Abstract
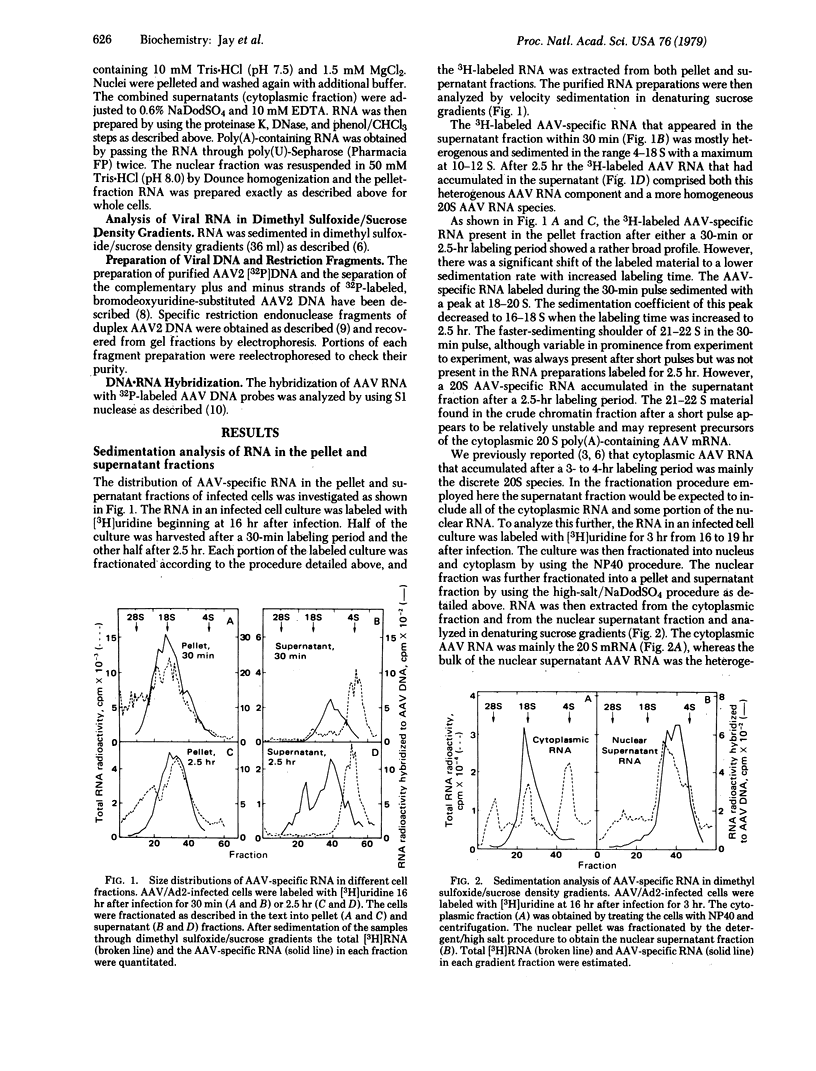
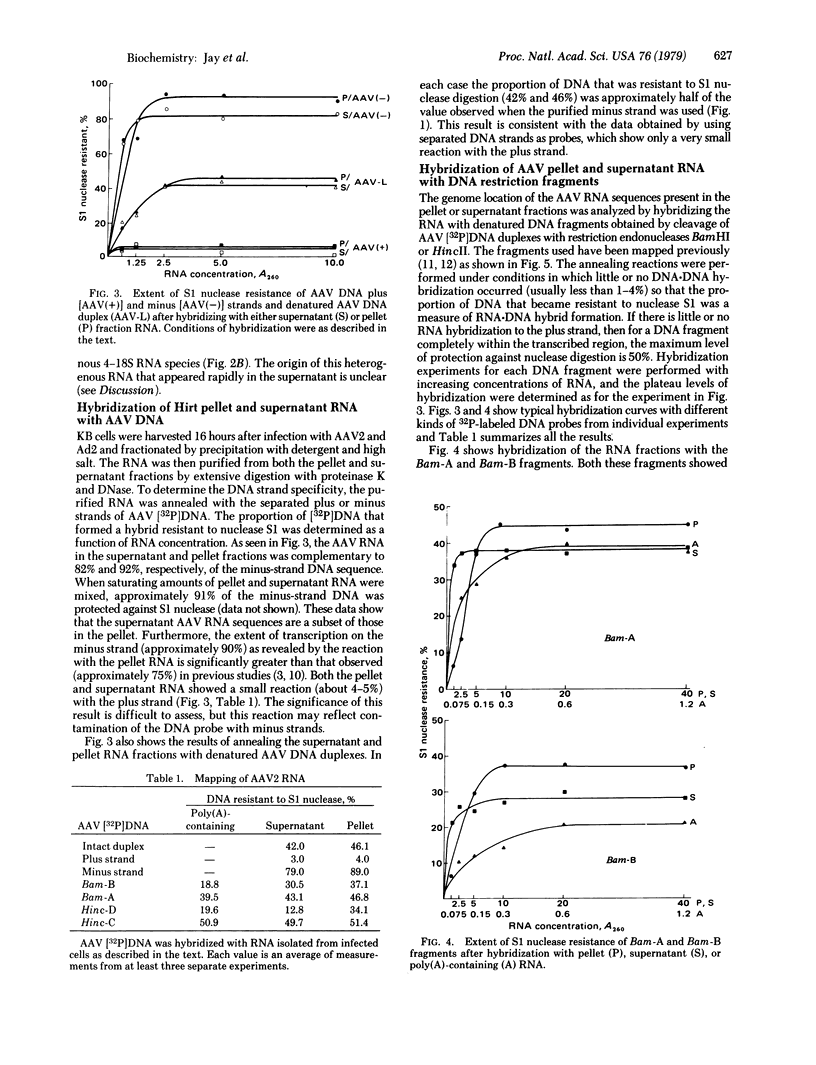
We report here a method of RNA preparation that may enrich for precursor RNA sequences and the results of an investigation of adeno-associated virus (AAV) RNA transcription that used this method. Whole cells were lysed with detergent and high salt and separated into supernatant and pellet (crude chromatin) fractions. These fractions were then separately deproteinized by proteolytic digestion and phenol extractions. DNA was removed from the preparation by two cycles of pancreatic DNase digestion and phenol extraction. Hybridization analyses of the RNA obtained from AAV/adenovirus-infected KB (human) cells revealed some AAV-specific RNA sequences that were not present in the mature 20S mRNA. These additional sequences were contained in AAV RNA molecules present in the pellet fraction, whereas the 20S AAV mRNA accumulated in the supernatant. A species of AAV-specific RNA (about 22S), which was associated only with the pellet fraction and was labeled only after a short pulse, appeared to have a kinetic relationship with the more stable cytoplasmic 20S mRNA. These putative AAV mRNA "precursors" and precursor sequences were not observed previously when conventional methods were used to obtain RNA from either whole cells or isolated nuclei.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Kort J., Fife K. H., Grogan E. W., Spear I. Study of the fine structure of adeno-associated virus DNA with bacterial restriction endonucleases. J Virol. 1975 Sep;16(3):712–719. doi: 10.1128/jvi.16.3.712-719.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Fife K. H., de la Maza L. M., Berns K. I. Genome localization of adeno-associated virus RNA. J Virol. 1976 Sep;19(3):1044–1053. doi: 10.1128/jvi.19.3.1044-1053.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J. Intracellular distribution and polyadenylate content of adeno-associated virus RNA sequences. Virology. 1976 Aug;73(1):273–285. doi: 10.1016/0042-6822(76)90080-5. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Denhardt D. T. Physical map and strand polarity of specific fragments of adenovirus-associated virus DNA produced by endonuclease R-EcoRI. J Virol. 1975 Sep;16(3):559–568. doi: 10.1128/jvi.16.3.559-568.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Rose J. A. Adenovirus-associated virus multiplication. IX. Extent of transcription of the viral genome in vivo. J Virol. 1972 Dec;10(6):1118–1125. doi: 10.1128/jvi.10.6.1118-1125.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G. Specific cleavage of adenovirus-associated virus DNA by restriction endonuclease R-EcoRI--characterization of cleavage products. Virology. 1975 Feb;63(2):523–538. doi: 10.1016/0042-6822(75)90325-6. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Transcription in vivo of a defective parvovirus: sedimentation and electrophoretic analysis of RNA synthesized by adenovirus-associated virus and its helper adenovirus. Virology. 1974 Sep;61(1):182–199. doi: 10.1016/0042-6822(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Gautschi M., Siegl G., Kronauer G. Multiplication of parvovirus LuIII in a synchronized culture system. IV. Association of viral structural polypeptides with the host cell chromatin. J Virol. 1976 Oct;20(1):29–38. doi: 10.1128/jvi.20.1.29-38.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Shimojo H. Viral DNA synthesis in vitro with nuclei isolated from adeno-associated virus type 1-infected cells. Virology. 1977 Mar;77(1):424–428. doi: 10.1016/0042-6822(77)90441-x. [DOI] [PubMed] [Google Scholar]
- Handa H., Shimojo H., Yamaguchi K. Multiplication of adeno-associated virus type 1 in cells coinfected with a temperature-sensitive mutant of human adenovirus type 31. Virology. 1976 Oct 1;74(1):1–15. doi: 10.1016/0042-6822(76)90123-9. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- MARKO A. M., BUTLER G. C. The isolation of sodium desoxyribonucleate with sodium dodecyl sulfate. J Biol Chem. 1951 May;190(1):165–176. [PubMed] [Google Scholar]
- Maza L. M., Carter B. J. Cleavage of adeno-associated virus DNA with Sali,Psti and Haeii restriction endonucleases. Nucleic Acids Res. 1976 Oct;3(10):2605–2616. doi: 10.1093/nar/3.10.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Siegl G., Gautschi M. Multiplication of parvovirus LuIII in a synchronized culture system. III. Replication of viral DNA. J Virol. 1976 Mar;17(3):841–853. doi: 10.1128/jvi.17.3.841-853.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. V. Immunocytochemical demonstration of separate chromatin-associated and inclusion-associated antigens. J Virol. 1977 Oct;24(1):353–362. doi: 10.1128/jvi.24.1.353-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. VI. simultaneous autoradiographic and immunochemical intranuclear localization of viral DNA synthesis and protein accumulation. J Virol. 1978 Jan;25(1):349–360. doi: 10.1128/jvi.25.1.349-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]


