Abstract
Background
Immobilization-induced loss of muscle mass is a complex phenomenon with several parallels to sarcopenic and cachectic muscle loss. Muscle is a large organ with a protein turnover that is orders of magnitude larger than most other tissues. Thus, we hypothesize that muscle loss and regain is reflected by peptide biomarkers derived from type VI collagen processing released in the circulation.
Methods
In order to test this hypothesis, we set out to develop an ELISA assay against an type VI collagen N-terminal globular domain epitope (IC6) and measured the levels of IC6 and an MMP-generated degradation fragment of collagen 6, (C6M) in a human immobilization–remobilization study setup with young (n = 11) and old (n = 9) men. They were subjected to 2 weeks of unilateral lower limb immobilization followed by 4 weeks of remobilization including thrice weekly resistance training, using the contralateral leg as internal controls. Subjects were sampled for strength, quadriceps muscle volume and blood at baseline (PRE), post-immobilization (2W), and post-remobilization (4W). Blood were subsequently analyzed for levels of the C6M and IC6 biomarkers. We subsequently tested if there was any correlation between C6M, IC6, or the C6M/IC6 ratio and muscle mass or strength at baseline. We also tested whether there was any relation between these biomarkers and changes in muscle mass or strength with immobilization or remobilization.
Results
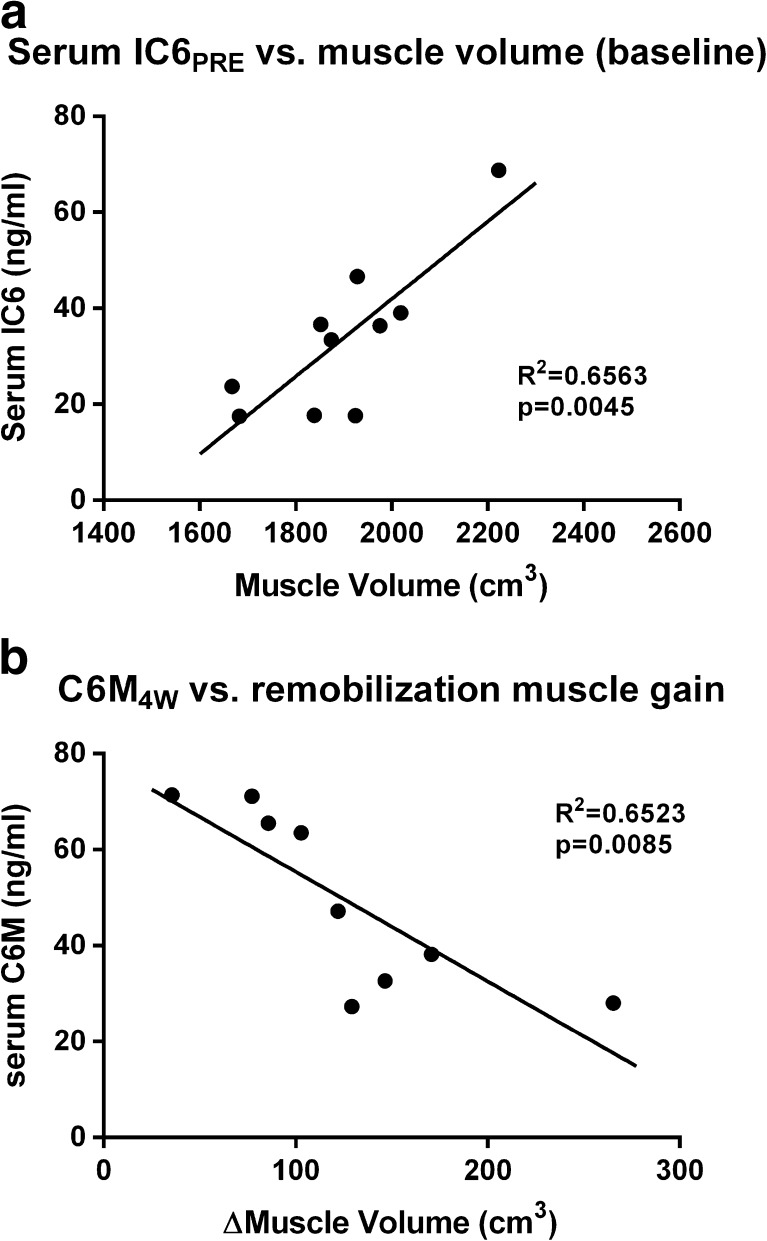
The model produced significant loss of muscle mass and strength in the immobilized leg. This loss was bigger in young subjects than in elderly, but whereas the young recovered almost fully, the elderly had limited regrowth of muscle. We found a significant correlation between IC6 and muscle mass at baseline in young subjects (R2 = 0.6563, p = 0.0045), but none in the elderly. We also found a significant correlation between C6M measured at the 4W time point and the change in muscle mass during remobilization, again only manifesting in the young men(R2 = 0.6523, p = 0.0085). This discrepancy between the young and the elderly may be caused in part by much smaller changes in muscle mass in the elderly and partly by the relative small sample size.
Conclusion
While we cannot rule out the possibility that these biomarkers in part stem from other tissues, our results strongly indicate that these markers represent novel biomarkers of muscle mass or change in muscle mass in young men.
Electronic supplementary material
The online version of this article (doi:10.1007/s13539-013-0114-x) contains supplementary material.
Keywords: Skeletal muscle, Biomarker, Immobilization, Disuse, Remobilization, Resistance exercise
Introduction
Awareness of muscle mass and/or loss as a health parameter and predictor of functional capacity and quality of life is rapidly increasing in the medical as well as the pharmaceutical communities. This is especially true as the proportion of the populations in the western world that can be considered elderly is increasing, as is the incidence of impaired musculoskeletal mass or function. Impaired muscle function reduces habitual physical activity levels, thereby negatively impacting metabolism and cardiovascular health. Also, as muscle tissue is important in the regulation of glucose metabolism and provides amino acids for intermediary metabolism in immune cells, loss of muscle mass impairs glycemic control and reduces immune system capacity. Thus, while there is no question that maintaining muscle mass and function is paramount to health and functional independence, it is unclear to what extent loss of muscle mass is a consequence of pathology and to what extent it may contribute to pathology.
Despite this fact, measuring muscle mass or muscle function is not a standardized part of clinical practice, probably due to the economical or logistical expense in relation to the perceived benefits of such measurements. The emerging interest in muscle loss as more than a symptom may change this, as may availability of logistically more feasible techniques, which holds true in particular for pharmaceutical trials where monitoring primary as well as adverse intervention effects in large cohorts is the main objective. Quantifying muscle pathology, mass, or changes therein has historically been reliant on biophysical tools: (1) mechanical measurements of limb girth or skinfold thickness, (2) conductance/absorbance of X-rays/sound in the body, or (3) magnetic resonance imaging [1, 2]. If a biochemical biomarker of muscle mass or function could be found (in blood or urine), the logistical demands of this technology would likely be of a magnitude that would facilitate its use in clinical practice and trials. Indeed, utilizing new and improved biomarker technologies to improve selection and monitoring of clinical trial cohorts has been proposed to be an important tool in reducing the otherwise exploding expenses associated with conducting clinical trials [3, 4]. Therefore, finding good muscle biomarkers whose measurement is of limited logistical complexity is essential to the clinical fields of sarcopenia and cachexia [1].
Type VI collagen (COL6) is a basement membrane protein expressed in most tissues, but highly abundant in muscle sarcolemma [5]. Defects in the corresponding gene have thus far only been associated with a range of congenital muscle dystrophy phenotypes, most notably but not restricted to, the Bethlem and Ullrich Myopathies, which indicates an indispensable role in muscle tissue [6–9]. Based on the involvement of COL6 in these muscle dystrophies, we set out to elucidate if serum levels of type VI collagen fragments could be used as biomarkers of muscle mass or change therein.
In order to pursue this concept, levels of two type VI collagen fragments were measured in relevant models of muscle loss and muscle gain: a previously described C6M biomarker ELISA kit [6–10] and a novel fragment sequence recognized by our novel antibody and ELISA kit (IC6). C6M is a C-terminal peptide produced through MMP-2/-9-mediated proteolysis of collagen VI. Activities of both MMP-2 and -9 are upregulated in several forms of muscle tissue loss [6, 8, 9, 11] and an increased abundance of this peptide in serum could be expected with immobilization-induced muscle loss. The IC6 recognizes an internal amino acid sequence of the N-terminal globular domain of type VI Collagen, i.e., recognizing fragments set free from partial proteolysis, not just from MMP cleavage. Thus, we expect IC6 fragments to be produced during tissue turnover and remodeling, possibly representing a biomarker of muscle mass in the steady state condition (as muscle tissue turnover is proportional to muscle mass in the steady state). Although collagen VI is present in other tissues, the sheer abundance, gross protein turnover, and indispensability in muscle may indicate that the majority of these peptides in blood are primarily derived from muscle tissue turnover.
The protein component that makes up the vast majority of the volume in otherwise healthy muscle is the myofibrillar proteins. As collagen VI is a sarcolemmal protein, any relation to muscle mass would depend on a similar change in turnover in myofibrillar proteins relative to extracellular matrix muscle proteins, i.e. that the ratio between myofibrillar proteins and ECM proteins are not skewed with interventions. While this ratio does seem to change in several animal studies, this does not seem to be the case with human muscle during immobilization [12, 13]. Also from a biological point of view, it makes sense that the total amount of sarcolemma (and thus sarcolemmal proteins) is related to the size of myofibers.
Summarizing, we hypothesized that IC6 is a biomarker of muscle tissue turnover or possibly muscle tissue, whereas C6M is a (positive) biomarker of muscle protein degradation.
Methods
Subjects
Twenty healthy men, 9 old (OM) and 11 young (YM) (Table 1), volunteered to participate in the study. Before immobilization, there was no difference in body weight between OM and YM, whereas OM had a larger percentage of body fat than YM [11]. No weight changes occurred during the study period. To minimize potential differences in physical activity as a confounding variable, subjects with similar activity levels were included (OM, 5.2 ± 1.4 h/week; YM, 5.0 ± 0.9 h/week), assessed by using an occupational and recreational activity questionnaire [14]. None of the subjects had previously participated in systematic resistance training. All subjects underwent medical evaluation, including review of previous medical history and physical examination before participation, and none had a previous record of acute or chronic illness or took any medication affecting skeletal muscle function. All subjects were informed of the risks associated with the investigation and provided their written, informed consent. The study (KF01-322606) was approved by the local Ethics Committee of Copenhagen in accordance with the Helsinki declaration.
Table 1.
Subject demographics. Demograhic stats for the young and old subjects included in the present study. Data are represented as means ± SEM
| Young | Old | |
|---|---|---|
| n | 11 | 9 |
| Age | 24.4 ± 0.5 | 67.3 ± 1.3 |
| Height | 181.4 ± 1.8 | 178.7 ± 2.6 |
| Weight | 72.2 ± 2.3 | 84.8 ± 3.4 |
| BMI | 22.1 ± 0.5 | 26.3 ± 0.5 |
| Body fat | 24.5 + 5.7 | 26.0 ± 3.9 |
| Activity level | 5.0 ± 0.9 | 5.2 ± 1.4 |
Experimental procedure
Familiarization with the testing procedures was carried out in separate sessions 2 weeks before the start of the study. All subjects underwent 2 weeks of lower limb cast immobilization, followed by 4 weeks of resistance training. Blood sampling and testing of muscle mechanical function was performed on separate days 1 week before (PRE) and 24 h after immobilization (2W), as well as 48 h after retraining (4W). Subjects were instructed not to engage in any vigorous physical activity 24 h before a test session. Following blood sampling, each test session included assessment of body height and weight, as well as measurements of selected parameters of knee extensor muscle mechanical function, which was always preceded by a brief low-intensity warm-up on a cycle ergometer (5 min, 50–150 W). To minimize the influence from diurnal variation, each subject was tested at the same time of day (±2 h). Strength testing and quadriceps muscle volumetry was performed as previously described [11, 15]. Briefly, muscle volume was calculated from serial T1-weighted MRI scans from the just below the femoral condyle to the knee three times by a blinded observer. Muscle strength was measure in a KinCom dynamometer at 70° knee angle from three successive attempts at generating maximal isometric force.
Immobilization
The immobilization procedure has previously been described in detail [7, 11]. In brief, immobilization was accomplished by unilateral whole leg casting (randomly selected limb) using a lightweight cast (X-lite, Allard) with the knee joint fixed at an angle of 30° (0° full extension). The cast was not removable at any time during the immobilization period. Subjects were carefully instructed to perform all ambulatory activities on crutches, to abstain from ground contact, and to refrain from performing muscle contractions in the immobilized leg. , To reduce the potential risk of venous thrombosis, subjects were informed to perform isolated, unloaded plantar and dorsal ankle flexions in the immobilized leg several times a day [10, 16–18].
Retraining
After removal of the cast, subjects received manual mobilization of their immobilized leg by a physiotherapist to ensure normal range of motion around the knee joint. Retraining began 2 days after cast removal and consisted of 4 weeks of unilateral strength training of the immobilized leg (three sessions/week) to yield a total of 12 training sessions. None of the subjects missed any training sessions. All training sessions were supervised, and subjects were continuously provided with feedback on the performed exercises. After adequate warm-up, subjects performed knee extension, leg press, and knee flexion in load-adjustable machines (Technogym International). Subjects performed three to five sets of 10–12 repetitions, decreasing in set length and increasing in set number and intensity in a progressive manner. Training loads were progressively adjusted in the first training session of each week by use of 5RM tests.
ELISA development
Development of the novel IC6 ELISA assay is described in the supplementary material. Briefly, the antigen of choice was used to raise antibodies in mice that were selected for antibody titer response. Following several booster shots, their spleens were removed and used to lymphocyte isolation. Spleen lymphocytes were used to create hybridomas. Hybridomas were selected for specificity and selectivity. The best candidate antibody from selected hybridomas entered into construction and optimization of a competitive ELISA assay. During biological characterization we have shown extraction of IC6 fragments from human and rat muscle samples (data not shown).
Biomarker assays
All biomarker assays were competitive ELISA assays based on proprietary antibodies against the relevant peptides. Each ELISA assay was conducted as follows: streptavidin-coated 96-well ELISA plates were incubated with a biotinylated coater in 100 μl coating buffer for 30 min at 20 °C on a shaker (at 300 rpm). Next, the plate was incubated with 20 μl of sample or standard/calibrator and 100 μl of HRP-conjugated monoclonal antibody diluted in Ab incubation buffer for a defined period of time in a fixed-temperature cabinet. After each incubation step, the plate was washed five times in washing buffer (20 mM Tris, 50 mM NaCl, pH 7.2). Finally, 100 μl tetramethylbenzinidine (TMB) (Kem-En-Tec cat. no. 438OH) was added and the plate was incubated for 15 min at 20 °C in the dark. The TMB reaction was stopped by adding 100 μl stopping solution (1 % H2SO4) and measured spectrophotometrically at 450 nm with 650 nm as the reference. The specifics of each assay can be found in Table 2.
Table 2.
ELISA assay technical specifications. Specifications of the competitive ELISA assays, IC6 and C6M, utilized in this study
| IC6 | C6M | |
|---|---|---|
| Coater peptide | Biotin-ADWGQSRDAEEIAISQ | YRGPEGPQGP-K-Biotin |
| Coater buffer | 25 mM PBS-BTB pH 7.4 | 25 mM Tris/BTB, 1 % BSA, 0.1 % Tween-20, pH 8.0 |
| Ab incubation buffer | 25 mM PBS-BTB pH 7.4 | 25 mM Tris/BTB, 1 % BSA, 0.1 % Tween-20, +5 % Liquid II, pH 8.0 |
| Ab incubation conditions | 20 h at 4 °C | 1 h at 20 °C on a shaker at 300 rpm |
| Standard/calibrator concentration range | 20–0.3 ng/ml | 242.6–0.5 ng/ml |
Statistical analysis
All biomarkers were compared to muscle strength (knee extensor MVC) and quadriceps volume (by MRI) at baseline. Also, each biomarker were compared to the change in muscle volume in the preceding period, e.g., biomarker levels at 2W vs. change in muscle mass or strength from PRE to 2W. All biomarkers analysis was done using Graphpad Prism 6 (v6.00) for Windows.
Results
Changes in muscle mass, strength, and biomarker levels over time
Levels of IC6 and C6M did not differ significantly between age groups or time points (Table 3). Strength and muscle mass data have been published previously [11], but are summarized briefly in Table 5 for reference with permission from the original publisher (APS). Briefly, muscle mass was lost in both groups, but muscle loss was more pronounced in YM than in OM. During remobilization, there was little muscle regain in OM, whereas there was almost complete recovery in YM. For strength, both groups lost strength and regained it fully upon recovery.
Table 3.
Absolute levels of biomarkers and muscle size/strength data. Overview of average levels of biomarkers, biomarker ratios, muscle volume, and strength over the course of the three sampling time points in the study. Data are shown as means and SD's for the YM and OM groups individually and for both groups pooled
| Young | Elderly | All combined | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| (ng/ml) | (ng/ml) | (ng/ml) | ||||
| IC6 (ng/ml) | ||||||
| PRE | 32.22 | 16.02 | 24.40 | 11.67 | 28.70 | 14.43 |
| 2W | 31.86 | 17.29 | 26.07 | 12.72 | 29.25 | 15.31 |
| 4W | 28.51 | 13.99 | 23.372 | 11.69 | 26.08 | 12.87 |
| C6M (ng/ml) | ||||||
| PRE | 46.88 | 16.44 | 51.85 | 17.37 | 49.11 | 16.60 |
| 2W | 47.40 | 15.36 | 53.80 | 19.71 | 50.28 | 17.27 |
| 4W | 52.88 | 20.69 | 55.86 | 20.32 | 54.29 | 20.00 |
| C6M/IC6 ratio (unitless) | ||||||
| PRE | 1.83 | 1.27 | 2.63 | 1.54 | 2.19 | 1.42 |
| 2W | 1.81 | 1.09 | 2.58 | 1.63 | 2.16 | 1.38 |
| 4W | 2.31 | 1.63 | 3.23 | 2.32 | 2.74 | 1.99 |
| Quadriceps volume (cm3) | ||||||
| PRE | 1898 | 161.0 | 1305 | 147.7 | 1654 | 336.6 |
| 2W | 1729 | 116.1 | 1250 | 155.9 | 1532 | 275.1 |
| 4W | 1872 | 155.3 | 1278 | 143.8 | 1628 | 334.8 |
| Knee extensor strength (Nm) | ||||||
| PRE | 215.2 | 28.41 | 139.1 | 21.15 | 183.9 | 45.97 |
| 2W | 171.3 | 24.26 | 117.8 | 23.88 | 149.2 | 35.78 |
| 4W | 227.3 | 30.89 | 146.9 | 30.60 | 194.2 | 50.50 |
Table 5.
Correlation matrices of serum biomarkers vs. muscle mass
| Correlation chart for individual biomarker measurements against changes in muscle massb | ||||||
|---|---|---|---|---|---|---|
| Young | Old | All | ||||
| Δm M,2W | Δm M,4W | Δm M,2W | Δm M,4W | Δm M,2W | Δm M,4W | |
| C6M2W | −0.297 | −0.089 | −0.230 | 0.235 | −0.004 | −0.084 |
| IC62W | −0.737* | 0.493 | −0.345 | 0.217 | −0.554* | 0.444 |
| C6M/IC6 ratio2W | 0.274 | −0.386 | 0.362 | −0.348 | 0.415 | −0.412 |
| C6M4W | 0.227 | −0.808** | 0.117 | −0.263 | 0.232 | −0.525* |
| IC64W | −0.120 | −0.183 | −0.346 | 0.283 | −0.336 | 0.100 |
| C6M/IC6 ratio4W | 0.181 | −0.393 | 0.469 | −0.583 | 0.464 | −0.477 |
Correlation chart for individual biomarker measurements against changes in muscle mass: Correlation matrix showing correlation coefficients (R) for correlations between measured biomarker levels (or ratio between them, C6M/IC6) measured at the 2W and 4W time points and changes in muscle mass from PRE to 2W time points and 2W to 4W time points. Δm indicates changes in muscle mass up to the specified period, e.g. Δm M,4W is the change in muscle mass from the 2W time point to the 4W time point
*0.01 < p < 0.05; **p < 0.01
Correlation to muscle mass or strength at baseline
We found significant correlations between IC6 levels and muscle mass at baseline, across all the subjects (p = 0.038). This is primarily driven by a strong correlation in the young (p = 0.004) (Fig. 1a), whereas none is present if the analysis is isolated to the elderly subjects (Table 4). No significant correlations to muscle strength manifested.
Fig. 1.
Biomarker correlations vs. muscle characteristics: a Serum IC6PRE vs. muscle volume (baseline): IC6 displays a highly significant positive correlation with muscle mass at baseline in young men. Although this is not conclusive proof that circulating IC6 is muscle-derived it substantiates that IC6 is a biomarker of muscle mass. Due to incomplete blood sample sets, there is only an n of 10 for this correlation. b C6M4W vs. remobilization muscle gain: C6M titer is a negative biomarker for muscle hypertrophy during retraining. Lower levels of C6M following 4 weeks of retraining correlates with bigger hypertrophy during the same period. Due to incomplete blood sample sets, there is only an n of 9 for this correlation
Table 4.
Correlation matrices of serum biomarkers vs. muscle mass or strength parameters
| Correlation to muscle mass or strength at baselinea | ||||||
|---|---|---|---|---|---|---|
| Young | Old | All | ||||
| m M, PRE | F M, PRE | m M, PRE | F M, PRE | m M, PRE | F M, PRE | |
| C6MPRE | 0.263 | −0.012 | 0.321 | 0.040 | −0.129 | −0.230 |
| IC6PRE | 0.810** | 0.151 | 0.108 | 0.007 | 0.507* | 0.298 |
| C6M/IC6 ratio | −0.378 | −0.090 | 0.422 | 0.334 | −0.382 | −0.313 |
Correlation to muscle mass or strength at baseline: Correlation matrix showing correlation coefficients (R) for correlations between measured biomarker levels and muscle mass and strength at baseline for young and old subjects as well as pooled. m M is muscle mass, F M is muscle strength (MVC force)
*0.01 < p < 0.05; **p < 0.01
Correlations to changes in muscle mass or strength
Furthermore, we observed a correlation between C6M levels at the 4W time point and change in muscle mass during remobilization (from the 2W time point to the 4W time point). This was present across all subjects pooled (p = 0.037), but again this was driven by a strong correlation present in the young subjects only (p = 0.008; Fig. 1b), whereas none could be found if the analysis was confined to the elderly (Table 5).
We also found a correlation between IC6 at the 2W time point displayed an inverse correlation with the change in muscle mass during immobilization. This was present across both age groups (p = 0.014), but driven by a correlation in the young men (p = 0.015). However, this correlation appeared to be driven very strongly by a single outlier. No significant correlation to changes in muscle strength manifested, probably due to the bigger variation in strength parameters, effectively making our study underpowered to detect such relationships.
Discussion
In the present study, we developed an antibody and corresponding ELISA assay detecting a novel Type VI collagen fragment (termed IC6) and measured said fragment and another previously described collagen type VI fragment, termed C6M, in a model of immobilization/remobilization-induced muscle loss and recovery in young and elderly subjects. This was done in order to elucidate whether the C6M and IC6 peptides are biomarkers of muscle mass and/or changes therein. We found that levels of the IC6 peptide correlated with muscle mass at baseline and furthermore that levels of the C6M peptide correlated with the anabolic response during remobilization, particularly in the young.
IC6 assay
We have developed a solid ELISA assay, showing a high degree of specificity for fragments containing a sequence unique to the N-terminal globular domain of the Collagen type VI α1 chain. We propose that IC6 epitopic fragments are released to the systemic circulation during increased tissue turnover in muscle, a process having been shown to occur during protein turnover in a number of other tissues [10, 19, 20].
Biomarkers of muscle mass
We report a significant correlation between the amount of the immunoreactive IC6 peptide and muscle mass at baseline, particularly in young men.
Previously, the best-characterized biomarker of muscle mass that could be measured in bodily fluids was creatinine. Creatinine is produced at a continuous rate from metabolism of creatine and excreted with no tubular reabsorption explaining its use a marker of glomerular filtration rate. As the majority of creatine is in muscle, the notion that creatinine could be a marker of muscle mass was presented as early as 1919 [21]. Later, this was shown in several human studies to hold true, most notably by work of Heymsfield et al. [22, 23]. However, as diuresis varies along the course of the day, this technique requires 24-h urine sampling. Furthermore, as meat contains creatine, which is converted to creatinine during digestion, meat intake will significantly perturb creatinine measurements. During optimal circumstances (average of 24-h urine of days 4 to 8 following the initiation of a meat-free diet), urine creatinine correlates strongly with measures of muscle mass, e.g., arm circumference (R2 = 0.80–0.85) [22, 24]. The same group have shown that 24-h urinary levels of the methylated amino acid 3-methyl histidine (3MH) produced in muscle also correlates well with muscle mass (R = 0.88) under similar conditions (last 3 days of a week on a meat-free diet) [25]. While these correlations may seem impressive, putting subjects in clinical trials or patients on meat-free diets, waiting for 4 days (for dietary creatinine to clear the system) and sampling 24-h urine continuously for several days makes this technology less than ideal.
Thus, the reported relation between IC6 levels and muscle mass is stronger than those previously reported for any biomarker technology of comparable logistical sampling requirements and we therefore propose this a novel biomarker of muscle mass in young subjects. Further validation in larger studies will show under what circumstances we can extend this relation to elderly subjects as well.
Given that IC6 seem to be a biomarker of muscle mass in itself, one might think that changes in the levels of IC6 should be a biomarker of change in muscle mass. While this may be the case for gross changes, analyzing for this was not part of the initial analysis for statistical reasons. As the inter- and intra-assay variations are 11.75 and 11.05 %, respectively, changes in biomarkers across time points will have the same variability. This means that small changes in the correlate, muscle mass, become very difficult to detect, as they are much smaller than the aforementioned 11 %, and therefore drowning in statistical noise.
Biomarkers of anabolic response
As of now, the only way to truly get a “snapshot” of the process of muscle anabolism or catabolism, the very processes of tissue accretion or degradation, is through stable isotope-based techniques, involving labeling tissues and measuring enrichment or dilution before and after an intervention. Thus, this technique requires repeated tissue (muscle biopsies) and blood samplings again representing a logistically challenging technique.
Urinary or serum 3MH has been proposed as an alternate marker of muscle protein degradation. This amino acid is produced in actomyosin complexes in all muscle tissues and not subject to reuse through tRNA transfer or intermediary metabolism and thus represents a good biomarker candidate in theory. However, data have been conflicting as interventions shown to increase protein degradation through stable isotope-based techniques, does not consistently result in increases in serum or microdialysis of 3MH [26–28].
In the present work, C6M was found to correlate negatively with the change of muscle mass during remobilization. This indicates that a downregulation of MMP-2 or -9 proteolysis leading to lower levels of the C6M peptide is associated with a better regrowth of muscle, i.e., lower degradation during remobilization leads to better regrowth, which is essentially in agreement with our initial hypothesis. Serum C6M has previously been found to increase with carbon tetrachloride (CCl4) administration and bile duct ligation [10] and circulating levels of collagen VI has been reported to be a biomarker of alcoholic liver disease [29]. In those studies, the presence of collagen VI fragments or the intact peptide were interpreted as markers of hepatic tissue changes. But liver failure, as all organ failures, causes sarcopenia or cachexia, depending on severity and the organ in question, in animals as well as humans [30, 31] and both of the models utilized in the Veidal study, CCl4 administration and bile duct ligation, have been shown to cause muscle loss in themselves [31–33]. Considering that in the present study C6M responds in the absence of an intervention that affects the liver significantly, we therefore consider it likely that the changes reported here could indeed stem from muscle remodeling rather than liver remodeling. As these MMPs have previously been shown to be upregulated with immobilization, this further strengthens the notion that the C6M peptide is a muscle-derived MMP cleavage fragment [6, 9]. Hence, we propose that the collagen VI fragment is a novel marker of anabolic response to training following immobilization. Further research will show if this extends to more generalized anabolic responses, e.g. during normal resistance training, treatment with selective androgen receptor modulators (SARMs) or other anabolic agents.
We also found an inverse correlation between IC6 at the 2W time point and the change in muscle mass during immobilization, but this correlation was driven by a single outlier in the young men. When the same analysis was performed with automatic outlier exclusion, this correlation disappeared (which was not the case for the other reported correlations). As IC6 levels were fairly stable over time, this indicates that the ones with the highest IC6 (and thus highest muscle mass), loses the most muscle, which does seem to hold some merit. But as mentioned, this correlation seems questionable due to the strong influence by a single measurement.
Correlations in young vs. old subjects
The biomarker associations that we report in this paper only manifested strongly in the young subjects in this study. There are several possible explanations to this.
First of all, the loss of muscle mass during immobilization and subsequent recovery during remobilization was significantly smaller in the old individuals than what was observed seen in the young. Thus, if our hypothesized collagen VI fragments are indeed biomarkers of muscle mass or change therein, the study may have been statistically underpowered for the purpose of validating these markers as muscle biomarkers in elderly subjects.
Secondly, the elderly subjects were more heterogenous as such. Almost all the young subjects were university students, whereas the elderly subjects represented more diverse occupations and habit. This was reflected in their habitual physical activity levels, for which the SEM was almost twice as big in the elderly as in the young (Table 1). It has previously been shown that with age absolute levels of biomarkers changes just like their variability is known to increase [34].
Third, given that the present biomarkers are derived from an extracellular matrix protein that is not fully exclusive to muscle, it must be expected that age-associated altered ECM turnover in other tissues can possibly contribute to levels of biomarkers in blood, thereby masking the part of the biomarker signal that is related to muscle.
Unilateral lower limb immobilization as a muscle loss model
The muscle tissue comprises 25–40 % of the total body mass in adults (20–32 kg in a normal 80-kg adult man). The unilateral immobilization through casting from the hip to the toes utilized in our study, affected approximately 25–30 % of the total muscle mass, given that the lower body is considered to contain 50–60 % of total muscle mass and only one leg is immobilized. However, a lesser degree of immobilization for the rest of the body should also be expected as the subjects were using crutches. Thus, the reported 4–9 % loss of quadriceps mass represents a much smaller change in total muscle mass. This actually reinforces the findings regarding the C6M response to reloading as this may indicate that full-body immobilization would have produced bigger changes in C6M levels.
Limitations
We acknowledge that at this point we cannot prove that the measured peptides are derived from muscle mass exclusively. While we can prove that these peptide fragments are produced in muscle, we cannot exclude the possibility that that they are also produced in other tissues. However, one must first consider the contribution of muscle mass to total body mass, relative to the contributions from liver, kidney, skin, fat, or other tissues, and second, it remains that the intervention utilized in this model produced changes in biomarker serum levels that (significantly) correlated with the muscle response to unloading and reloading, supporting the notion that these biomarkers are indeed products of changes in muscle ECM secondary to changes in muscle mass.
Another limiting factor, not on the present findings, but on the concept and application of peptide fragment biomarkers like these, is the absolute absence of knowledge about the kinetics. To the knowledge of the authors, there is no literature describing the rate of appearance, plasma binding/volume of distribution and rate of elimination. Also, there does not appear to be proposed consensus elimination mechanisms for peptide fragment biomarkers as such. This may limit the application of biomarkers in different diseases, where serum binding varies, e.g., through an effect on albumin, or where kidney and liver metabolism is perturbed.
Conclusion
Summarizing, we present two novel biomarkers of muscle mass and the anabolic response to reloading following immobilization in young men. Both can be sampled without any particular logistical requirements and both explain up to 70–80 % of the variation in muscle mass or change in muscle mass. Further research will be undertaken to prove tissue origin of these biomarkers, with other interventions, in more heterogeneous populations and to determine if and under what circumstances these markers work such populations.
Electronic supplementary material
(JPEG 25 kb)
(DOC 58 kb)
Acknowledgments
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle, 2010;1:7–8 (von Haehling S, Morley JE, Coats AJ and Anker SD). The study was supported by grants from the Danish National Research Council, the Danish Rheumatology Association, Faculty of Health Sciences, University of Copenhagen, and the Danish Ministry of Culture.
Conflict of interest
Anders Nedergaard is funded in part by Nordic Bioscience through an industrial post doc collaboration with the Department of Sports Medicine Copenhagen and the Danish Advanced Technology Foundation. The principal business of Nordic Bioscience is biomarker discovery and development of ELISA assays against these biomarkers. Shu Sun is funded in part by Nordic Bioscience, through an industrial PhD collaboration with University of Southern Denmark. Morten Karsdal is CEO of Nordic Bioscience. Kim Henriksen is employed by Nordic Bioscience. Yunyun Luo is employed by Nordic Bioscience. Yi He is funded in part by Nordic Bioscience through an industrial PhD collaboration with University of Southern Denmark. Qiulong Zheng is employed by Nordic Bioscience. Charlotte Suetta and Michael Kjær declare no conflict of interest.
References
- 1.for the International Working Group on Sarcopenia. Cesari M, Fielding RA, Pahor M, Goodpaster B, Hellerstein M, et al. Biomarkers of sarcopenia in clinical trials—recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutrition. 2009;13:724–728. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA Administration: Challenge and opportunity on the critical path to new medical products, 2nd edn. U.S. Department of Health and Human Services, Food and Drug Administration 2004.
- 4.Karsdal MA, Henriksen K, Leeming DJ, Mitchell P, Duffin K, Barascuk N. Biochemical markers and the FDA critical path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 5.Zou Y, Zhang R-Z, Sabatelli P, Chu M-L, Bönnemann CG. Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol. 2008;67:144–154. doi: 10.1097/nen.0b013e3181634ef7. [DOI] [PubMed] [Google Scholar]
- 6.Reznick AZ, Menashe O, Bar-Shai M, Coleman R, Carmeli E. Expression of matrix metalloproteinases, inhibitor, and acid phosphatase in muscles of immobilized hindlimbs of rats. Muscle Nerve. 2003;27:51–59. doi: 10.1002/mus.10277. [DOI] [PubMed] [Google Scholar]
- 7.Bönnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011;7:379–390. doi: 10.1038/nrneurol.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X. Emerging ideas: matrix metalloproteinase-2 in muscle atrophy. Clin Orthop Relat Res. 2011;469:1797–1799. doi: 10.1007/s11999-010-1726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannelli G, De Marzo A, Marinosci F, Antonaci S. Matrix metalloproteinase imbalance in muscle disuse atrophy. Histol Histopathol. 2005;20:99–106. doi: 10.14670/HH-20.99. [DOI] [PubMed] [Google Scholar]
- 10.Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Larsen MR, Nguyen QHT, et al. MMP mediated degradation of type VI collagen is highly associated with liver fibrosis–identification and validation of a novel biochemical marker assay. PLoS One. 2011;6:e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, et al. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107:1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 12.Lemoine JK, Haus JM, Trappe SW, Trappe TA. Muscle proteins during 60-day bedrest in women: impact of exercise or nutrition. Muscle Nerve. 2009;39:463–471. doi: 10.1002/mus.21189. [DOI] [PubMed] [Google Scholar]
- 13.Haus JM, Carrithers JA, Carroll CC, Tesch PA, Trappe TA. Contractile and connective tissue protein content of human skeletal muscle: effects of 35 and 90 days of simulated microgravity and exercise countermeasures. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1722–7. doi: 10.1152/ajpregu.00292.2007. [DOI] [PubMed] [Google Scholar]
- 14.Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38:1104–1115. doi: 10.1161/01.CIR.38.6.1104. [DOI] [PubMed] [Google Scholar]
- 15.Hvid L, Aagaard P, Justesen L, Bayer ML, Andersen JL, Ortenblad N, et al. Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining. J Appl Physiol. 2010;109:1628–1634. doi: 10.1152/japplphysiol.00637.2010. [DOI] [PubMed] [Google Scholar]
- 16.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bleeker MWP, Hopman MTE, Rongen GA, Smits P. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1176–R1177. doi: 10.1152/ajpregu.00718.2003. [DOI] [PubMed] [Google Scholar]
- 18.Sochart DH, Hardinge K. The relationship of foot and ankle movements to venous return in the lower limb. J Bone Joint Surg Br. 1999;81:700–704. doi: 10.1302/0301-620X.81B4.8909. [DOI] [PubMed] [Google Scholar]
- 19.Bay-Jensen AC, Leeming DJ, Kleyer A, Veidal SS, Schett G, Karsdal MA. Ankylosing spondylitis is characterized by an increased turnover of several different metalloproteinase-derived collagen species: a cross-sectional study. Rheumatol Int. 2011;32:3565–3572. doi: 10.1007/s00296-011-2237-8. [DOI] [PubMed] [Google Scholar]
- 20.Bay-Jensen A-C, Sondergaard BC, Christiansen C, Karsdal MA, Madsen SH, Qvist P. Biochemical markers of joint tissue turnover. ASSAY and Drug Development Technologies. 2010;8:118–124. doi: 10.1089/adt.2009.0199. [DOI] [PubMed] [Google Scholar]
- 21.Bürger MZ. Beiträge zum Kreatininstoffwechsel: I. Die Bedeutung des KreatininKoefizienten für die quantitative Bewertung der Muskulatur als Körpergewichtskomponente. II. die Störungen des Muskelstoffwechsels. Zeitschrift fd ges Exper Med. 1919;9:361–399. doi: 10.1007/BF03002912. [DOI] [Google Scholar]
- 22.Wang ZM, Gallagher D, Nelson ME, Matthews DE, Heymsfield SB. Total-body skeletal muscle mass: evaluation of 24-h urinary creatinine excretion by computerized axial tomography. Am J Clin Nutr. 1996;63:863–869. doi: 10.1093/ajcn/63.6.863. [DOI] [PubMed] [Google Scholar]
- 23.Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478. [DOI] [PubMed] [Google Scholar]
- 24.Heymsfield S, McManus C, Smith J. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–690. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Deurenberg P, Matthews DE, Heymsfield SB. Urinary 3-methylhistidine excretion: association with total body skeletal muscle mass by computerized axial tomography. JPEN J Parenter Enteral Nutr. 1998;22:82–86. doi: 10.1177/014860719802200282. [DOI] [PubMed] [Google Scholar]
- 26.Calles-Escandon J, Cunningham JJ, Snyder P, Jacob R, Huszar G, Loke J, et al. Influence of exercise on urea, creatinine, and 3-methylhistidine excretion in normal human subjects. Am J Physiol. 1984;246:E334–8. doi: 10.1152/ajpendo.1984.246.4.E334. [DOI] [PubMed] [Google Scholar]
- 27.Chinkes DL. Methods for measuring tissue protein breakdown rate in vivo. Curr Opin Clin Nutr Metab Care. 2005;8:534–537. doi: 10.1097/01.mco.0000170754.25372.37. [DOI] [PubMed] [Google Scholar]
- 28.Rennie MJ, Phillips S, Smith K. Reliability of results and interpretation of measures of 3-methylhistidine in muscle interstitium as marker of muscle proteolysis. J Appl Physiol. 2008;105:1380–1. doi: 10.1152/japplphysiol.90782.2008. [DOI] [PubMed] [Google Scholar]
- 29.Stickel F, Urbaschek R, Schuppan D, Poeschl G, Oesterling C, Conradt C, et al. Serum collagen type VI and XIV and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig Dis Sci. 2001;46:2025–2032. doi: 10.1023/A:1010616021659. [DOI] [PubMed] [Google Scholar]
- 30.Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list—its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 31.Lin S-Y, Chen W-Y, Lee F-Y, Huang C-J, Sheu WH-H. Activation of ubiquitin-proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: potential role of TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E493–501. doi: 10.1152/ajpendo.00186.2004. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki Y, Miyazaki T, Ohkoshi N, Miyakawa S, Bouscarel B, Tanaka N. Degeneration of skeletal muscle fibers in the rat administrated carbon tetrachloride: similar histological findings of the muscle in a 64-year-old patient of LC with muscle cramp. Hepatol Res. 2002;24:368–378. doi: 10.1016/S1386-6346(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 33.Weber FL, Macechko PT, Kelson SR, Karajiannis E, Hassan MO. Increased muscle protein catabolism caused by carbon tetrachloride hepatic injury in rats. Gastroenterology. 1992;102:1700–1706. doi: 10.1016/0016-5085(92)91733-k. [DOI] [PubMed] [Google Scholar]
- 34.Iki M, Akiba T, Matsumoto T, Nishino H, Kagamimori S, Kagawa Y, et al. JPOS Study Group: reference database of biochemical markers of bone turnover for the Japanese female population. Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos Int. 2004;15:981–991. doi: 10.1007/s00198-004-1634-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPEG 25 kb)
(DOC 58 kb)