Abstract
Insulin acts in the hypothalamus, decreasing food intake (FI) by the IR/PI3K/Akt pathway. This pathway is impaired in obese animals and endoplasmic reticulum (ER) stress and low-grade inflammation are possible mechanisms involved in this impairment. Here, we highlighted the amygdala as an important brain region for FI regulation in response to insulin. This regulation was dependent on PI3K/AKT pathway similar to the hypothalamus. Insulin was able to decrease neuropeptide Y (NPY) and increase oxytocin mRNA levels in the amygdala via PI3K, which may contribute to hypophagia. Additionally, obese rats did not reduce FI in response to insulin and AKT phosphorylation was decreased in the amygdala, suggesting insulin resistance. Insulin resistance was associated with ER stress and low-grade inflammation in this brain region. The inhibition of ER stress with PBA reverses insulin action/signaling, decreases NPY and increases oxytocin mRNA levels in the amygdala from obese rats, suggesting that ER stress is probably one of the mechanisms that induce insulin resistance in the amygdala.
Keywords: Insulin, Amygdala, Phosphatidylinositol 3-kinase, Obesity, Inflammation, Endoplasmic reticulum stress, NPY, Oxytocin
Abbreviations: AGRP, agouti-related peptide; AMY, amygdala; BW, body weight; JNK, c-Jun N-terminal kinase; CNS, central nervous system; CRH, corticotrophin-releasing hormone; ER, endoplasmic reticulum; FKBP51, FK506 binding protein 51; FI, food intake; HFD, high-fat diet; HPRT, hypoxanthine phosphoribosyl transferase; IKKβ, I kappa B kinase; IRE1α, inositol-requiring kinase alpha; IR, insulin receptor; IRS-1, insulin substrate 1; LGI, low-grade inflammation; NPY, neuropeptide Y; PBA, 4-phenyl butyric acid; PI3K, phosphoinositide 3-kinase; PKB or Akt, protein kinase B; PERK, RNA-activated protein kinase-like ER resident kinase
Highlights
-
•
Lower food intake in response to insulin in the amygdala is dependent on the PI3K/Akt pathway.
-
•
Insulin decreases NPY and increases oxytocin mRNA levels via PI3K in vivo.
-
•
Insulin receptor and Akt phosphorylation in the amygdala are disrupted in obese rats.
-
•
Insulin resistance, ER stress and inflammation are present in the amygdala of obese rats.
-
•
The inhibition of ER stress with PBA reverses insulin resistance in the amygdala from obese rats.
1. Introduction
Insulin is an important peptide hormone that regulates the brains control of food intake and energy expenditure [1–3]. Deletion of the insulin receptor (IR) in the central nervous system (CNS) of mice induced obesity and altered metabolism in vivo [3,4]. Most studies consider the hypothalamus as the main region in the CNS that regulates energetic metabolism in response to insulin. However, insulin has effects in other brain regions, such as the ventral tegmental area, substantia nigra, and amygdala [5–10].
In the hypothalamus, insulin acts through IR inducing insulin substrate 1 (IRS-1) tyrosine phosphorylation. IRS-1 tyrosine phosphorylation activates phosphoinositide 3-kinase (PI3K), which is required for the effects of insulin on feeding [11]. PI3K phosphorylates generates phosphatidylinositol-3,4,5-triphosphate [3–5], which activates protein kinase B (PKB or Akt). The effect of IR/PI3K/Akt pathway on food intake is well described in the hypothalamic nuclei [1,11]. In arcuate nucleus, insulin inhibits transcription of agouti-related peptide (AGRP) and neuropeptide Y (NPY), which are orexigenic neuropeptides [1]. On the other hand, hyperinsulinemia increases corticotrophin-releasing hormone (CRH) mRNA expression in the paraventricular nucleus of hypothalamus [12] which may contribute to induce hypophagia [13]. However, it has not been investigated if, in the amygdala, insulin may regulate IR/PI3K/Akt, and whether this pathway might modulate the expression of neuropeptides in physiologic conditions.
In obese animal models insulin-induced IR/PI3K/Akt pathway is impaired in the hypothalamus. Endoplasmic reticulum (ER) stress and low-grade inflammation (LGI) are possible molecular mechanisms involved in this impairment. Both ER stress and LGI activate serine kinases such as c-Jun N-terminal kinase (JNK) and I kappa B kinase (IKKβ) which induce inhibitory IRS-1 serine 307 phosphorylation trigging hypothalamic insulin resistance in obese states [1,14]. However, whether a high fat diet induces LGI and/or ER stress in the amygdala is not known.
Thus, the aim of the present study was to investigate whether insulin activates IR/PI3K/Akt pathway in the amygdala and whether this activation controls feeding and neuropeptides expression. In addition, we aimed to investigate whether a high fat diet induces ER stress and LGI in parallel to insulin resistance in the amygdala.
2. Materials and methods
2.1. Material
Eight week old male Wistar rats were obtained from Central Breeding Center of the State University of Campinas, São Paulo, Brazil. Human recombinant insulin was from Eli Lilly and Co. (Indianapolis, IN, USA). Routine reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless specified elsewhere. Antibodies against beta-actin (sc376421), phospho-IR (sc25103), phospho-JNK (sc55642), phospho-IKKα/β (sc21660) and phospho-PERK (sc32577) were from Santa Cruz Biotechnology (California, USA). Phospho-IRE1ser724 (ab48187) was from Abcam (Massachussetts, USA). Phospho-Aktser473 (9271S) was from Cell Signaling Technology (Massachussetts, USA). FKBP51 (Q13451) was from Enzo Life Science (New York, USA).
2.2. Animal characterization
All experiments were approved by the Ethics Committee of the State University of Campinas. Eight week old male Wistar rats were maintained in cycles of 12 h dark/light at 21 ºC. Animals were randomly divided into two groups with similar body weights (BW) (280 ± 4 g) according to the diet. Standard rodent chow (chow) (70% carbohydrate, 20% protein and 10% fat) and a high-fat diet (HFD) (55% saturated fat (lard), 29% carbohydrates and 16% protein) from Nuvilab (Sogorb Industria e Comércio Ltda, Brazil) were used for 2 months as previously described [14,15]. Food and water were available ad libitum.
2.3. ITT (insulin tolerance test)
Awakened fasted rats were submitted to insulin tolerance test. Briefly, 1.5 IU/kg of insulin was injected intraperitoneally and glycemia was measured at 0, 5, 10, 15, 20, 25, and 30 min thereafter [15].
2.4. Food intake measurements
BW was measured 24 h after insulin injection in the amygdala. Food intake was recorded in a metabolic cage (Tecniplast 3701M081, Buguggiate, VA, Italy) for 4, 8, 12 and 24 h after insulin injection in the amygdala.
2.5. Cannula implantation
Free fed rats were anesthetized with 1 mg/kg IP injections of a mixture of 70 mg/kg ketamine (Fort Dodge Animal Health, USA) and 2 mg/kg xylazine (Lloyd Laboratories, USA) and placed in a stereotaxic instrument (Ultra Precise – model 963 – Kopf). Briefly, rats were implanted with unilateral cannulas (26-gauge stainless-steel guide cannula) (Plastics One, USA) aimed to the central nucleus of the amygdala (CeA): [coordinates (AP/L/DV to bregma) −2.16/−4.00/−7.18 mm] according to Paxinos and Watson and pilots experiments. Cannulas were fixed using two screws, special glue and acrylic cement. BW was monitored daily for 5–7 days following the surgery. We performed a pilot experiment to confirm the site of the cannulation. Briefly, rats received 2 μL injection of methylene blue dye. Rats were killed immediately after the injection, and brains were collected on ice. Brains were sectioned 1 mm in a coronal stainless steel matrix with razor blades to check the position of the cannula and the site of injection under microscopy. To further confirm the dissections we re-blotted the membranes from immunoblotting experiments with an antibody against to co-chaperone FK506 binding protein 51 (FKBP51). This protein is expressed in the CeA and is not expressed in the striatum which is a close region to the amygdala.
2.6. Insulin injections
After overnight fast cannulated rats received insulin (2 μL) or saline (2 μL) injections between 0700 and 0900 h for tissues collections or food intake measurements. To inhibit PI3K we used LY-294002 (50 μM from Calbiochem) or its vehicle (5% DMSO in saline) [16,17].
2.7. Tissue collection for immunoblotting
Rats have been fasted for 12 h and then received insulin or saline injection. After 15 min the amygdala was quickly dissected in a stainless-steel matrix with razor blades on ice [9,10]. A pool of 5 rats per sample and four samples (n = 4) per group was used. Samples were immediately homogenized in buffer (1% Triton X-100, 100 mM Tris, pH 7.4, 100 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mM phenylmethanesulphonylfluoride (PMSF) and 0.1 mg/ml aprotinin at 4 °C. The immunoblotting was performed as described before [14–17].
2.8. RNA isolation and real time-PCR
Rats have been fasted for 12 h and received LY-294002 or vehicle injection into the amygdala 1 h prior of insulin or saline injections. Twelve hours later rats were killed by decapitation and fresh AMY was quickly collected on a stainless-steel matrix with razor blades on ice. Total RNA was isolated using RNeasy® Mini Kit (Cat. 74106, Qiagen Inc., CA, USA). Relative quantitative RT-PCR was performed using TaqMan RT-PCR Master Mix (Applied Biosystems) in an Mx3000P thermocycler (Stratagene). The Mx3000P software was used to calculate the cycle threshold for each reaction. Relative expression levels were determined using the comparative Ct method with normalization of target gene expression levels to hypoxanthine phosphoribosyl transferase (HPRT). Primers and probes sequences were purchased from Applied Biosystems and were: Oxytocin (Rn00564446-g1), neuropeptide Y (NPY) (Rn01410145_m1) and Crh (Rn01462137-m1), HPRT (Rn01527840_m1) for rat. The PCR conditions were 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 60 s. Real time data were analyzed using the engine provided by Applied Biosystems.
2.9. 4-Phenyl butyric acid (PBA) treatment
Rats fed a HFD were implanted with cannulas in the amygdala as described before. After the recovery period (5–7 days), they were divided into two groups: vehicle and PBA treated rats. Chemical chaperone PBA (1 μg/kg of body weight) was dissolved in 4.6 mg/ml of DMSO into 1 mL of 0.9% saline and administered twice daily by gavage for 7 days as previously described [18,19]. The vehicle group received the same amount of DMSO diluted in saline. Following the PBA or vehicle treatment, body weight, insulin action and signaling, ER stress, NPYand oxytocin mRNA levels were measured.
2.10. Statistical analysis
Data are expressed as means ± SEM of the number of independent experiments indicated. For statistical analysis, groups were compared using a two tailed t-test. The level of significance adopted was p < 0.05.
3. Results
3.1. Food intake is decreased and IR and Akt phosphorylation are increased in response to insulin in the amygdala of rats on chow
Insulin injection in the amygdala did not alter 24 h-body weight of rats fed with standard chow (Fig. 1A). To evaluate whether insulin in the amygdala affects food intake, we evaluated food intake 4, 8, 12 and 24 h after the hormone administration. Insulin injected in the amygdala did not decrease food ingestion after 4 h. However, food intake was lower in response to insulin after 8, 12 and 24 h in rats on chow (Fig. 1B). IR tyrosine phosphorylation and Akt serine phosphorylation were increased in response to insulin injected in the amygdala compared to saline injected rats (Fig. 1C and D). In order to confirm whether the dissections of the amygdala were corrected, we re-blotted the membranes with anti-co-chaperone FK506 binding protein 51 (FKBP51) antibody. We observed the presence of FKBP51 in our membranes, indicating that the dissections of the amygdala were appropriate (Fig. 1C). FKBP51 is expressed in the amygdala but not in striatum (Fig. 1E).
Fig. 1.
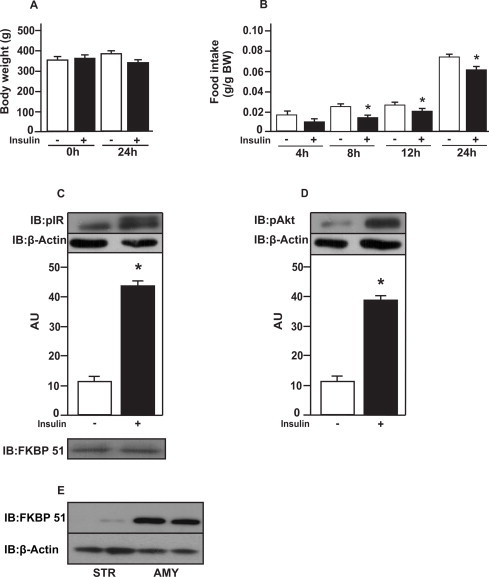
Food intake and insulin signaling in amygdala from rats on chow. (A) body weight (BW) after insulin injections; (B) food intake in g/g of BW in response to insulin (2 μL) injected in amygdala; (C) insulin receptor (IR); (D) protein kinase B (PKB or Akt) phosphorylation in response to insulin; and (E) protein expression of co-chaperone FK506 binding protein 51 (FKBP51) in amygdala (AMY) and striatum (STR). Data are means ± SD from 10 rats per group. To performed immunoblotting (IB) of amygdala (C and D) We used a pool of 5 rats per sample and four samples (n = 4) per group. We used β-actin as a loading control. FKBP51 was blot to confirm AMY dissections. Two tailed T test was used. *P < 0.05 versus saline injected rat.
3.2. Insulin decreases food intake and increases Akt phosphorylation in the amygdala via PI3K
Insulin plus LY or vehicle injections in the amygdala did not alter 24 h-body weight of rats fed with standard chow (Fig. 2A). To evaluate whether insulin in the amygdala affects food intake is dependent of PI3K, we evaluated food intake 8 h after the hormone administration with prior LY or vehicle treatment. As expected, food intake was lower in response to insulin after 8 h in rats on chow. However, the administration of LY prior to insulin in the amygdala abolished this effect (Fig. 2B). Akt serine phosphorylation was increased in response to insulin in the amygdala compared to saline injected rats. The administration of LY prior to insulin in the amygdala impaired Akt phosphorylation in response to insulin (Fig. 2C). We observed the presence of FKBP51 in our membranes, indicating that the dissections of the amygdala were appropriate (Fig. 2C).
Fig. 2.
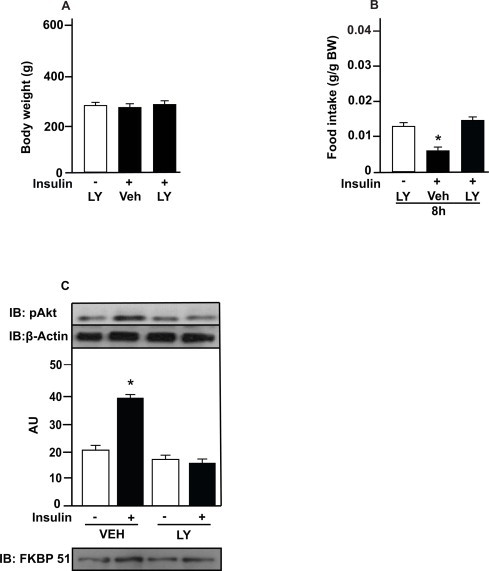
Insulin decreases food intake via PI3K in the amygdala. (A) body weight (BW) after insulin (2 μg), and LY (294002, 50 μM) injections; (B) food intake in g/g of BW in response to insulin (2 μg) or saline with prior injection of LY or vehicle (5% DMSO in saline) in amygdala; and (C) protein kinase B (PKB or Akt) phosphorylation in response to insulin with prior injection of LY or vehicle in amygdala. Data are presented as means ± SD from 10 rats per group. To performed immunoblotting (IB) of amygdala (C) we used a pool of 5 rats per sample and four samples (n = 4) per group. We used β-actin as a loading control. FKBP51 was blot to confirm AMY dissections. Two tailed T test was used. *P < 0.05 versus other groups; *P < 0.05 versus saline injected rats. Veh: vehicle.
3.3. Insulin modulates NPY and oxytocin gene expression in the amygdala
In order to gain insight regarding molecules regulated by insulin in the amygdala, we investigated weather insulin regulates neuropeptides expression in the amygdala from control rats. Insulin decreased NPY mRNA expression and increased oxytocin mRNA expression in the amygdala from chow group. LY injection blunted those effects (Fig. 3A and B). CRH mRNA expression was not different between the groups (Fig. 3C).
Fig. 3.
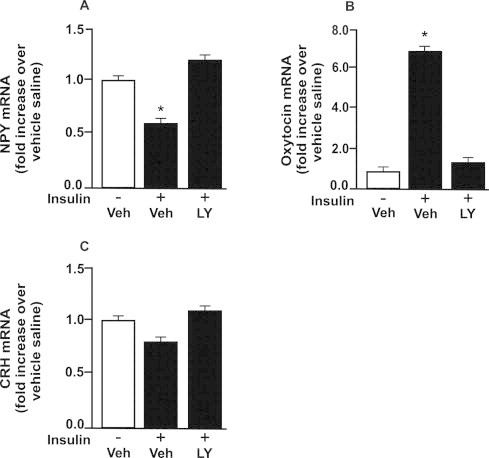
Insulin modulates NPY and oxytocin expression in amygdala. Fasted control rats were injected with insulin (2 μg) or saline with prior injection of LY or vehicle (5% DMSO in saline) in amygdala. (A) neuropeptide Y (NPY); (B) oxytocin and (C) corticotropin-releasing hormone (CRH) mRNA levels in amygdala. To perform RT-PCR of amygdala we used a pool of 5 rats per sample and 4 samples per group (n = 4). One-Way ANOVA was used. *P < 0.05 versus other groups. Veh: vehicle.
3.4. Insulin action and signaling in the amygdala are impaired in rats fed with HFD
Body weight was increased in rats fed with HFD compared to control rats (Fig. 4A). Insulin tolerance test showed that obese rats were insulin resistant compared to control rats (Fig. 4B). To evaluate whether HFD induces insulin resistance in the amygdala, we evaluated food intake 4, 8, 12 and 24 h in response to insulin or saline. Insulin injected in the amygdala did not decrease food intake after 4, 8, 12, 24 h in obese rats (Fig. 4C). IR tyrosine phosphorylation and Akt serine phosphorylation were increased in response to insulin in the amygdala in control rats compared to saline injected rats. However, this effect was blunted in rats on HFD (Fig. 4D and E). We observed the presence of FKBP51 in our membranes, indicating that the dissections of the amygdala were appropriate (Fig. 4E).
Fig. 4.
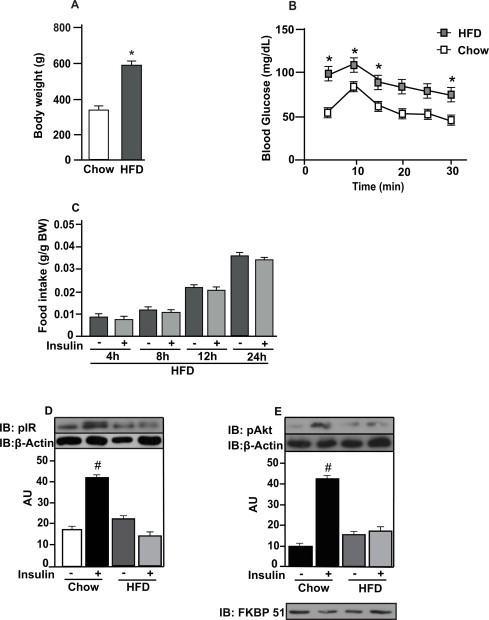
High fat diet (HFD) impairs insulin action and signaling in amygdala. (A) body weight (BW) of rats on chow or HFD; (B) blood glucose during insulin tolerance test (ITT) of awake rats on chow or HFD; (C) food intake in g/g of BW in response to insulin (2 μg) injected in amygdala of HFD animals; (D) insulin receptor (IR) and (E) protein kinase B (PKB or Akt) phosphorylation in response to insulin (2 μg) of rats on chow or HFD. Data are presented as means ± SD from 10 rats. To performed immunoblotting (IB) of amygdala (d and e) We used a pool of 5 rats per sample and four samples (n = 4) per group. We used β-actin as a loading control. FKBP51 was blot to confirm AMY dissections. Two tailed T test was used. *P < 0.05 versus chow; #P < 0.05 versus other groups.
3.5. HFD induces ER stress and low-grade inflammation in the amygdala
These results were obtained from rats without cannulas to reduce a possible interference of the surgery and chronic cannulas implantation on inflammatory and ER stress conditions. In order to evaluate ER stress, we investigated whether high fat feeding alters the phosphorylation of RNA-activated protein kinase-like ER resident kinase (PERK) and inositol-requiring kinase alpha (IRE1α). PERK and IRE1α phosphorylation were increased in the amygdala of rats fed a HFD compared to control rats, suggesting increased ER stress in the amygdala of obese rats (Fig. 5A and B). JNK phosphorylation was increased in the amygdala of rats fed with a HFD compared to control animals (Fig. 5C). IKKalpha/beta phosphorylation was also increased in the amygdala of obese rats (Fig. 5D). We observed the presence of FKBP51 in our membranes, indicating that the dissections of the amygdala were appropriate (Fig. 5A).
Fig. 5.
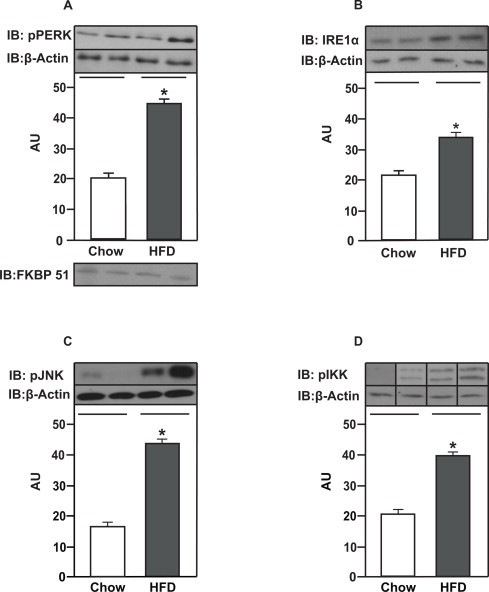
HFD induces ER stress and low-grade inflammation in amygdala. (A) PERK and (B) IRE1α phosphorylation in amygdala from rats on chow or HFD. (C) JNK and (D) IKK α/β phosphorylation in amygdala from rats on chow or HFD. Data are presented as means ± SD. To performed immunoblotting (IB) of amygdala we used a pool of 5 non-cannulated rats per sample and three samples per group. FKBP51 was blot to confirm AMY dissections. Two tailed T test was used. *P < 0.05 versus chow.
3.6. The inhibition of ER stress restores insulin action/signaling, decreases NPY and increases oxytocin mRNA levels in the amygdala from obese rats
We investigated whether the inhibition of ER stress with chemical chaperone PBA improves insulin resistance in the amygdala from rats fed a HFD. PBA treatment reduced PERK phosphorylation in the amygdala from obese rats, suggesting a reduction of ER stress in this brain region (Fig. 6A). PBA treatment also reduced body weight of obese rats when compared with vehicle treated rats (Fig. 6B). PBA treatment restored insulin action in the amygdala from obese rats since food intake was reduced after insulin injection into the amygdala (Fig. 6C). In parallel, we observed that Akt phosphorylation was increased in response to insulin in the amygdala from obese rats treated with PBA (Fig. 6D). Insulin decreased NPY mRNA expression and increased oxytocin mRNA expression in the amygdala from obese rats treated with PBA compared to vehicle obese rats (Fig. 7A and B).
Fig. 6.
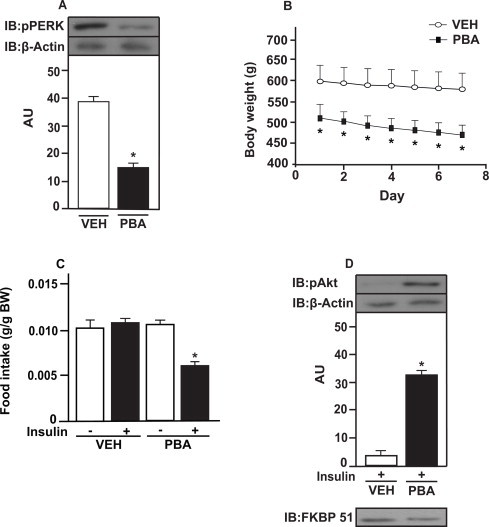
The inhibition of ER stress improves insulin resistance in amygdala from obese animals. (A) PERK phosphorylation in the amygdala from rats on HFD treated with 4-phenylbutyric acid (PBA) or vehicle (DMSO in saline); (B) body weight during PBA treatment; (C) 8 h-food intake (g/g of Body Weight (BW); and (D) Akt phosphorylation in response to insulin (2 μg) or saline injected in the amygdala from rats on a HFD treated with PBA or vehicle. Data are presented as means ± SD from 10 rats per group. To performed immunoblotting (IB) of amygdala we used a pool of 5 rats per sample and four samples (n = 4) per group. We used β-actin as a loading control. FKBP51 was blot to confirm AMY dissections. Two tailed T test and Two-Way ANOVA were used. *P < 0.05 versus other groups. Veh: vehicle.
Fig. 7.
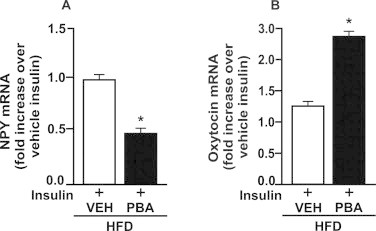
The inhibition of ER stress decreases NPY and increases oxytocin mRNA levels in amygdala from obese rats. Rats on high fat diet (HFD) were treated with PBA or vehicle (VEH) (5% DMSO in saline) for 7 days to investigate neuropeptides expression in the amygdala. 24 h-fasted HFD rats were injected with insulin (2 μg) in amygdala. (A) neuropeptide Y (NPY) and (B) oxytocin mRNA levels in response to insulin in amygdala from rats treated with PBA or VEH. To perform RT-PCR of amygdala we used a pool of 5 rats per sample and 4 samples per group (n = 4). T test was used to analyze the differences between groups. *P < 0.05 versus vehicle.
These results suggest that the inhibition of ER stress reverses insulin resistance in the amygdala from obese animals. We observed the presence of FKBP51 in our membranes, indicating that the dissections of the amygdala were appropriate (Fig. 6D).
4. Discussion
Our data indicate that insulin signaling in amygdala may have an important role in the control of food intake, and this effect is mediated by PI3K pathway. Insulin also decreased NPY and increased oxytocin mRNA levels in amygdala from control rats. In addition, we showed that in high fat feeding rats there is an increase in inflammatory pathways and ER stress in the amygdala, and in parallel, insulin signaling is reduced in this brain region. The inhibition of ER stress reversed insulin resistance in amygdala.
The anorexigenic effects of insulin are well described in the hypothalamus. In the hypothalamus, insulin decreases food ingestion by signaling through IR/PI3K/Akt pathway [1,17]. Insulin receptors have also been found to be abundant in the amygdala [7,8], more recently, amygdala was highlighted as an important site to regulate food intake.
Amygdala is involved in the control of emotion and cognitive functions as memory, learning, fear, anxiety, aversion and food preferences [20–24]. Inhibition of melanocortin into amygdala increased food intake. In contrast, injections of melanocortin agonist or enterostatin in the amygdala reduced food ingestion [10,25–27].
Our data showed that insulin in the amygdala diminishes food intake in rats on chow. Similar result was obtained by Boghossian et al. (2009) [9]. However, in their study they did not investigate which pathway may account for the effect of insulin in amygdala [9,10]. In our study we showed that LY injection abolished the anorexigenic effect of insulin, suggesting that the effect of insulin in the amygdala is mediated by PI3K/Akt pathway. In arcuate nucleus, the activation of neurons coexpressing agouti-related protein (AGRP), and NPY induces feeding [29,30]. Here, we demonstrated that insulin decreased the orexigenic NPY mRNA expression in amygdala via PI3K, which may contribute to decrease food intake. Oxytocin is an anorexigenic peptide and has been found to be abundant in the amygdala [31]. It was shown that insulin modulated oxytocin gene expression, at least, in vitro [32]. Indeed, we observed that insulin increased oxytocin mRNA expression via PI3K in amygdala in vivo of control rats. Together, these results suggest that the reduction on food intake in response to insulin was mediated, at least in part, by the decrease of NPY and increase of oxytocin mRNA levels.
It is well known that HF feeding induces insulin resistance in the CNS of rodents [28]. Indeed, we observed that insulin in the amygdala failed to reduce food intake in rats fed with HFD for 2 months. In parallel, we observed that Akt phosphorylation was faint in response to insulin in the amygdala of obese animals, suggesting insulin resistance in this region of CNS triggered by obesity.
Several mechanisms may contribute to the dysregulation of the insulin signaling pathway in the CNS of obese rodents [28,33–35]. It is well known that obesity may induce ER stress in peripheral tissues and also in the hypothalamus [14,35,36]. Elevated caloric intake due to diets enriched in lipids such as saturated fatty acid and cholesterol elicits ER stress via alteration of calcium homeostasis and production of toxic metabolites [37–42].
Increased PERK and IRE1α phosphorylation are indicators of ER stress [43]. Diet-induced obesity and ob/ob mice have higher levels of PERK and IRE1α phosphorylation in multiple tissues [36,44]. Enhanced PERK and IRE1α phosphorylation are also seen in the hypothalamus of obese mice [14,35,45]. In our study, we observed that PERK and IRE1α phosphorylation were increased in the amygdala of rats on HFD, suggesting that in addition to the hypothalamus [1,14] high fat feeding induces ER stress also in the amygdala. The inhibition of ER stress, with PBA treatment, reduced PERK phosphorylation in the amygdala in parallel to increase insulin action and signaling in this brain site. Furthermore, insulin was able to decrease NPY and increase oxytocin mRNA expression in the amygdala from obese rats treated chronically with PBA, suggesting a strong relationship between ER stress, insulin resistance and food intake controlled by NPY and oxytocin levels. Together, these results suggest that ER stress induced by HFD is probably one of the mechanisms of insulin resistance in the amygdala.
ER stress and inflammatory pathways have many links [46–48]. IRE1α induces JNK activation in many tissues which triggers a modulation of several inflammatory genes [49]. In addition, both IRE1α and PERK activate IKKβ/nuclear factor kappa B (NF-κB) pathway driving inflammatory response [48].
The activation of JNK and IKKβ induce inhibitory IRS-1 serine phosphorylation leading to insulin resistance in peripheral tissues and also in the hypothalamus [28,49,50,51]. Genetic disruption of IKKβ in AgRP neurons protects mice from diet induced obesity [52]. Conditional deletion of JNK1 in the CNS of mice improved insulin signaling and action in the hypothalamus upon high fat diet [53,54].
Our data demonstrated an increase in JNK and IKKβ phosphorylation, in agreement with reduced Akt phosphorylation in response to insulin in the amygdala of obese rats, suggesting that these serine kinases may have important role downregulating insulin signaling in this brain region.
In summary, our results suggested that amygdala is an important region for food intake regulation in response to insulin and this regulation is disrupted in obese rats. We showed that food intake is regulated in a PI3K/Akt manner in the amygdala similarly to that occurs in the hypothalamus. Insulin also decreases NPY and increases oxytocin mRNA levels via PI3K in vivo, which may contribute to hypophagia. Further, we provide data suggesting that obese rats may have low-grade inflammation and ER stress in parallel to insulin resistance in the amygdala. The inhibition of ER stress with PBA improves insulin action and signaling in the amygdala from obese rats, suggesting that ER stress is probably one of the mechanisms of insulin resistance in the amygdala.
Acknowledgements
The present work received financial support of FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo): 2008/55674-8, Sao Paulo, Brazil and INCT (Instituto Nacional Ciência e Tecnologia em Obesidade e Diabetes): 573856/2008-7.
The authors would like to thank Dioze Guadagnini, Andrey dos Santos, Alexandre H. B. de Matos, Iscia Lopes-Cendes, L. Janeri, J. Pinheiro, (Department of Internal Medicine, UNICAMP, Campinas, Sao Paulo) for their technical assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Belgardt B.F., Bruning J.C. CNS leptin and insulin action in the control of energy homeostasis. Ann. N. Y. Acad. Sci. 2010;1212:97–113. [Google Scholar]
- 2.Benoit S.C., Clegg D.J., Seeley R.J., Woods S.C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud H.R. Interactions between the "cognitive" and "metabolic" brain in the control of food intake. Physiol. Behav. 2007;9:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Bruning J.C., Gautam D., Burks D.J., Gillette J., Schubert M., Orban P.C., Klein R., Krone W., Muller-Wieland D., Kahn C.R. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist J.K. CNS regulation of energy balance and body weight: insights from rodent models. Lab. Anim. Sci. 1998;48:630–637. [PubMed] [Google Scholar]
- 6.Figlewicz D.P., Evans S.B., Murphy J., Hoen M., Baskin D.G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 7.Havrankova J., Schmechel D., Roth J., Brownstein M. Identification of insulin in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1978;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havrankova J., Roth J., Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 9.Boghossian S., Lemmon K., Park M., York D.A. High-fat diets induce a rapid loss of the insulin anorectic response in the amygdala. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1302–R1311. doi: 10.1152/ajpregu.00252.2009. [DOI] [PubMed] [Google Scholar]
- 10.Boghossian S., Park M., York D.A. Melanocortin activity in the amygdala controls appetite for dietary fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R385–R393. doi: 10.1152/ajpregu.00591.2009. [DOI] [PubMed] [Google Scholar]
- 11.Niswender K.D., Morrison C.D., Clegg D.J., Olson R., Baskin D.G., Myers M.G., Jr., Seeley R.J., Schwartz M.W. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Chan O., Inouye K., Akirav E., Park E., Riddell M.C., Vranic M., Matthews S.G. Insulin alone increases hypothalamo–pituitary–adrenal activity, and diabetes lowers peak stress responses. Endocrinology. 2005;146:1382–1390. doi: 10.1210/en.2004-0607. [DOI] [PubMed] [Google Scholar]
- 13.Hillebrand J.J., de Wied D., Adan R.A. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides. 2002;23:2283–2306. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 14.Ropelle E.R, Flores M.B., Cintra D.E., Rocha G.Z., Pauli J.R., Morari J., de Souza C.T., Moraes J.C., Prada P.O., Guadagnini D., Marin R.M., Oliveira A.G., Augusto T.M., Carvalho H.F., Velloso L.A., Saad M.J., Carvalheira J.B. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol. 2010;8(8):):1–20. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Tsukumo D.M., Carvalho-Filho M.A., Carvalheira J.B., Prada P.O., Hirabara S.M., Schenka A.A., Araújo E.P., Vassallo J., Curi R., Velloso L.A., Saad M.J. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 16.Prada P.O., Ropelle E.R., Mourão R.H., de Souza C.T., Pauli J.R., Cintra D.E., Schenka A., Rocco S.A., Rittner R., Franchini K.G., Vassallo J., Velloso L.A., Carvalheira JB, Saad MJ. EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes. 2009;58:2910–2919. doi: 10.2337/db08-0506. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.De Souza C.T., Frederico M.J., da Luz G., Cintra D.E., Ropelle E.R., Pauli J.R., Velloso L.A. Acute exercise reduces hepatic glucose production through inhibition of the Foxo1/HNF-4alpha pathway in insulin resistant mice. J. Physiol. 2010;588:2239–2253. doi: 10.1113/jphysiol.2009.183996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcan L. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Won J.C., Jang P.G., Namkoong C., Koh E.H., Kim S.K., Park J.Y., Lee K.U., Kim M.S. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring). 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 20.Figlewicz D.P., MacDonald Naleid A., Sipols A.J. Modulation of food reward by adiposity signals. Physiol. Behav. 2007;91:473–478. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass M.J., Billington C.J., Levine A.S. Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R86–R92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- 22.Glass M.J., Billington C.J., Levine A.S. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- 23.Kelley A.E. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chem. Senses. 2007;32:105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]
- 25.Primeaux S.D., York D.A., Bray G.A. Neuropeptide Y administration into the amygdala alters high fat food intake. Peptides. 2006;27:1644–1651. doi: 10.1016/j.peptides.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Lin L., Park M., Hulver M., York D.A. Different metabolic responses to central and peripheral injection of enterostatin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R909–R915. doi: 10.1152/ajpregu.00045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L., Park M., York D.A. Enterostatin inhibition of dietary fat intake is modulated through the melanocortin system. Peptides. 2007;28:643–649. doi: 10.1016/j.peptides.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prada P.O., Zecchin H.G., Gasparetti A.L., Torsoni M.A., Ueno M., Hirata A.E., Corezola do Amaral M.E., Hoer N.F., Boschero A.C., Saad M.J. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146:1576–1587. doi: 10.1210/en.2004-0767. [DOI] [PubMed] [Google Scholar]
- 29.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., Plum L., Balthasar N., Hampel B., Waisman A., Barsh G.S., Horvath T.L., Brüning J.C. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 30.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 31.Chaves V.E., Tilelli C.Q., Brito N.A., Brito M.N. Role of oxytocin in energy metabolism. Peptides. 2013;45:9–14. doi: 10.1016/j.peptides.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Lioutas C., Einspanier A., Kascheike B., Walther N., Ivell R. An autocrine progesterone positive feedback loop mediates oxytocin upregulation in bovine granulosa cells during luteinization. Endocrinology. 1997;138:5059–5062. doi: 10.1210/endo.138.11.5650. [DOI] [PubMed] [Google Scholar]
- 33.Benoit S.C., Kemp C.J., Elias C.F., Abplanalp W., Herman J.P., Migrenne S., Lefevre A.L., Cruciani-Guglielmacci C., Magnan C., Yu F. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J. Clin. Invest. 2009;119:2577–2589. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park-York M., Boghossian S., Oh H., York D.A. PKC theta expression in the amygdala regulates insulin signaling, food intake and body weight. Obesity (Silver Spring) 2012;21(4):755–764. doi: 10.1002/oby.20278. [DOI] [PubMed] [Google Scholar]
- 35.Ozcan L., Ergin A.S., Lu A., Chung J., Sarkar S., Nie D., Myers M.G., Jr., Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 37.Listenberger L.L., Ory D.S., Schaffer J.E. Palmitate-induced apoptosis canoccur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 38.Holland W.L., Brozinick J.T, Wang L.P, Hawkins E.D, Sargent KM. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Unger R.H., Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim. Biophys. Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 40.Ariyama H., Kono N., Matsuda S., Inoue T., Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel V.T., Petersen K.F., Shulman G.I. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu S., Yang L., Li P., Hofmann O., Dicker L. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity . Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsutsumi A., Motoshima H., Kondo T., Kawasaki S., Matsumura T., Hanatani S., Igata M., Ishii N., Kinoshita H., Kawashima J. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochem. Biophys. Res. Commun. 2011;404:339–344. doi: 10.1016/j.bbrc.2010.11.120. [DOI] [PubMed] [Google Scholar]
- 45.Won J.C., Jang P.G., Namkoong C., Koh E.H., Kim S.K., Park J.Y., Lee K.U., Kim M.S. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 46.Deng J., Lu P.D., Zhang Y., Scheuner D., Kaufman R.J., Sonenberg N., Harding H.P., Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu P., Han Z., Couvillon A.D., Kaufman R.J., Exton J.H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hummasti S., Hotamisligil G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010;107:579–591. doi: 10.1161/CIRCRESAHA.110.225698. [DOI] [PubMed] [Google Scholar]
- 49.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 50.Lee Y.H., Giraud J., Davis R.J., White M.F. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 2003;278:2896–2902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- 51.Aguirre V., Werner E.D., Giraud J., Lee Y.H., Shoelson S.E., White M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belgardt B.F., Mauer J., Wunderlich F.T., Ernst M.B., Pal M., Spohn G., Bronneke H.S., Brodesser S., Hampel B., Schauss A.C. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unger E.K., Piper M.L., Olofsson L.E., Xu A.W. Functional role of c-Jun-N-terminal kinase in feeding regulation. Endocrinology. 2010;151:671–682. doi: 10.1210/en.2009-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]


