Abstract
In the central nervous system, alteration of glial cell differentiation can affect brain functions. Polychlorinated biphenyls (PCBs) are persistent environmental chemical contaminants that exert neurotoxic effects in glial and neuronal cells. We examined the effects of a commercial mixture of PCBs, Aroclor1254 (A1254) on astrocytic differentiation of glial cells, using the rat C6 cell line as in vitro model. The exposure for 24 h to sub-toxic concentrations of A1254 (3 or 9 μM) impaired dibutyryl cAMP-induced astrocytic differentiation as showed by the decrease of glial fibrillary acidic protein (GFAP) protein levels and inhibition in change of cell morphology toward an astrocytic phenotype. The A1254 inhibition was restored by the addition of a protein kinase C (PKC) inhibitor, bisindolylmaleimide (bis), therefore indicating that PCBs disturbed the cAMP-induced astrocytic differentiation of C6 cells via the PKC pathway. The phosphorylation of signal transducer and activator of transcription 3 (STAT3) is essential for cAMP-induced transcription of GFAP promoter in C6 cells. Our results indicated that the exposure to A1254 (3 or 9 μM) for 24 h suppressed cAMP-induced STAT3 phosphorylation. Moreover, A1254 reduced cAMP-dependent phosphorylation of STAT3 requires inhibition of PKC activity. Together, our results suggest that PCBs induce perturbation in cAMP/PKA and PKC signaling pathway during astrocytic differentiation of glial cells.
Keywords: Astrocytic differentiation, C6 glial cell line, Aroclor1254, Glial fibrillary acidic protein (GFAP), Protein kinase C (PKC), Signal transducer and activator of transcription 3 (STAT3)
Abbreviations: PCBs, polychlorinated biphenyls; dbcAMP, N6,2′-O-dibutyryl cAMP; A1254, Aroclor 1254; DMSO, dimethyl sulfoxide; DMEM, Dulbecco’s Modified Eagle’s Medium; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; NMDA, N-methyl-d-aspartate; nNOS, neuronal nitric oxide; CNS, central nervous system; ROS, reactive oxygen species; GFAP, glial fibrillary acidic protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DAPI, 4′,6-diamidino-2-phenylindole; PKA, protein kinase A; PKC, protein kinase C; bis, 2-[1-(3-dimethylamino-propyl)indol-3-yl]-3-(indol-3-yl) maleimide; STAT3, signal transducer and activator of transcription 3; CRE, cAMP responsive element; CREB, cAMP-response element binding protein; TRE, CRE transcriptional response element
Highlights
-
•
A1254, a polychlorinated biphenyls mixture, exerts neurotoxic effects in C6 glial cells.
-
•
Sub-toxic concentrations of A1254 decrease levels of glial fibrillary acidic protein.
-
•
Sub-toxic concentrations of A1254 inhibit cAMP-induced astrocytic differentiation.
-
•
A1254 reduces cAMP-dependent phosphorylation of STAT3 on Ser727 via protein kinase C.
-
•
Polychlorinated biphenyls may affect astrocytic differentiation of glial cells.
1. Introduction
Astrocytes, the main class of neuroglia, are the most abundant cells in the central nervous system (CNS) providing an architecture for neurons and secreting growth factors and cytokines in the response to injury [1]. Besides, astrocytes are the major cell type that preferentially sequestrates metals and accumulates toxic agents [2], therefore suggesting a possible role in the control and/or modulation of neurotoxic effects.
Polychlorinated biphenyls (PCBs) are widespread and persistent environmental contaminants accumulating in food chain in polluted areas [3] that can affect nervous system development and functions [4]. PCBs were produced for use as non-flammable dielectrics in electronic parts, lubricants, plasticizers, vehicles for pesticide application, and pigment suspension agents in carbonless copy paper [5]. PCBs produce neurochemical alterations in several experimental models [6], behavioral changes in learning, motor activity and sexual behavior [7]. In addition, PCBs greatly affect cell viability, brain functions and have been associated with neurodegenerative disorders [8]. PCBs induce mitochondrial dysfunction and reactive oxygen species (ROS) production [9] and in turn, alterations of dopaminergic neurons [10], death of cerebellar granule cells via N-methyl-d-aspartate (NMDA) receptor activation [11] and of neuroblastoma cells via the involvement of neuronal nitric oxide (nNOS) [12]. Furthermore, chronic exposure to these pollutants can affect the development of the CNS [4] and neuronal plasticity [13]. Aroclor 1254 (A1254), a commercial mixture of PCBs [14], most commonly found in various foods and in human specimens at contaminated sites [15], has been widely utilized in studying PCBs toxicity [9–14].
Although several scientific studies have been conducted on the neurotoxicity triggered by PCBs, the effects of these pollutants on astrocytic differentiation has been poorly investigated. The rat C6 glial cell line [16] has been widely used as model for study of factors that modulate differentiation of glial cells [17]. The treatment of C6 cells with dibutyryl(db)-cAMP, leads to inhibition of cell growth and increased expression of the astrocytic marker glial fibrillary acidic protein (GFAP) correlated to change in cell morphology from an epithelial-like to a process-bearing morphology [18]. In this study, we determined A1254 cytotoxicity and the effects of sub-toxic concentrations on dbcAMP-induced astrocytic differentiation in C6 cells, by evaluation of GFAP levels and monitoring cell morphology.
Furthermore, since protein kinase C (PKC) signaling is involved in A1254 neurotoxicity [19–21], we also assessed the effects of a selective PKC inhibitor [21,22] on A1254-induced toxic effects. cAMP-induced GFAP expression in C6 cells also requires activation of signal transducer and activator of transcription 3 (STAT3) pathway [23,24], we also investigated the effect of A1254 on the activation status of STAT3.
2. Materials and methods
2.1. Materials
Aroclor 1254 (Cat. N. 48586, Lot N. LB58885) was purchased from Supelco (Italy, 99% purity) and dissolved in dimethyl sulfoxide (DMSO, cell culture tested, Sigma–Aldrich, Italy). The protein kinase C (PKC) inhibitor, 2-[1-(3-dimethylamino-propyl)indol-3-yl]-3-(indol-3-yl) maleimide (bis) was purchased from Cell Signaling Technology (Cat. N. 9841, Euroclone, Italy) and dissolved in DMSO. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, MTT and N6,2′-O-dibutyryl cAMP (dbcAMP) were obtained from (Cat. N. D0260, Sigma–Aldrich, Italy).
2.2. Cell cultures and treatments
The C6 rat glial cell line [16] (American Type Culture Collection, ATTC CCL-107) was cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Life Technologies, Italy), 1.5 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin under humidified atmosphere of 5% CO2 at 37 °C. Cells were sub-cultured twice a week by 3–5-fold dilution with culture medium. Treatments of sub-confluent cells were performed replacing the culture medium with those containing increasing concentrations of A1254 (0.05–90 μM) or protein kinase C inhibitor, bisindolylmaleimide [21,22] (0.05–12 μM). Astrocytic differentiation was induced using serum-free DMEM containing 1 mM dbcAMP. Co-exposure experiments were performed by adding simultaneously A1254 (3 or 9 μM) and/or bis (0.125 μM) during dbcAMP (1 mM) stimulation of C6 cells for 24 h. All the treatments were performed under serum-free conditions in presence of 0.1% (v/v) DMSO used as vehicle for A1254 and bis.
2.3. Viability assay
Cells were seeded onto 96-well plates (2 × 104 cells per well) and after the treatments, their viability was evaluated as mitochondrial activity using the MTT assay [25]. Briefly, the medium was removed and cells incubated with 100 μl MTT (0.5 mg/ml) for 1 h. After that, the solution was removed, formazan solubilized in 100 μl DMSO and the absorbance measured at 540 nm using a microplate reader (Labsystems Multiskan, MS). Results were expressed as percentage of cell survival vs. control cells cultured in serum-free medium with 0.1% (v/v) DMSO (vehicle) (which represent the 100% survival).
2.4. Immunocytochemistry and phase-contrast analysis
Cells grown on coverslips were treated and fixed by a 20 min exposure to cold 4% paraformaldehyde in PBS, and then subjected to immunocytochemistry and phase-contrast analysis. For immununocytochemistry, cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min. After blocking with 10% donkey serum for 1 h, the coverslips were incubated with a mouse anti-GFAP antibody (Cat. N. G3893, Sigma–Aldrich, Italy), thereafter, a fluorescein isothiocyanate (FITC)-secondary antibody (Jeckson-Li StarFish, Italy) was applied. After washing, coverslips were mounted with Vectashield medium containing 1.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, CA, USA) to visualize the nuclei. Immunofluorescence analysis was performed using a Leica DM LB microscope (Plan 20×/0.40 objective) connected to a Leica DFC 345 FX digital camera and images were captured using the Leica Application Suite 3.6 software (Version 3.6.0) (Switzerland). Phase-contrast images were captured using a Zeiss Axiovert 40 CFL inverted microscope (Carl Zeiss, Milan, Italy) (LD A-Plan 40×/0.50 Ph 2 objective). The microscope was equipped with a 12.1-megapixel CCD digital videocamera (Canon, PowerShot G9, Italy) with a digital image software (Remote Capture DC, Canon). Images were imported into ImageJ software 1.43u, NIH.
2.5. Western blotting
Cells seeded in 6-well plates (2 × 105 per well) were subjected to different treatments and then lysed at 4 °C in 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Igepal, 0.5% sodium deoxicolate, protease (Roche, Italy) and phosphatase inhibitor cocktails (Calbiochem, Italy). The total protein concentration was determined by a Bradford protein assay [26] using bovine serum albumin as a standard. Equal amounts of proteins (20 μg) were subjected to 12% SDS–PAGE performed as described by Laemmli [27]. The proteins were transferred to a nitrocellulose membrane (BA85; Schleicher & Schull) and incubated with a primary antibody: mouse anti-GFAP, anti-mouse STAT3 (Cat. N. 9139), anti-rabbit phospho-STAT3 (Ser727) (Cat N. 9134), anti-rabbit phospho-PKC substrates (Cat. N. 2261S) from Cell Signaling Technology (Euroclone, Italy) followed by incubation with an appropriated anti-mouse (Cat. N. 31430) or anti-rabbit (Cat. N. 31460) peroxidase-conjugated secondary antibody (Pierce, Thermo Scientific, Italy) in PBS containing 5% dry milk. The signals were visualized using an Enhanced Chemiluminescence (ECL) detection kit (Cat. N. RPN 2209, GE Healthcare, Italy). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Cat. N. AM4300, Ambion, Applied Biosystems, Italy) was used as protein loading control. Signal quantification was performed with ImageJ software 1.43u, NIH.
2.6. Statistical analysis
Statistical significance of treated samples against control cells (cultured in serum-free medium with vehicle 0.1% v/v DMSO) was determined by One-way analysis of Variance (ANOVA), followed by Dunnett's test. Each value represents the mean ± SEM of at least three independent experiments performed in triplicate (*p < 0.05; §p < 0.01; #p < 0.001).
3. Results
3.1. Effect of A1254 exposure on C6 cell survival
Cytotoxic effect of A1254 was assessed in C6 glial cells by treatments with increasing amounts of the mixture, in agreement with literature [21]. The exposure to increasing concentrations of A1254 (0.05–90 μM) for 24 h induced a concentration dependent reduction of cell viability, evaluated as mitochondrial activity by MTT assay; the addition of DMSO, used as vehicle for A1254, at a final concentration of 0.1% (v/v) by itself did not cause any cell toxicity (Fig. 1A). Moreover, the highest concentrations of A1254 (30, 45 and 90 μM) significantly (p < 0.001) decreased the cell viability reaching a maximal effect of 80–98% lethality. Based upon these results, the non-toxic concentration of 3 μM and the concentration of 9 μM that caused about 25% lethality were chosen to study the effect of a prolonged treatment on cell viability. The concentration of A1254 3 μM caused about 25–50% reduction on cell viability at 48 and 72 h. Furthermore, the concentration of A1254 9 μM caused a higher decrease of cell survival reduction of cell viability (about 50%) at 48 and 72 h (Fig. 1B). Therefore, the concentrations of A1254 3 and 9 μM and an exposure time of 24 h were used for the subsequent treatments for cellular and biochemical studies.
Fig. 1.
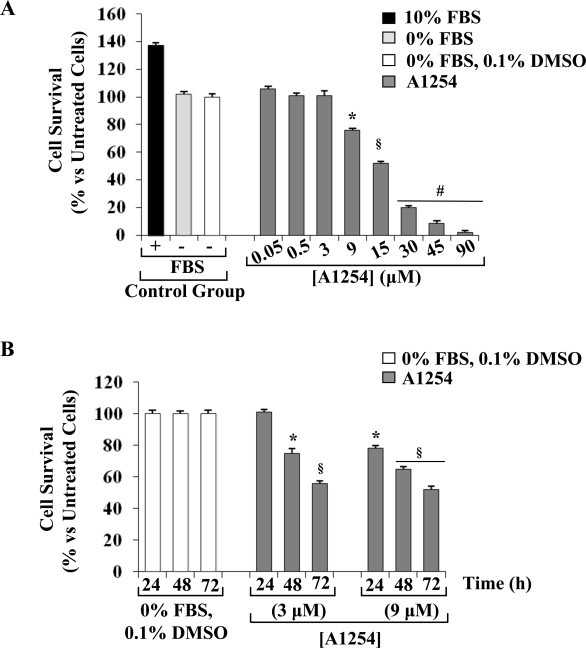
Exposure to A1254 affected cell viability in a concentration and time-dependent manner in C6 cells. (A) Cells were exposed to increasing concentrations of A1254 (0.05–90 μM) for 24 h in serum-free medium with 0.1% DMSO (vehicle). (B) C6 cells were treated with A1254 (3 or 9 μM) for 48 and 72 h. After incubation, cell viability was evaluated using the MTT assay, as described in Section 2.3. The cell viability was calculated vs untreated control cells, cultured in serum-free-DMEM with 0.1% DMSO (vehicle) at the respective time of incubation (24, 48 or 72 h) set as 100%. Results are presented as percentage (mean ± SEM) (n = 3) of the control cells. Significant difference from the untreated control; *p < 0.05; §p < 0.01; #p < 0.001.
3.2. Effects of A1254 on dbcAMP-induced GFAP expression levels and astrocytic differentiation in C6 cells
To examine whether the exposure to sub-toxic concentrations of A1254 might affect dbcAMP induced GFAP expression levels, C6 cells were exposed simultaneously to both agents and GFAP protein expression levels were determined by western blotting (Fig. 2). C6 cells cultured in DMEM with 10% FBS expressed low GFAP protein levels that were similar to those expressed by untreated cells cultured under serum-free condition, with or without 0.1% (v/v) DMSO or cells exposed to A1254 alone. The stimulation with dbcAMP (1 mM) for 24 h greatly elevated GFAP protein expression levels (about 8-fold increase) according to previous results [18]. Cells co-exposed to dbcAMP and A1254 showed an inhibition of GFAP expression; in particular, while the concentration of 3 μM A1254 did not significantly affect the GFAP protein levels (about 1.2-fold decrease), the 9 μM led to a significant decrease on GFAP expression levels (about 4-fold decrease), in comparison with cells treated with dbcAMP alone. Moreover, the effects of A1254 on dbcAMP-induced GFAP expression and astrocytic phenotype were evaluated by immunocytochemistry (Fig. 3) and phase-contrast analysis (Fig. 4). C6 cells cultured in DMEM with 10% serum showed a very low GFAP immunoreactivity (Fig. 3, A–C) and had a flat and epithelial-like undifferentiated morphology (Fig. 4, A). The treatment of C6 cells with 1 mM dbcAMP for 24 h, resulted in differentiated astrocytic cells with round cell bodies and long cell processes (Fig. 4, F), expressing a strong GFAP immunoreactivity (Fig. 3, P–R). In contrast, the co-exposure to A1254 (3 or 9 μM) and dbcAMP impaired astrocytic differentiation. In fact, GFAP immunoreactivity was significantly attenuated (Fig. 3, S–X) and a decrease of the length of extended processes in each cell body was observed (Fig. 4, G and H), particularly at the concentration of 9 μM (Fig. 3, V–X; Fig. 4, H). In the absence of dbcAMP, the addition of A1254 (3 or 9 μM) did not influence either GFAP immunoreactivity (Fig. 3, J–O) or cell morphology (Fig. 4, D and E) that were similar to that of control cells, cultured in serum-free medium with (Fig. 3, G–I; Fig. 4, C) or without (Fig. 3, D–F; Fig. 4, B) 0.1% (v/v) DMSO (vehicle). These results indicate that the exposure to sub-toxic concentrations of A1254 impair dbcAMP-induced astrocytic differentiation in C6 cells.
Fig. 2.
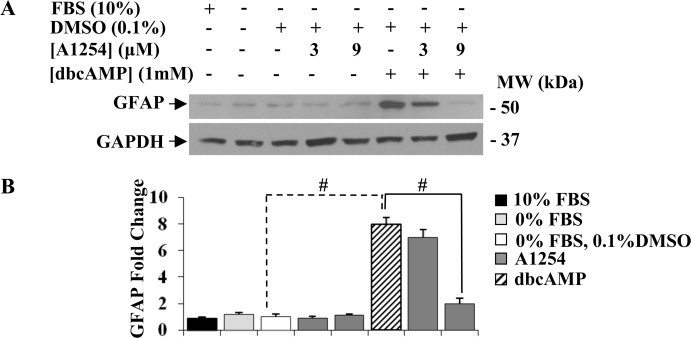
Exposure to A1254 decreased dbcAMP-induced GFAP protein expression levels in C6 cells. Control and C6 cells treated with A1254 (3 or 9 μM) in presence or absence of dbcAMP (1 mM) were harvested after 24 h and assayed for GFAP protein expression levels. Equal amounts of protein cell lysates (20 μg) were subjected to protein analysis by 12% SDS–PAGE. (A) Western blotting showing GFAP protein expression levels. GAPDH was used as loading control for cell lysates. Signals were revealed by immunostaining and ECL, as described in Section 2.5. (B) Densitometric analysis of GFAP expression levels. Fold change in GFAP levels was calculated by first normalizing to GAPDH levels in individual samples and then relative to untreated control (cells cultured in serum-free DMEM with 0.1% (v/v) DMSO, vehicle) set as 1. Each bar represents the mean ± SEM (n = 3). Columns with (#) were statistically different from control and dbcAMP-differentiated cells (#p < 0.001).
Fig. 3.
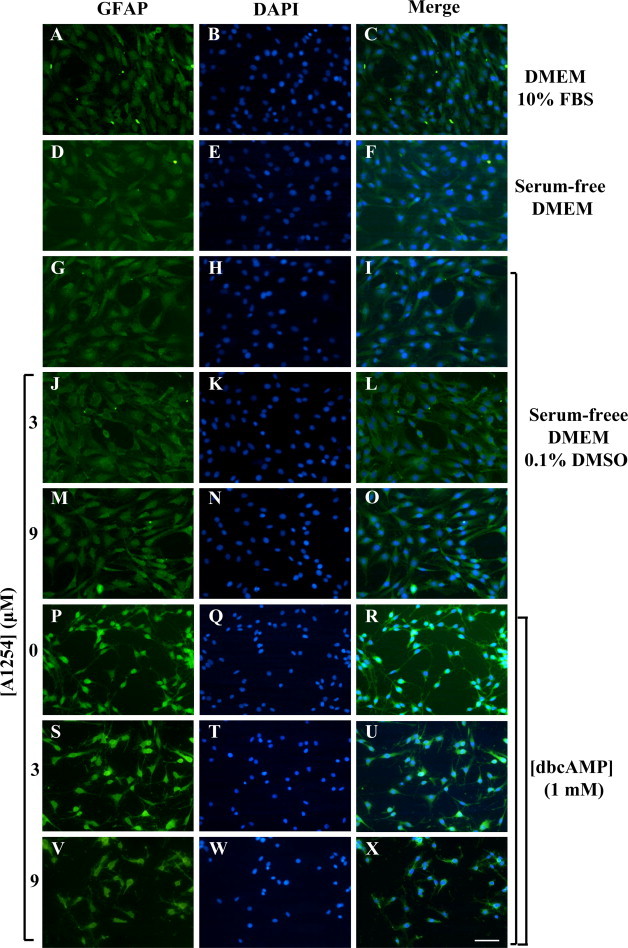
Effects of A1254 exposure on GFAP immunoreactivity in dbcAMP differentiating C6 cells. C6 cells were subjected to different treatments, fixed and then immunocytochemistry was performed as detailed in Section 2.4. C6 cells were cultured in DMEM with 10% FBS (A–C) or kept in serum-free DMEM in presence (G–I) or absence (D–F) of 0.1% (v/v) DMSO (vehicle). Cells exposed to: 3 μM A1254 (J–L); 9 μM A1254 (M–O); 1 mM dbcAMP (P–R). dbcAMP-differentiating cells exposed to A1254 3 μM (S–U) or 9 μM (V–X). After the treatments, cells were subjected to GFAP immunostaining (green) (A, D, G, J, M, P, S and V). DAPI-nuclear stain (blue) of the same field (B, E, H, K, N, Q, T and W). Merge for composite images (C, F, I, L, O, R, U and X). All the treatments were performed for 24 h under serum-free conditions in presence of 0.1% (v/v) DMSO used as vehicle for A1254. Scale bar = 50 μm. (For interpretation of color in Fig. 3, the reader is referred to the web version of this article.)
Fig. 4.
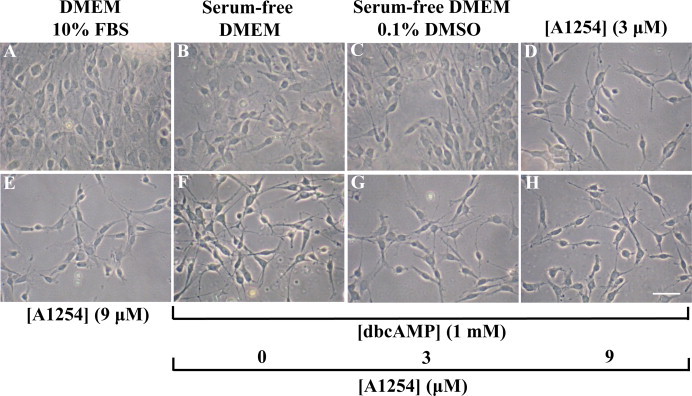
Effects of A1254 exposure on morphological change in dbcAMP-differentiating C6 cells. C6 cells were subjected to different treatments, fixed and then subjected to phase- contrast analysis, as detailed in Section 2.4. Phase-contrast micrographs of C6 cells cultured in DMEM with 10% serum (A) or kept in serum-free DMEM with (C) or without (B) of 0.1% (v/v) DMSO (vehicle). Cells exposed to: 3 μM A1254 (D); 9 μM A1254 (E); 1 mM dbcAMP (F). dbcAMP-differentiating cells exposed to A1254 3 μM (G) or 9 μM (H). All the treatments were performed for 24 h under serum-free conditions in presence of 0.1% (v/v) DMSO, used as vehicle for A1254. Scale bar = 25 μm.
3.3. Effect of the protein kinase C inhibitor, bisindolylmaleimide, (bis) on A1254-inhibition of dbcAMP-stimulated GFAP expression levels in C6 cells
Since PCBs neurotoxic effects have been reported to involve PKC signaling [28,29], we evaluated whether a selective PKC inhibitor, bis [21,22] might affect the A1254-induced impairment of dbcAMP-induced GFAP expression and astrocytic morphology.
We first examined the affect of bis exposure on cell viability. To this end, C6 cells were treated with increasing amounts of bis for 24 h, and then subjected to MTT assay (Fig. 5A). We observed a concentration-dependent cytotoxic effect, with an IC50 value of 10 μM in agreement with the literature [21]. The concentration of 0.125 μM bis, that did not affect cell survival (about 98% viability), was chosen for further experiments. In addition, we also assessed cell viability after co-exposure to bis and A1254 (3 or 9 μM) in dbcAMP induced cells (Fig. 5B). The treatment with bis (0.125 μM) in combination with dbcAMP (1 mM) did not affect significantly cell viability (88% cell survival) compared to untreated cells (serum-free DMEM with 0.1% (v/v) DMSO) as well as to cells stimulated with dbcAMP alone (96% cell viability). Interestingly, the co-exposure to bis (0.125 μM) and A1254 (3 or 9 μM) in presence of dbcAMP did not change cell survival respect to A1254 alone (3 or 9 μM) (Fig. 1A) or in combination with bis (Fig. 5B).
Fig. 5.
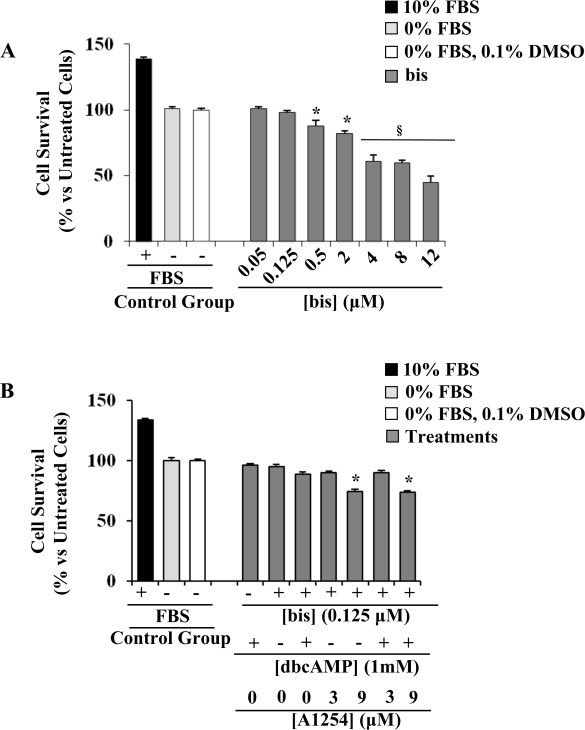
Effect of exposure to the protein kinase C inhibitor, bisindolylmaleimide, (bis) on C6 cell survival. (A) Concentration-dependent cytotoxic effects were evaluated by exposure for 24 h to increasing concentrations of A1254 (0.05–90 μM) in serum-free medium with 0.1% (v/v) DMSO (vehicle). (B) Effects of co-exposure to bis (0.125 μM) and A1254 (3 or 9 μM) of dbcAMP stimulated cells. Control treatments were performed as reported in the figure. After the treatments, cell survival was determined by MTT assay, as reported in Section 2.3. Results are presented as percentage (mean ± SEM) (n = 3) of the control cells cultured in serum-free DMEM with 0.1% (v/v) DMSO (vehicle) set as 100%. Significant difference from the control; *p < 0.05; §p < 0.01.
We then examined the effect of co-exposure to bis and A1254 on dbcAMP induced GFAP expression levels by western blotting (Fig. 6). The treatment with bis was able to revert the decrease of GFAP levels triggered by A1254 in dbcAMP stimulated C6 cells, with a maximal recovery (about 9-fold increase) for cells treated with 9 μM A1254. The treatment with bis (0.125 μM) alone did not change significantly the basal expression of GFAP levels (about 1.6-fold increase) compared to that of untreated control cells (serum-free-DMEM with 0.1% (v/v) DMSO).
Fig. 6.
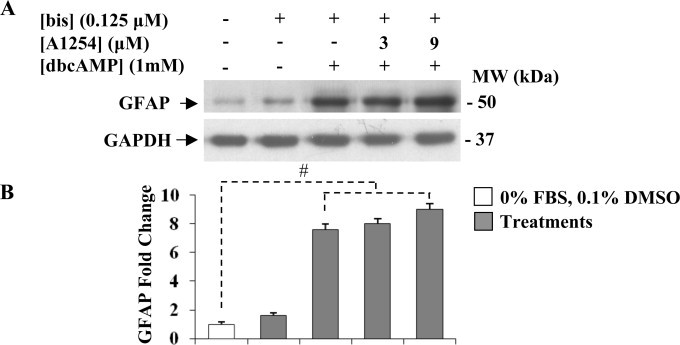
Effect of the protein kinase C inhibitor, bisindolylmaleimide (bis) on GFAP protein expression levels in dbcAMP-stimulated C6 cells exposed to A1254. Cells were treated with bis (0.125 μM) or co-exposed to bis (0.125 μM) and A1254 (3 or 9 μM) in presence of dbcAMP (1 mM) in serum-deprived medium containing 0.1% (v/v) DMSO, used as vehicle for A1254 and bis. After 24 h incubation, treated and untreated control cells were harvested and assayed for GFAP protein expression levels. Equal amounts of protein cell lysates (20 μg) were subjected to protein analysis by 12% SDS–PAGE. (A) Western blotting showing GFAP protein expression levels. GAPDH was used as loading control for cell lysates. Signals were revealed by immunostaining and ECL, as described in Section 2.5. (B) Densitometric analysis of GFAP protein expression levels. Fold change in GFAP protein levels was calculated by first normalizing to GAPDH levels in individual samples and then relative to untreated control cells cultured in serum-free DMEM with 0.1% (v/v) DMSO, (vehicle) set as 1. Each bar represents the mean ± SEM (n = 3). Columns with (#) were statistically different from untreated control cells or dbcAMP-differentiated cells (# p<0.001).
The effect of bis (0.125 μM) was also evaluated on GFAP immunostaining and morphological changes in C6 cells co-treated with dbcAMP (1 mM) and A1254 (3 or 9 μM) (Fig. 7). The treatment with bis alone did not affect GFAP immunoreactivity (Fig. 7A, D–F) and cell morphology (Fig. 7B, B) was similar to that of control cells (Fig. 7A, A–C; Fig. 7B, A). Furthermore, the co-treatment with bis and dbcAMP (Fig. 7A, G–I; Fig. 7B, C) did not influence dbcAMP induced astrocytic differentiation (see Figs. 3, P–R and 4, F). Moreover, in presence of bis and A1254 (3 or 9 μM), we observed a strong GFAP immunoreactivity (Fig. 7A, J–O) and extension of long astrocytic processes (Fig. 7B, D and E). Taken together, these results strongly suggest the involvement of PKC signalling in the impairment of astrocytic differentiation triggered by A1254 in C6 cells.
Fig. 7.
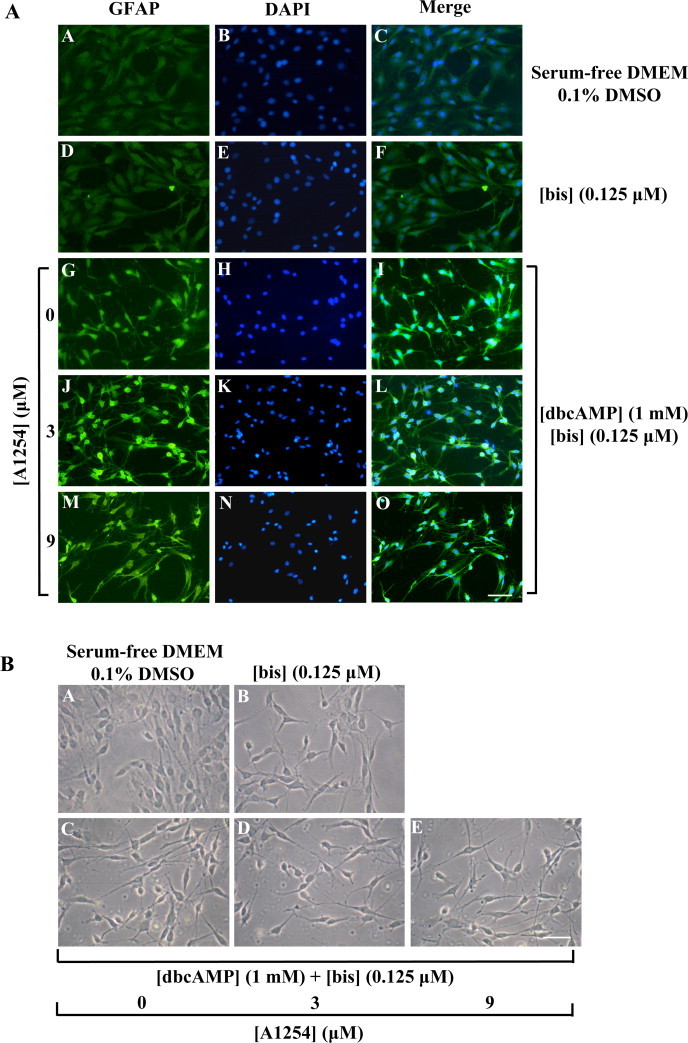
Effect of co-exposure to A1254 and PKC inhibitor, bis, on dbcAMP-induced GFAP immunoreactivity and morphological change in C6 cells. C6 cells were subjected to different treatments, fixed and then immunocytochemistry (A) and phase-contrast (B) analysis were performed as detailed in Section 2.4. Cells were treated with bis (0.125 μM) or co-exposed to bis (0.125 μM) and A1254 (3 or 9 μM) in presence of dbcAMP (1 mM) in serum-deprived medium containing 0.1% (v/v) DMSO. After 24 h incubation, cells were subjected to immunofluorescence analysis to reveal GFAP immunoreactivity (green). Immunocytochemistry of cells treated with 0.1% (v/v) DMSO (vehicle) (A–C); cells exposed to bis (0.125 μM) (D–F). Astrocytic differentiation induced by treatment with dbcAMP (1 mM) in presence of bis (0.125 μM) (G–I). dbcAMP-differentiated cells co-exposed to: bis (0.125 μM) and 3 μM A1254 (J–L): bis (0.125 μM) and 9 μM A1254 (M–O). GFAP immunostaining (A, D, G, J and M). DAPI-nuclear stain (blue) of the same field (B, E, H, K, and N). Merge for composite images (C, F, I, L and O). Scale bar = 50 μm. (B) Phase-contrast micrographs of C6 cells cultured in serum-free DMEM in presence (B) or absence (A) of bis (0.125 μM). dbcAMP-differentiating cells were treated with bis (0.125 μM) alone (C) or in combination with A1254 3 μM (D) or 9 μM (E). All the treatments were performed for 24 h under serum-free conditions in presence of 0.1% (v/v) DMSO used as vehicle for A1254 and bis. Scale bar = 25 μm. (For interpretation of color in Fig. 7, the reader is referred to the web version of this article.)
3.4. Effect of A1254 on PKC activity and cAMP-dependent activation of STAT3
To confirm that A1254 inhibits dbcAMP-stimulated astrocytic differentiation via PKC pathway, the PKC activity was evaluated. We used a Phospho-(Ser) PKC substrate antibody that recognizes the motif: Arg or Lys-X-Serphospho-Hyd-Arg or Lys in western blotting analysis performed on total protein extracts from C6 cells subjected to different treatments for 24 h (Fig. 8A). Undifferentiated C6 glioma cells (cultured in serum-free DMEM–0.1% DMSO, with or without A1254, 3 or 9 μM) had a basal level of phosphorylation on several protein bands that was more prominent on some bands (Mr of 200, 78, 65 and 28 kDa). The treatments with dbcAMP (1 mM) or bis (0.125 μM) and the co-exposure to dbcAMP and bis in presence or absence of A1254 (3 or 9 μM) completely eliminated these phosphorylations. Conversely, the co-exposure to dbcAMP and A1254, at the concentration of 9 μM, led to a PKC activation, as revealed by the presence of phosphorylated protein bands that had a pattern similar to that of undifferentiated C6 cells. This finding indicated that A1254 effects are also mediated by PKC activation that plays a critical role in counteracting the cAMP/PKA positive signaling during astrocytic differentiation in C6 cells.
Fig. 8.
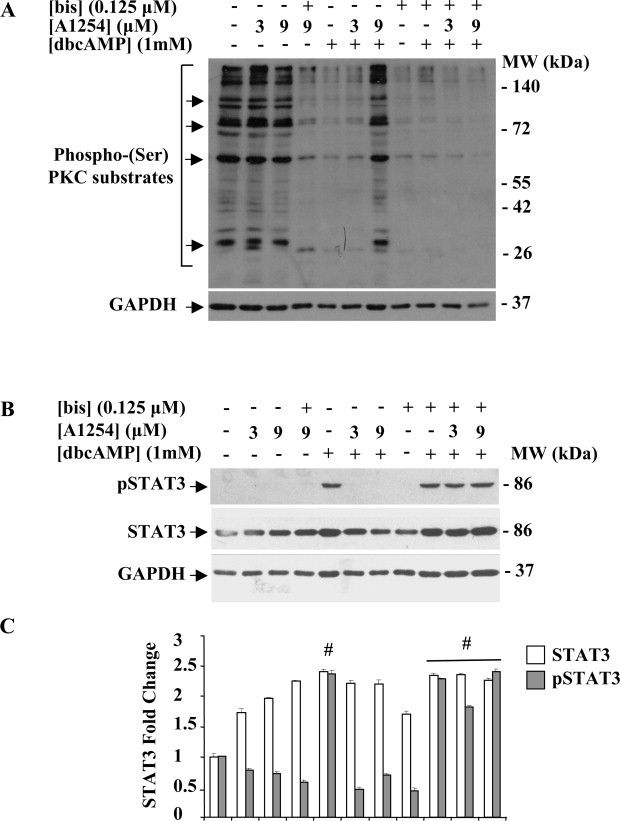
Effects of A1254 treatment on PKC activity (A) and activation status of STAT3 (B) in bis co-treated dbcAMP C6 cells. C6 cells were treated or not with A1254 (3 or 9 μM) in presence or absence of the PKC inhibitor, bis (0.125 μM) during dbcAMP (1 mM) stimulation in serum-deprived medium containing 0.1% (v/v) DMSO, used as vehicle for A1254 and bis. After 24 h incubation, control and treated cells were harvested and equal amounts of protein cell lysates (20 μg) were separated by 12% SDS–PAGE and subjected to immunostaining, as described in Section 2.5. (A) Western blotting using an anti-phospho-(Ser) PKC substrates showing PKC substrates phosphorylation. Arrows indicated the more abundant phosphorylated protein bands (Mr of 200, 78, 65 and 28 kDa). (B) Western blotting showing phospho-Serine727 form of STAT3 and STAT3 total protein expression levels. GAPDH was used as loading control for cell lysates. Signals were revealed by immunostaining and ECL, as described in Section 2.5. (C) Densitometric analysis of phospho-STAT3 and total protein expression levels; fold changes were calculated by first normalizing to GAPDH levels in individual samples and then relative to untreated control (cells cultured in serum-free DMEM with 0.1% (v/v) DMSO, vehicle) set as 1. Each bar represents the mean ± SEM (n = 3). Columns with (#) were statistically different from untreated control or dbcAMP-differentiated cells (#p < 0.001).
Since cAMP induced GFAP expression in C6 cells is accompanied with an increase in phosphorylation level of STAT3 (pSTAT3), which is a transcription activator for GFAP promoter [23,24,30], next we examined whether PCBs treatment modulates the phosphorylation status of STAT3 during dbcAMP induced astrocytic differentiation in C6 cells. Our results (Fig. 8B) demonstrated that the stimulation with dbcAMP (1 mM) for 24 h induced a great elevation in phosphorylation level of STAT3 on Ser727 compared with that of undifferentiated C6 glial cells, cultured in serum-free DMEM–0.1% DMSO, with or without A1254 (3 or 9 μM). The exposure to A1254 (3 and 9 μM) almost completely suppressed dbcAMP induced STAT3 phosphorylation. The treatment with the PKC inhibitor, bis, alone or in combination with dbcAMP, did not alter the activation status of STAT3 compared to control and dbcAMP stimulated cells. Interestingly, the co-exposure to A1254 and bis of dbcAMP-stimulated cells increased either STAT3 protein and phosphorylation levels, that were similar to those induced by dbcAMP. This finding indicates that A1254 reduced cAMP-dependent phosphorylation of STAT3 on Ser727 requires inhibition of PKC activity.
4. Discussion
Astrocytes are the main class of neuroglia involved in the regulation of brain microenvironment, in particular as regards neurotransmitter and ionic homeostasis, metabolic support of neurons, regulation of energy metabolism, synaptic transmission and neuronal excitability, synaptic generation, detoxification, free-radical scavenging, metal sequestration, development, and maintenance of blood–brain barrier, guidance of neuronal migration and immune function [31]. These cells are among the first lines of defense in the nervous system and are involved in activities which maintain an environment optimally suited for neuronal functions.
A1254 and polybrominated diphenyl ethers (PBDEs), compounds that have similar structure to PCBs, exert differential cytotoxic effects on human astrocytoma cells [20]. There are few studies in literature that explain the role of PCBs on astrocytes, mainly the effect of PCBs mixture, called Aroclor, is poorly understood.
We used the rat C6 glioma cell line that is commonly used to perform differentiation studies, because these cells possesses progenitor properties that reflect oligodendrocytic and astrocytic phenotypes [32,33]. In addition, C6 cells have been extensively used for the study of factors and conditions which play a role in the proliferation and differentiation of glial cells [33]. In the present study, we first demonstrated that the exposure to A1254 induced cytotoxic effect in C6 cells, in a concentration and time-dependent manner (Fig. 1). Interestingly, the IC50 value was lower than that detected in neuroblastoma cell line [14], thus suggesting a higher sensitivity of C6 glial cells to PCBs. However, the most intriguing finding of the present study is the evidence for a consistent down-regulation of dbcAMP-induced GFAP expression levels following exposure of C6 cells to A1254 (Figs. 2 and 3). These results strongly suggest that PCBs exposure is able to significantly impair astrocytic differentiation of glial cells. Cell differentiation requires the modulation of multiple biochemical pathways that must be coordinate. The exposure of C6 cells to dbcAMP is known to inhibit cell growth and to induce a change of morphology toward an astrocytic phenotype [34] as reflected by increased expression of the astrocytic markers, GFAP [35,36]. In C6 cells, induction of GFAP synthesis is a crucial event that promotes cytoskeleton reorganization required for the formation of spindle shape processes during astrocytic differentiation. The signaling cascade of cAMP-induced astrocytic differentiation is not yet fully elucidated, and the role of PKA in C6 cell differentiation is still under investigation. Although all components of the PKA-CRE signaling pathway are present in C6 cells, some authors reported that cAMP-induced activation of GFAP synthesis is independent of PKA activation [35,37,38]. In fact, the CRE-like sequence (TGACCTCA) present in the mouse and human GFAP promoters is not conserved in rat [39–41] and no other CRE sequences are detected in the cAMP-dependent enhancer region of the GFAP promoter [37]. However, PKA plays an important part in GFAP induction via activation of the Jak-STAT3 pathway mediated by the transcription of cAMP-response element (CRE) genes in differentiated C6 cells [28,30].
Although some of the effects induced by cAMP are exerted through PKA, some actions have been reported to be mediated by PKC. In fact, in C6 cells, GFAP expression is also regulated by a PKC-dependent mechanism [35,42]. Besides, activation of PKC leads to the phosphorylation of proteins which are involved in proliferation and differentiation of glial cells [43,44].
C6 cells express specific PKC isoforms that correlate to different phenotypes through modulation of GFAP expression: PKCα induces a strong inhibition of GFAP expression whereas PKCβ and γ increase the expression of this protein [45].
In fact, it has been shown that in C6 cells the treatment with dbcAMP causes a decrease of PKC α isoform resulting in an increase of the astrocytic marker GFAP [45]. On the other hand, it has been demonstrated that PCBs are able to activate PKC translocation [27,46] and more recently, Madia et al. [20] have highlighted that A1254 exposure induces just the translocation of PKCα and ɛ isoforms. Therefore, it is plausible to suggest that the effect of PCBs on PKC isoforms antagonizes that of dbcAMP resulting in the detected GFAP decrease with a consequent inhibition of astrocytic differentiation. This hypothesis was confirmed using a specific inhibitor of PKC, bis, that reverted the impairment on dbcAMP differentiation triggered by A1254 in C6 cells (Figs. 6 and 7).
As regards the possible molecular mechanism by which PCBs can affect astrocytic differentiation in C6 cells, we hypothesized that the reduction of GFAP expression levels exerted by PCBs might involve an effect of these agents on PKC synthesis and/or activity. The involvement of PKC in PCB-induced decrease of GFAP expression induced by dbcAMP is further proved by the results showing that while the treatment with dbcAMP (1 mM) completely eliminated phosphorylation of PKC-specific substrates, the co-exposure to dbcAMP and A1254, at the concentration of 9 μM, led to a PKC activation, as revealed by the presence of phosphorylated protein bands that had a pattern similar to that of undifferentiated C6 cells (Fig. 8A). This finding clearly indicates that A1254 effects are also mediated by PKC activation that plays a critical role in counteracting the cAMP/PKA positive signaling during astrocytic differentiation in C6 cells.
Another issue that deserves to be discussed is the effect of A1254 exposure on the well known increase of phosphorylation level of STAT3 that accompanies cAMP-induced GFAP expression in glial and C6 cells [28,30] and, how this effect may be associated with the involvement of PKC in the mechanism of action of PCBs in our experimental model. Indeed, the results of the present study showed that A1254 exposure inhibited the increase of STAT3 phosphorylation induced by cAMP and, the PKC inhibitor, bis, blocked this inhibition. This result suggests that A1254-reduced cAMP-dependent phosphorylation of STAT3 on Ser727 requires inhibition of PKC activity (Fig. 8).
It is interesting to underline that in the same range of concentrations, A1254 caused an inhibition of skeletal muscle differentiation evaluated as fusion of myoblasts into multinucleated myotubes and on the basis of the increase of creatine-kinase activity [47]. On the other hand, it has been also demonstrated that PCBs could interfere with the process of neural differentiation. In fact, it was found that PCB 118, a congener known to widely contaminate human population, might alter the course of oligodendrocyte formation in primary Normal Human Neural Progenitor (NHNP) cells [48].
In summary, our results indicate that PCBs are cytotoxic to C6 glial cells and that, at sub-toxic concentrations, they may affect glial differentiation causing either a reduction of GFAP expression levels or an inhibition of change in cell morphology toward an astrocytic phenotype. Furthermore, our results suggest that one of the possible mechanisms involved in the negative effect exerted by PCBs on glial differentiation might be an interference with PKC pathway. Further studies will be necessary to clarify whether regulation of GFAP expression can be mediated by direct effect of PKC/PKA on this protein, a cross-talk between them or indirectly by interactions with other signaling pathway.
Acknowledgments
We are grateful to Dr. C. Muscoli and Prof. V. Mollace for helpful discussion (University “Magna Graecia”, Catanzaro, Italy).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Compston A., Zajicek J., Sussman J., Webb A., Hall G., Muir D., Shaw C., Wood A., Scolding N. Glial lineages and myelination in the central nervous system. J. Anat. 1997;190:161–200. doi: 10.1046/j.1469-7580.1997.19020161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschner M., Syversen T., Souza D.O., Rocha J.B., Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz. J. Med. Biol. Res. 2007;40:285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- 3.Korrick S., Altshul L. High breast milk levels of polychlorinated biphenyls (PCBs) among four women living adjacent to a PCB-contaminated waste site. Environ. Health Perspect. 1998;106:513–518. doi: 10.1289/ehp.98106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilson H.A., Jacobson J.L., Rogan W.J. Polychlorinated biphenyls and the developing nervous system: cross-species comparisons. Neurotoxicol. Teratol. 1990;12:239–248. doi: 10.1016/0892-0362(90)90095-t. [DOI] [PubMed] [Google Scholar]
- 5.McKinney J., Walter C. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Health Perspect. 1994;102:290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faroon O., Jones D., de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol. Ind. Health. 2001;16:305–333. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- 7.Chung Y.W., Nunez A.A., Clemens L.G. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol. Behav. 2001;74:363–370. doi: 10.1016/s0031-9384(01)00579-0. [DOI] [PubMed] [Google Scholar]
- 8.Caudle W.M., Richardson J.R., Delea K.C., Guillot T.S., Wang M., Pennell K.D., Miller G.W. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol. Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- 9.Aly H.A., Domènech O. Aroclor 1254 induced cytotoxicity and mitochondrial dysfunction in isolated rat hepatocytes. Toxicology. 2009;262:175–183. doi: 10.1016/j.tox.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Lee D.W., Opanashuk L.A. Polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Mariussen E., Myhre O., Reistad T., Fonnum F. The polychlorinated biphenyl mixture aroclor 1254 induces death of rat cerebellar granule cells: the involvement of the N-methyl-D-aspartate receptor and reactive oxygen species. Toxicol. Appl. Pharmacol. 2002;179:137–144. doi: 10.1006/taap.2002.9353. [DOI] [PubMed] [Google Scholar]
- 12.Canzoniero L.M., Adornetto A., Secondo A., Magi S., Dell’aversano C., Scorziello A., Amoroso S., Di Renzo G. Involvement of the nitric oxide/protein kinase G pathway in polychlorinated biphenyl-induced cell death in SH-SY 5Y neuroblastoma cells. J. Neurosci. Res. 2006;84:692–697. doi: 10.1002/jnr.20971. [DOI] [PubMed] [Google Scholar]
- 13.Inglefield J.R., Mundy W.R., Shafer T.J. Inositol 1,4,5-triphosphate receptor-sensitive Ca(2+) release, store-operated Ca(2+) entry, and cAMP responsive element binding protein phosphorylation in developing cortical cells following exposure to polychlorinated biphenyls. J. Pharmacol. Exp. Ther. 2001;297:762–773. [PubMed] [Google Scholar]
- 14.Webb R.G., McCall A.C. Identities of polychlorinated biphenyl isomers in Aroclors. J. Assoc. Offic. Anal. Chem. 1972;55:746–752. [PubMed] [Google Scholar]
- 15.Tilson H.A., Kodavanti P.R. Neurochemical effects of polychlorinated biphenyls: an overview and identification of research needs. Neurotoxicology. 1997;18:727–743. [PubMed] [Google Scholar]
- 16.Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 17.Parker K.K., Norenberg M.D., Vernadakis A. Transdifferentiation of C6 glial cells in culture. Science. 1980;208:179–181. doi: 10.1126/science.6102413. [DOI] [PubMed] [Google Scholar]
- 18.Hu W., Onuma T., Birukawa N., Abe M., Ito E., Chen Z., Urano A. Change of morphology and cytoskeletal protein gene expression during dibutyryl cAMP-induced differentiation in C6 glioma cells. Cell Mol. Neurobiol. 2008;4:519–528. doi: 10.1007/s10571-007-9229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodavanti P.R., Narayanan K., Nobuyoshi Y., Derr-Yellin E.C., Ward T.R., Burgin D.E., Tilson H.A., Birnbaum L.S. Differential effects of two lots of Aroclor 1254: congener-specific analysis and neurochemical end points. Environ. Health Perspect. 2001;109:1153–1161. doi: 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madia F., Giordano G., Fattori V., Vitalone A., Branchi I., Capone F., Costa L.G. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol. Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Gurley G.H., Jelaso A.M., Ide C.F., Spitsbergen J.M. Effects of polychlorinated biphenyls (PCBs) of neurotrophic factors in C6 glial cells in culture. Neurotoxicology. 2007;28:1264–1271. doi: 10.1016/j.neuro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Asano H., Horinouchi T., Mai Y., Sawada O., Fujii S., Nishiya T., Minami M., Katayama T., Iwanaga T., Terada K., Miwa S. Nicotine- and tar-free cigarette smoke induces cell damage through reactive oxygen species newly generated by PKC-dependent activation of NADPH oxidase. J. Pharmacol. Sci. 2012;118:275–287. doi: 10.1254/jphs.11166fp. [DOI] [PubMed] [Google Scholar]
- 23.Takanaga H., Yoshitake T., Hara S., Yamasaki C., Kunimoto M. cAMP-induced astrocytic differentiation of C6 glioma cells is mediated by autocrine interleukin-6. J. Biol. Chem. 2004;279:15441–15447. doi: 10.1074/jbc.M311844200. [DOI] [PubMed] [Google Scholar]
- 24.Takanaga H., Yoshitake T., Yatabe E., Hara S., Kunimoto M. Beta-naphthoflavone disturbs astrocytic differentiation of C6 glioma cells by inhibiting autocrine interleukin-6. J. Neurochem. 2004;90:750–757. doi: 10.1111/j.1471-4159.2004.02681.x. [DOI] [PubMed] [Google Scholar]
- 25.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein dye binding. Ann. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Kodavanti P.R., Shafer T.J., Ward T.R., Mundy W.R., Freudenrich T., Harry G.J., Tilson H.A. Differential effects of polychlorinated biphenyl congeners on phosphoinositide hydrolysis and protein kinase C translocation in rat cerebellar granule cells. Brain Res. 1994;662:75–82. doi: 10.1016/0006-8993(94)90797-8. [DOI] [PubMed] [Google Scholar]
- 29.Kodavanti P.R., Tilson H.A. Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways. Ann. NY Acad. Sci. 2000;919:97–105. doi: 10.1111/j.1749-6632.2000.tb06872.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.S., Han J., Lee S.H., Park J.A., Kim K.W. Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J. Cell Sci. 2010;123:1959–1968. doi: 10.1242/jcs.063784. [DOI] [PubMed] [Google Scholar]
- 31.Markiewicz I., Lukomska B. The role of astrocytes in the physiology and pathology of the central nervous system. Acta Neurobiol. Exp. 2006;66:343–358. doi: 10.55782/ane-2006-1623. [DOI] [PubMed] [Google Scholar]
- 32.Vernadakis A., Kentroti S., Brodie C., Mangoura D., Sakellaridis N. C-6 glioma cells of early passage have progenitor properties in culture. Adv. Exp. Med. Biol. 1991;296:181–195. doi: 10.1007/978-1-4684-8047-4_18. [DOI] [PubMed] [Google Scholar]
- 33.Coyle D.E. Adaptation of C6 glioma cells to serum-free conditions leads to the expression of a mixed astrocyte–oligodendrocyte phenotype and increased production of neurite-promoting activity. J. Neurosci. Res. 1995;41:374–385. doi: 10.1002/jnr.490410310. [DOI] [PubMed] [Google Scholar]
- 34.Hu W., Onuma T., Birukawa N., Abe M., Ito E., Chen Z., Urano A. Change of morphology and cytoskeletal protein gene expression during dibutyryl cAMP-induced differentiation in C6 glioma cells. Cell Mol. Neurobiol. 2008;4:519–528. doi: 10.1007/s10571-007-9229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Messens J., Slegers H. Synthesis of glial fibrillary acidic protein in rat C6 glioma in chemically defined medium: cyclic AMP-dependent transcriptional and translational regulation. J. Neurochem. 1992;58:2071–2080. doi: 10.1111/j.1471-4159.1992.tb10948.x. [DOI] [PubMed] [Google Scholar]
- 36.Arcone R., Pagliuca M.G., Chinali A., Grimaldi M., Schettini G., Gast A., Pietropaolo C. Thrombin mutants with altered enzymatic activity have an impaired mitogenic effect on mouse fibroblasts and are inefficient modulators of stellation of rat cortical astrocytes. Biochim. Biophys. Acta. 1999;1451:173–186. doi: 10.1016/s0167-4889(99)00086-5. [DOI] [PubMed] [Google Scholar]
- 37.Anciaux K., Van Dommelen K., Nicolai S., Van Mechelen E., Slegers H. Cyclic AMP-mediated induction of the glial fibrillary acidic protein is independent of protein kinase A activation in rat C6 glioma. J. Neurosci. Res. 1997;48:324–333. [PubMed] [Google Scholar]
- 38.Roymans D., Vissenberg K., De Jonghe C., Grobben B., Claes P., Verbelen J.P., Van Broeckhoven C., Slegers H. Phosphatidylinositol 3-kinase activity is required for the expression of glial fibrillary acidic protein upon cAMP-dependent induction of differentiation in rat C6 glioma. J. Neurochem. 2001;76:610–618. doi: 10.1046/j.1471-4159.2001.00077.x. [DOI] [PubMed] [Google Scholar]
- 39.Miura M., Tamura T.A., Mikoshiba K. Cell-specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis- and trans-acting promoter elements for astrocyte specific expression. J. Neurochem. 1990;55:1180–1188. doi: 10.1111/j.1471-4159.1990.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 40.Besnard F., Brenner M., Nakatani Y., Chao R., Purohit H.J., Freese E. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J. Biol. Chem. 1991;266:18877–18883. [PubMed] [Google Scholar]
- 41.Kaneko R., Hagiwara N., Leader K., Sueoka N. Glial-specific cAMP response of the glial fibrillary acidic protein gene in the RT4 cell lines. Proc. Natl. Acad. Sci. USA. 1994;91:4529–4533. doi: 10.1073/pnas.91.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafit-Zagardo B., Kume-Iwaki A., Goldman J.E. Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia. 1988;1:346–354. doi: 10.1002/glia.440010507. [DOI] [PubMed] [Google Scholar]
- 43.Baltuch G.H., Dooley N.P., Villemure J.G., Yong V.W. Protein kinase C and growth regulation of malignant gliomas. Can. J. Neurol. Sci. 1995;22:264–271. doi: 10.1017/s0317167100039457. [DOI] [PubMed] [Google Scholar]
- 44.Baltuch G.H., Dooley N.P., Rostworowski K.M., Villemure J.G., Yong V.W. Protein kinase C isoform alpha overexpression in C6 glioma cells and its role in cell proliferation. J. Neurooncol. 1995;24:241–250. doi: 10.1007/BF01052840. [DOI] [PubMed] [Google Scholar]
- 45.Brodie C., Kuperstein I., Acs P., Blumberg P.M. Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res. Mol. Brain Res. 1998;56:108–117. doi: 10.1016/s0169-328x(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 46.Yang J.H., Derr-Yellin E.C., Kodavanti P.R. Alterations in brain protein kinase C isoforms following developmental exposure to a polychlorinated biphenyl mixture. Brain Res. Mol. Brain Res. 2003;111:123–135. doi: 10.1016/s0169-328x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- 47.Coletti D., Palleschi S., Silvestroni L., Cannavò A., Vivarelli E., Tomei F., Molinaro M., Adamo S. Polychlorobiphenyls inhibit skeletal muscle differentiation in culture. Toxicol. Appl. Pharmacol. 2001;175:226–233. doi: 10.1006/taap.2001.9237. [DOI] [PubMed] [Google Scholar]
- 48.Fritsche E., Cline J.E., Nguyen N.H., Scanlan T.S., Abel J. Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ. Health Perspect. 2005;113:871–876. doi: 10.1289/ehp.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]


