stranded RNA. These results suggest that the interferon-mediated ribosome-associated human protein kinase catalyzes the phosphorylation of eIF-2α in a site-specific manner that is apparently identical with the reaction catalyzed by the hemin-regulated rabbit reticulocyte kinase; hence, the phosphorylation of eIF-2 may play a role in regulating the initiation of translation in interferon-treated cells.
Keywords: translational control, double-stranded RNA, reovirus, antiviral agents
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Effect of interferon pretreatment on coupled transcription and translation in cell-free extracts of primary chick embryo cells. Virology. 1978 Feb;84(2):496–508. doi: 10.1016/0042-6822(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. E., Lebleu B., Kawakita M., Shaila S., Sen G. C., Lengyel P. Increased endonuclease activity in an extract from mouse Ehrlich ascites tumor cells which had been treated with a partially purified interferon preparation: dependence of double-stranded RNA;. Biochem Biophys Res Commun. 1976 Mar 8;69(1):114–122. doi: 10.1016/s0006-291x(76)80280-x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., Lebleu B., Nudel U., Zilberstein A., Berissi H., Revel M. Blocks in elongation and initiation of protein synthesis induced by interferon treatment in mouse L cells. Eur J Biochem. 1975 May;54(1):1–10. doi: 10.1111/j.1432-1033.1975.tb04106.x. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Farrell P. J. Extracts of interferon-treated cells can inhibit reticulocyte lysate protein synthesis. Biochem Biophys Res Commun. 1977 Jul 11;77(1):124–131. doi: 10.1016/s0006-291x(77)80173-3. [DOI] [PubMed] [Google Scholar]
- Datta A., de Haro C., Sierra J. M., Ochoa S. Role of 3':5'-cyclic-AMP-dependent protein kinase in regulation of protein synthesis in reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1463–1467. doi: 10.1073/pnas.74.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein D. A., Samuel C. E. Mechanism of interferon action. Properties of an interferon-mediated ribonucleolytic activity from mouse L929 cells. Virology. 1978 Aug;89(1):240–251. doi: 10.1016/0042-6822(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Falcoff R., Falcoff E., Sanceau J., Lewis J. A. Influence of preincubation on the development of the inhibition of protein synthesis in extracts from interferon-treated mouse L cells. Action on tRNA. Virology. 1978 May 15;86(2):507–515. doi: 10.1016/0042-6822(78)90089-2. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. I. Patterns of gene expression by mutants of groups C, D, and E. Virology. 1972 Oct;50(1):189–201. doi: 10.1016/0042-6822(72)90359-5. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuler A. M. Reovirus core transcriptase and ethidium bromide: a continuous fluorimetric assay for polynucleotide synthesis based on the secondary structure of mRNA. Biochim Biophys Acta. 1971 May 13;238(2):363–368. doi: 10.1016/0005-2787(71)90105-5. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Friedman R. M., Brown R. E., Ball L. A., Brown J. C. Inhibition of Protein Synthesis in Cell-Free Systems from Interferon-Treated, Infected Cells: Further Characterization and Effect of Formylmethionyl-tRNA(F). J Virol. 1974 Jan;13(1):9–21. doi: 10.1128/jvi.13.1.9-21.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J. R., Baglioni C. Inhibition of protein synthesis by double-stranded RNA and phosphorylation of initiation factor, eIF-2. J Biol Chem. 1978 Jun 25;253(12):4219–4223. [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U., Parajsz C., Jungwirth C., Bodo G. Interferon-induced translation defects in a cell-free protein-synthesizing system from mouse erythroleukemia cells. Virology. 1978 Jul 1;88(1):54–61. doi: 10.1016/0042-6822(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Metz D. H. Interferon and interferon inducers. Adv Drug Res. 1975;10:101–156. [PubMed] [Google Scholar]
- Ohtsuki K., Dianzani F., Baron S. Decreased initiation factor activity in mouse L cells treated with interferon. Nature. 1977 Oct 6;269(5628):536–538. doi: 10.1038/269536a0. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M., Das A., Dasgupta A., Majumdar A., Ralston R., Roy R., Gupta N. K. Regulation of protein synthesis in rabbit reticulocyte lysates by the heme-regulated protein kinase: inhibition of interaction of Met-tRNAfMet binding factor with another initiation factor in formation of Met-tRNAfMet.40S ribosomal subunit complexes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):745–749. doi: 10.1073/pnas.75.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Farris D. A. Mechanism of interferon action. Species specificity of interferon and of the interferon-mediated inhibitor of translation from mouse, monkey, and human cells. Virology. 1977 Apr;77(2):556–565. doi: 10.1016/0042-6822(77)90481-0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action. Studies on the mechanism of interferon-mediated inhibition of reovirus messenger RNA translation in cell-free protein synthesis systems from mouse ascites tumor cells. Virology. 1976 Nov;75(1):166–176. doi: 10.1016/0042-6822(76)90015-5. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A., Sharp S. B., Lundak T. S., Safer B., Merrick W. C. Effect of hemin on site-specific phosphorylation of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):789–793. doi: 10.1073/pnas.75.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Dudock B., Berissi H., Revel M. Control of messenger RNA translation by minor species of leucyl-transfer RNA in extracts from interferon-treated L cells. J Mol Biol. 1976 Nov;108(1):43–54. doi: 10.1016/s0022-2836(76)80093-9. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]