Abstract
Purpose
Due to their properties and characteristics human mesenchymal stem cells (MSCs) appear to have great therapeutic potential. Many different populations of MSCs have been described and to understand whether they have equivalent biological properties is a critical issue for their therapeutic application.
Methods
We proposed to analyze the in vitro growth kinetics of MSCs derived from different body sites (iliac crest bone marrow, vertebrae bone marrow, colon mucosa, dental pulp).
Results
Mesenchymal stem cells derived from vertebrae can be maintained in culture for a greater number of steps and they also generate mature cells of all mesenchymal lineages with greater efficiency, when induced into osteogenic, adipogenic and chondrogenic differentiation.
Conclusions
The ability of vertebrae-derived MSCs in terms of expansion and differentiation is very interesting at the light of a clinical application for bone fusion in spine surgery.
Keywords: Mesenchymal stem cells, Vertebra-derived bone marrow, In vitro growth, Differentiation, Spine fusion
Introduction
Adult mesenchymal stem cells (MSCs) represent a heterogeneous population of cells, with a positive immunophenotype for STRO-1, CD73, CD146 and CD106 and a negative immunophenotype for CD11b, CD45, Cd34, Cd31 and CD117 [1]. MSCs act via multifaceted pathways that are not completely understood to date to perform two mainly physiological functions: the secretory or “trophic” function, including the secretion of a wide spectrum of factors with immunomodulatory, anti-inflammatory, antiapoptotic, proangiogenic, proliferative or chemoattractive capacities; the orchestration of differentiation processes together with differentiated or undifferentiated resident cells for functional tissue restoration. In recent years, many studies have been focused on MSCs because of their biological characteristics particularly suitable for clinical application in a wide spectrum of diseases [2]. They can be isolated from different tissues, grown ex vivo, and induced to differentiate in vitro into multiple cell types, including bone, cartilage, fat and stroma [3]. Considering this ability MSCs have been used clinically for several regenerative approaches in the musculoskeletal system, using different methods for cell harvesting from a donor site and cell delivery to the musculoskeletal system [4]. MSCs can be derived from blood by venous puncture or derived from bone marrow by, for example, aspiration from iliac crest or by surgical removal from a donor site, with increasing levels of invasiveness. The simplest means of cell delivery to the musculoskeletal system is direct injection of cells into the diseased tissue, under strict compliance with good medical practice [5, 6]. Alternatively, MSCs can be harvested from blood, bone marrow or tissues and then maintained in tissue culture for amplification under controlled good medical practice conditions. This method allows the monitoring of the cells under safety and quality aspects, as well as further cell selection. The use of unprocessed as well as ex vivo processed cells can be enhanced and supplemented by the use of biomaterial scaffold, soluble factors, nucleic acid or mechanical stimulation. For this purpose, large numbers of natural or synthetic biomaterials have been tested specifically for almost every target cells and tissue [7].
Spine fusion is frequently used to treat traumatic, degenerative and oncological spine diseases. Autologous bone graft has been considered the gold standard for spine fusion procedures because of its osteogenic, osteoinductive and osteoconductive ability. However, its use is associated with significant disadvantages including donor site pain, increased operative time, insufficient availability, and nonunion post-lumbar fusion [8]. Allograft bone has been the most widely used substitute to avoid the complications of donor site morbidity, but it has increased risks of infection and rejection and poor osteoinductive properties [9]. Various bone substitutes have been developed to promote spinal fusion [10, 11].
Mesenchymal stem cells have been used as a model to characterize in vitro the cytocompatibility and the biological features of different biomaterials, which are proposed as possible candidates for spine fusion improvement (bone graft substitutes or three-dimensional scaffolds) [12, 13]. Moreover, for spinal fusion MSCs have been successfully tested in several small and large animal models [14].
Considering that MSCs, originally identified in the bone marrow, can be isolated also from other tissues, many different populations of MSCs that differ for the embryonic origin and the anatomical localization have been described and to understand whether they have equivalent biological properties is a critical issue for their therapeutic use. We proposed to analyze cellular and molecular characteristics of human adult MSCs derived from different body locations, such as bone marrow from iliac crest (Ic-MSCs), sternum (St-MSCs) and vertebrae (vMSCs), as well as colon (Co-MSCs) and dental pulp (DPSCs). We previously investigated whether homeobox genes of the HOX and TALE subfamilies might provide suitable markers to identify distinct stromal cell populations, as HOX proteins control cell positional identity and, together with their co-factors TALE, are involved in orchestrating differentiation of adult tissues [15]. We observed that stromal populations from different sources, although immunophenotypically similar, display distinct HOX and TALE signatures [16]. Our data suggest that cell populations derived from different body sites may not represent equivalent cell sources for cell-based therapeutic strategies for regeneration and repair of specific tissues. In the light of these observations, we analyzed and compared the in vitro proliferation and differentiation ability of MSCs derived from different body sites, to identify the cell population showing better biological properties for spine fusion clinical application.
Materials and methods
Isolation and cultures of human stromal cells
Bone marrow samples were either purchased from CAMBREX Poietics cell systems (Gaithersburg, MD, USA) or harvested under local anesthesia from iliac crest, sternum and vertebrae of different patients, undergoing routine orthopedic surgery, following informed consent.
A total of 20 mL of bone marrow aspirate were combined with saline solution (ratio 1:3), to set up primary bone marrow cell cultures. Bone marrow aspirates were carefully layered over Lymphoprep density gradient (Axis-Shields, Oslo, Norway), and centrifuged at 400×g for 25 min. The low-density mononuclear fraction was collected and washed twice with saline solution. After resuspention in Dulbecco’s modified Eagle medium low-glucose (DMEM, Invitrogen, UK), supplemented with 10 % of fetal bovine serum (FBS; Invitrogen), 100 μg/mL gentamicin (Sigma Aldrich, Saint Louis, MO, USA), 2 mM l-glutamine (Biowittaker Cambrex, Walkersville, MD, USA), cells were subsequently seeded at 2 × 105 cells/cm2 in T-75 tissue culture flasks (Corning Inc. Corning, NY, USA). Cells were grown at 37 °C in a humidified atmosphere containing 5 % CO2. In order to remove non-adherent cells fresh medium was replaced after 48–72 h, with refeeding 3 times a week until 80 % confluence was reached.
Alternatively, bone marrow samples were treated for 20 min at 20 °C with RosetteSep human MSC enrichment cocktail (StemCell Technologies, Vancouver, BC, Canada) composed by CD3, CD14, CD19, CD38, CD66b, Glycophorin A tetrameric antibody (Ab) complexes to obtain lineage depletion, diluted and centrifuged over Ficoll-Hypaque gradient for 25 min at 300×g. Enriched cells were collected, washed, and residual red blood cells were removed after treatment with NH4Cl (StemCell) for 10 min in ice. Moreover, MACS column (Miltenyi, Bergisch Gladbach, Germany) has been used to remove CD34+ cells. Cells were then cultured at sub-clonal/low-density concentration (1–10 cells/cm2) for 3 weeks in α-medium (Invitrogen, Carlsbad, CA, USA), with 20 % fetal calf serum (FCS; StemCell), in T-75 flasks at 37 °C in 5 % CO2 atmosphere. Half medium was replaced twice a week. When adherent layers of MSCs reached confluence, cells were trypsinized and plated at low density for further expansion.
Colon surgical specimens were thoroughly washed with PBS supplemented with 5× antibiotic/antimicotic (A/A) solution (Invitrogen), maintained in PBS 5× A/A at 4 °C, treated with 30–45 mL 1 mM EDTA/EGTA PBS for 75 min at 20 °C, vigorously shaken, then processed as described for bone marrow.
Human dental pulp cells were obtained from molars of healthy subjects, after informed consent. A Gracey curette was used to obtain radicular dental pulps, from healthy and non-carious teeth. Pulp tissue explants were placed in T-25 tissue culture flasks (Corning Inc. Corning, NY), in the presence of Minimum Essential Medium alpha modification (α-MEM, Sigma Aldrich) supplemented with 20 % of FBS (Sigma Aldrich), 100 IU/mL penicillin (Pharmacia & UpJohn SpA, Italy), and 2 mM l-glutamine (Cambrex Bioscience Inch., Baltimore, USA). Cells were then cultured as described for bone marrow.
For growth curve assays, cumulative population doubling in arbitrary units was calculated according to the following formula:
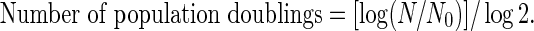 |
where N is number of cells at the end of a period of growth and N0 is number of cells plated.
Immunophenotypic analysis
Before incubation for 30 min at 4 °C with fluorescein isothiocyanate (FITC)-conjugated anti-CD105 antibody, phycoerythrin (PE)-conjugated anti-HLA-DR and PE-Cy5-conjugated anti-CD90; (BD Bioscience, San Jose, CA), primary cells were washed with D-PBS added with 0.1 % NaN3 (Sigma) and 0.05 % of bovine serum albumin (BSA; Sigma). After washing, cells were analyzed by flow cytometry, using a FACScan flow cytometer (BD Bioscience) and CellQuest Analysis Software (BD Bioscience).
Mesenchymal stem cells were detached at confluence and characterized as CD105+ CD45− CD34− by triple staining using FITC-conjugated anti-CD105 antibody, PE-conjugated anti-CD34, and peridinin chlorophyll protein-conjugated anti-CD45.
In vitro differentiation assays
Differentiation assay was performed at passage 2 when MSCs reached 80 % confluence.
Osteogenic differentiation was induced by culturing 3 × 103 MSC/cm2 in MSCGM medium (Cambrex, Poietics Cell Systems) for 24 h at 37 °C in 5 % CO2 atmosphere. MSCGM was replaced with osteogenesis induction medium (Cambrex), and changed for 3 weeks every 3–4 days. Alizarin Red S staining was carried out on plates to evaluate the percentage of calcium deposition. Briefly, after 3 weeks in osteoinductive medium cells were washed with D-PBS, fixed with 4 % formaldehyde for 30 min at room temperature and stained with 1 % Alizarin Red S solution (Sigma) pH 4.2 for 15 min, followed by multiple washes with distilled water.
To promote adipogenic differentiation, confluent MSCs were cultured with Adipogenesis induction medium (Cambrex) for 3 days, followed by 1–3 days of culture in Adipogenic maintenance medium (Cambrex). Following 3 times of this treatment, consisting in induction/maintenance conditions, cells were cultured for an additional week with maintenance medium. Oil Red O staining was performed to highlight the presence of lipid droplets, typical of growing adipocytes. The adipogenic cultures were fixed with 4 % formaldehyde for 30 min at room temperature and stained for 15 min with fresh Oil Red O working solution consisting of three parts stock solution (0.5 % Oil Red O in isopropyl alcohol; Sigma) and two parts of water, and subsequently washed with distilled water.
Chondrogenic differentiation was performed spinning 2.5 × 105 MSC twice at 150×g for 5 min at 20 °C with incomplete chondrogenic medium (Cambrex), and then resuspending in 0.5 mL complete medium (Cambrex) in 15 mL polypropylene tube. Cells were cultured for 3 weeks at 37 °C in 5 % CO2 atmosphere. Medium were replaced 2–3 days. Pellets were formalin-fixed and paraffin embedded or froze-sectioned. Thin sections mounted on a slide were stained with Safranin O or Alcian Blue.
Results
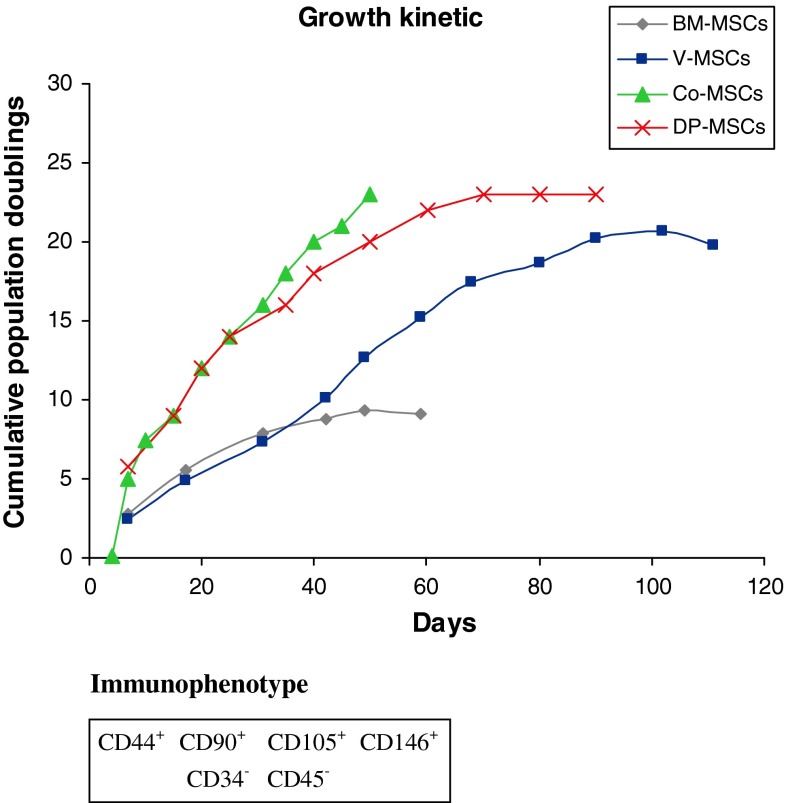
Mesenchymal stem cells derived from different sources (bone marrow samples from iliac crest, sternum or vertebrae, colon-derived MSCs, human dental pulp-derived MSCs) were tested for in vitro growth in the presence of cell culture medium, calculating the cumulative population doublings for each type of MSCs. As reported in Fig. 1, MSCs from different sources presented very different growth kinetics, even if they shared a common immunophenotype, where CD44, CD90, CD105, CD146 antigens were significantly expressed and Cd34, CD45 antigens were absent. While iliac crest and sternum-derived MSCs could be maintained in culture for about 3 months, undergoing a limited expansion, mesenchymal cultures derived from dental pulp and colon grew much more rapidly, but could not be maintained longer. Strikingly, MSCs obtained from vertebrae continued to propagate at high rate even after 3.5 months ex vivo.
Fig. 1.
Immunophenotype characterization and in vitro growth kinetics of mesenchymal stem cells from different body sites. Representative growth curves of MSCs isolated from iliac crest and sternum (Ic/St-MSCs), vertebrae (vMSCs), dental pulp (DPSCs) and colon (Co-MSCs). For growth curve assay, MSCs were cultured to reach confluence, detached (usually once a week), counted and replated at the initial plating density. The growth kinetic of a cell population is plotted as the cumulative population doubling (PD) versus days in culture. MSCs derived from different sources exhibit different growth kinetics
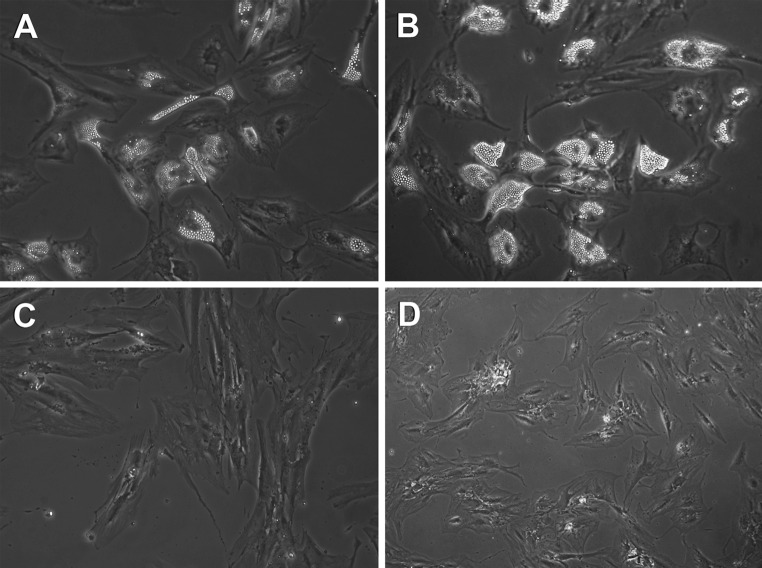
Mesenchymal stem cells from different sources were also induced to osteogenic, adipogenic and chondrogenic differentiation following exposure to specific inducing agents. As summarized in Table 1, we observed that vMSCs generate mature cells of all mesenchymal lineages with greater efficiency, thus resulting the best population both in terms of expansion and differentiation. Representative pictures of BM-derived MSCs and vMSCs induced to adipogenic and osteogenic differentiation are reported in Fig. 2.
Table 1.
Differentiation ability of mesenchymal stem cells derived from different sources following different lineages
| Lineages | BM-MSCs | vMSCs | Co-MSCs | DP-MSCs |
|---|---|---|---|---|
| Osteocytes | + | ++ | + | + |
| Adipocytes | + | ++ | +/− | +/− |
| Chondrocytes | + | ++ | +/− | + |
BM-MSCs bone marrow mesenchymal stem cells, vMSCs vertebral mesenchymal stem cells, Co-MSCs colon mucosa mesenchymal stem cells, DP-MSCs dental pulp mesenchymal stem cells
Fig. 2.
MSCs derived from iliac crest bone marrow (a, c) and vertebrae bone marrow (b, d) are induced to differentiate into adipogenic (a, b) and osteogenic (c, d) lineages following exposure to inducing agents for 16 days
Discussion
Numerous spinal pathologies are treated with surgical procedures involving bone fusion of spine segments. However, the development of these procedures is associated to the increase in related failures and complications. In particular, 10 to 15 % of vertebral arthrodesis is correlated to nonunion (pseudoarthrosis), with persistent pain, loss of functional recovery, implant failure (hardware mobilization or breakdown), need of re-intervention. The hardware employed, if correctly positioned, provides an immediate primary stability; however, it is not able to bear alone the physiological load to which the spine is subjected and if bone fusion is not achieved the hardware fails. The success of the spine fusion depends on many factors, some related to the patient (age, smoking, previous spine surgery, thoracolumbar kyphosis, coexistence of hip osteoarthritis) [17], other related to the surgical treatment, among them the choice of bone graft or bone graft substitutes having adequate osteogenic, osteoinductive and osteoconductive properties. Autograft, in particular iliac crest bone graft, is considered the gold standard for bone fusion. However, the problems associated to bone graft, such as donor site morbidity and insufficient availability, have led to the development of different biomaterials, with potential osteogenic, osteoinductive and osteoconductive properties, to be used as graft substitutes or extenders [9–11].
Another promising line of research concerns the use of multipotent adult MSCs able to differentiate into different mesenchymal cell lines and tested nowadays in orthopedics surgery for bone and cartilage regeneration. Preclinical and clinical results show that, independently from the method used to deliver MSCs into the musculoskeletal system, the direct application of these cells on a loss of bone substance stimulates bone regeneration [4]. Recently, Fernandez-Bances et al. [18] treated seven patients with pseudoarthrosis of a long bone through introduction of MSCs derived from iliac crest bone marrow of the same patient supported by allogenic cancellous bone graft (from the local bone bank), obtaining consolidation in all the cases.
This finding has been evidenced also for bone fusion improvement following spine surgical procedures in preclinical studies. Bone marrow-derived MSCs, combined with biomaterial scaffolds (hydroxyapatite, b-tricalcium phosphate, demineralized bone matrix), were successfully used to achieve spine fusion in comparison with conventional iliac crest bone autograft [19–22]. Although most of the studies involve MSCs derived from bone marrow, some authors have recently shown that MSCs derived from adipose tissue can efficiently promote bone regeneration in animal models for the healing of femoral or vertebral fractures [23, 24] and for the acceleration of spinal arthrodesis [25]. As the adipose tissue can be found in abundance with reduced donor site morbidity, the problems associated to bone marrow harvesting could be overcome.
Following our observation that MSCs from different sources display different expression profiles of the homogenes of HOX and TALE subfamilies [16], we analyzed here MSCs derived from different sources for proliferative and differentiating properties in vitro, in the light of their possible clinical applications. In particular, vertebral bone marrow is rich in MSCs, such as iliac crest bone marrow. The preliminary results reported here show that vertebral MSCs grow and differentiate in vitro with greater efficiency in comparison to MSCs derived from iliac crest bone marrow or from other tissues examined (colon and dental pulp). This finding could be very interesting and open new perspectives for the improvement of spine fusion, which is a mandatory step for the success of spinal surgical procedures.
In the course of a surgical procedure for spinal fusion, vertebral bone marrow can be harvested in amount proportional to the length of the arthrodesis, simultaneously with the preparation of the site for pedicle screw insertion. Then multipotent MSCs can be isolated from vertebral bone marrow during the progress of the surgical procedure and reintroduced in the fusion site (using homologous or autologous bone tissue or biomaterials as scaffold), without any additional surgical time nor any other donor site involvement.
Acknowledgments
The authors thank Carlo Piovani for his technical assistance in providing bone marrow samples during surgery at Maggiore Hospital and Rizzoli Orthopedics Institute, Bologna, Italy.
Conflict of interest
None.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4(7):371–380. doi: 10.1038/ncprheum0816. [DOI] [PubMed] [Google Scholar]
- 6.Hogan MV, Bagayoko N, James R, Starnes T, Katz A, Chhabra AB. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg. 2011;19(3):134–142. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Nöth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62(7–8):765–783. doi: 10.1016/j.addr.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Rhim R, Li L, et al. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009;9(11):886–892. doi: 10.1016/j.spinee.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Whang PG, Wang JC. Bone graft substitutes for spinal fusion. Spine J. 2003;3(2):155–165. doi: 10.1016/S1529-9430(02)00539-9. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Williams K, Umscheid CA, et al. Osteoinductive bone graft substitutes for lumbar fusion: a systematic review. J Neurosurg Spine. 2009;11(6):729–740. doi: 10.3171/2009.6.SPINE08669. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah KG, Steinmetz MP, Benzel EC, et al. The state of lumbar fusion extenders. Spine (Phila Pa 1976) 2011;36(20):E1328–E1334. doi: 10.1097/BRS.0b013e318209952b. [DOI] [PubMed] [Google Scholar]
- 12.Barbanti Brodano G, Mazzoni E, Tognon M, Griffoni C, Manfrini M. Human mesenchymal stem cells and biomaterials interaction: a promising synergy to improve spine fusion. Eur Spine J. 2012;21(Suppl 1):S3–S9. doi: 10.1007/s00586-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manfrini M, Di Bona C, Canella A, Lucarelli E, Pellati A, D’Agostino A, Barbanti-Bròdano G, Tognon M. Mesenchymal stem cells from patients to assay bone graft substitutes. J Cell Physiol. 2013;228(6):1229–1237. doi: 10.1002/jcp.24276. [DOI] [PubMed] [Google Scholar]
- 14.Pneumaticos SG, Triantafyllopoulos GK, Chatziioannou S, Basdra EK, Papavassiliou AG. Biomolecular strategies of bone augmentation in spinal surgery. Trends Mol Med. 2011;17(4):215–222. doi: 10.1016/j.molmed.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol. 2006;291(2):193–206. doi: 10.1016/j.ydbio.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Picchi J, Trombi L, Spugnesi L, Barachini S, Maroni G, Brodano GB, Boriani S, Valtieri M, Petrini M, Magli MC. HOX and TALE signatures specify human stromal stem cell populations from different sources. J Cell Physiol. 2013;228(4):879–889. doi: 10.1002/jcp.24239. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine. 2006;31(20):2329–2336. doi: 10.1097/01.brs.0000238968.82799.d9. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Bances I, Perez-Basterrechea M, Perez-Lopez S, Nunez Batalla D, Fernandez Rodriguez MA, Alvarez-Viejo M, Ferrero-Gutierrez A, Menendez–Menendez Y, Garcia-Gala JM, Escudero D, Paz Aparicio J, Carnero Lopez S, Lopez Fernandez P, Gonzalez Suarez D, Otero Hernandez J (2013) Repair of long bone pseudoartrthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy; in press [DOI] [PubMed]
- 19.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 2006;31(18):E636–E640. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 20.Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973–3982. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Kerr EJ, 3rd, Jawahar A, Wooten T, Kay S, Cavanaugh DA, Nunley PD. The use of osteo-conductive stem-cells allograft in lumbar interbody fusion procedures: an alternative to recombinant human bone morphogenetic protein. J Surg Orthop Adv. 2011;20(3):193–197. [PubMed] [Google Scholar]
- 22.Ammerman JM, Libricz J, Ammerman MD. The role of osteocel plus as a fusion substrate in minimally invasive instrumented transforaminal lumbar interbody fusion. Clin Neurol Neurosurg. 2013;115(7):991–994. doi: 10.1016/j.clineuro.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Shoj T, Ii M, Mifune Y, Matsumoto T, Kawamoto A, Kwon SM, Kuroda T, Kuroda R, Kurosaka M, Asahara T. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest. 2010;90:637–649. doi: 10.1038/labinvest.2010.39. [DOI] [PubMed] [Google Scholar]
- 24.Sheyn D, Kallai I, Tawackoli W, Yakubovich DC, Oh A, Su S, Da X, Lavi A, Kimelman-Bleich N, Zilberman Y, Li N, Bae H, Gazit Z, Pelled G, Gazit D. Gene modified adult stem cells regenerate vertebral bone defect in a rat model. Mol Pharm. 2011;8(5):1592–1601. doi: 10.1021/mp200226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez MJ, McIntosh KR, Spencer ND, Borneman JN, Horsewell R, Anderson P, Yu G, Gaschen L, Gimble JM. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. J Orthop Res. 2009;27(3):366–373. doi: 10.1002/jor.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]