Abstract
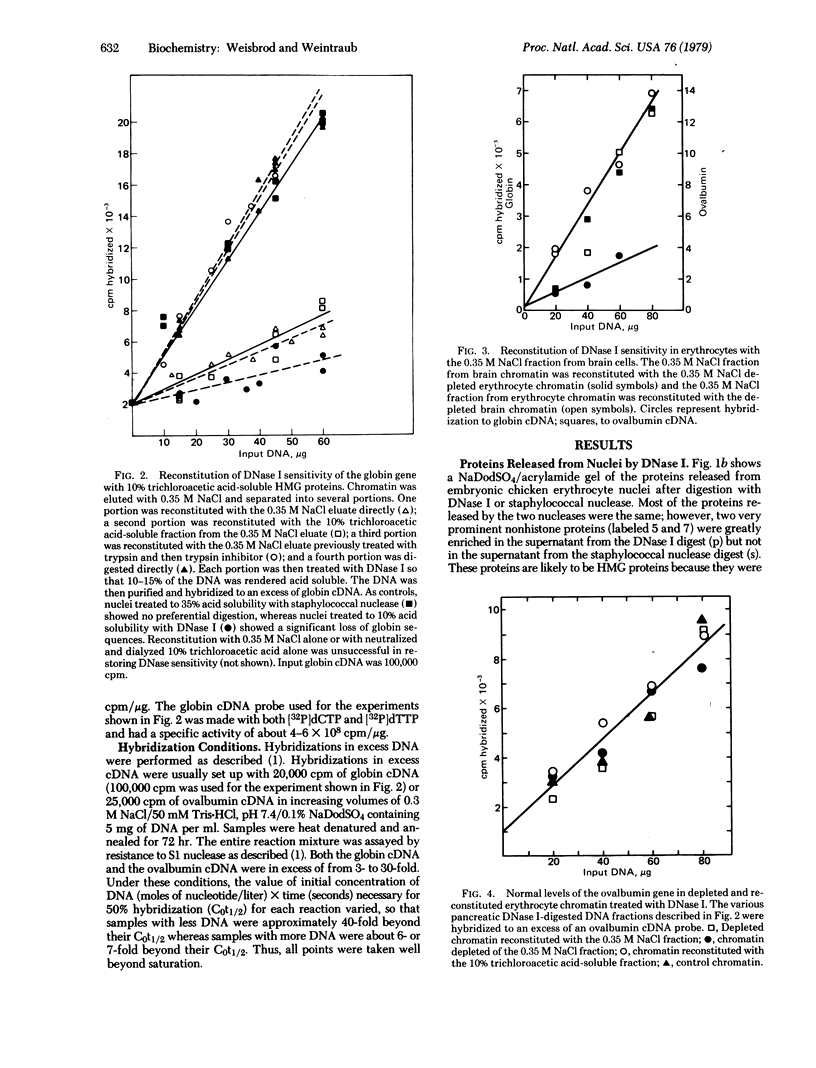
The globin gene is preferentially sensitive to digestion by DNase I in erythrocyte chromatin but not in brain, fibroblast, or oviduct chromatin. Elution of the erythrocyte chromatin with 0.35 M NaCl leads to no detectable change in the gross structure of individual nucleosomes; however, in this depleted chromatin the globin gene is no longer preferentially sensitive to DNase I. Reconstitution of the depleted chromatin with either the entire 0.35 M NaCl fraction or a subclass from this fraction greatly enriched in two high mobility group proteins (nos. 14 and 17) results in the successful reconstitution of DNase I sensitivity of the globin gene. For all of these preparations, the inactive ovalbumin gene exhibited no preferential sensitivity to DNase I. Reconstitution of the erythrocyte 0.35 M NaCl fraction with depleted brain chromatin resulted in no preferential sensitivity of the globin gene in brain chromatin; however, reconstitution of the brain 0.35 M NaCl fraction with depleted erythrocyte chromatin led to successful reconstitution of DNase I sensitivity of the globin gene. Thus, the eluted proteins responsible for conferring DNase I sensitivity are probably not tissue-specific and probably do not recognize specific DNA sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustin M., Hopkins R. B., Isenberg I. Immunological relatedness of high mobility group chromosomal proteins from calf thymus. J Biol Chem. 1978 Mar 10;253(5):1694–1699. [PubMed] [Google Scholar]
- Flint S. J., Weintraub H. M. An altered subunit configuration associated with the actively transcribed DNA of integrated adenovirus genes. Cell. 1977 Nov;12(3):783–794. doi: 10.1016/0092-8674(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973 Dec 3;40(1):215–219. doi: 10.1111/j.1432-1033.1973.tb03188.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Das S., Neiman P., Weintraub H. Regulation of expression and chromosomal subunit conformation of avian retrovirus genomes. Cell. 1978 Aug;14(4):865–878. doi: 10.1016/0092-8674(78)90342-2. [DOI] [PubMed] [Google Scholar]
- Groudine M., Holtzer H., Scherrer K., Therwath A. Lineage-dependent transcription of globin genes. Cell. 1974 Nov;3(3):243–247. doi: 10.1016/0092-8674(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy W B., Wong N. C., Dixon G. H. Selective association of the trout-specific H6 protein with chromatin regions susceptible to DNase I and DNase II: possible location of HMG-T in the spacer region between core nucleosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2810–2814. doi: 10.1073/pnas.74.7.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Renaturation kinetics of cDNA complementary to cytoplamic polyadenylated RNA from rainbow trout testis. Accessibility of transcribed genes to pancreatic DNase. Nucleic Acids Res. 1977 Apr;4(4):883–898. doi: 10.1093/nar/4.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Gorovsky M. A. Structure of rDNA-containing chromatin of Tetrahymena pyriformis analyzed by nuclease digestion. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):773–778. doi: 10.1101/sqb.1978.042.01.077. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Serunian L. A., Silver L. M., Elgin S. C. A protein released by DNAase I digestion of drosophila nuclei is preferentially associated with puffs. Cell. 1978 Jul;14(3):539–544. doi: 10.1016/0092-8674(78)90240-4. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Panet A., Cedar H. Selective degradation of integrated murine leukemia proviral DNA by deoxyribonucleases. Cell. 1977 Aug;11(4):933–940. doi: 10.1016/0092-8674(77)90304-x. [DOI] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Johns E. W. High mobility group non-histone chromosomal proteins from chicken erythrocytes. Biochem Biophys Res Commun. 1978 Mar 30;81(2):351–358. doi: 10.1016/0006-291x(78)91540-1. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Allfrey V. G. Selective release of chromosomal proteins during limited DNAase 1 digestion of avian erythrocyte chromatin. Cell. 1977 Oct;12(2):409–415. doi: 10.1016/0092-8674(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Palter K., Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975 Sep;6(1):85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]