Abstract
Purpose
Computer-assisted navigation systems are largely used for pedicle screws positioning in degenerative and traumatic spine surgery. In oncologic spine surgery its use is still developing and could be extended for tumor identification and excision. Aim of this paper is to present our experience.
Methods
Seven selected patients (5 females, 2 males), mean age 44 years (min 17–max 62) affected by primary benign or malignant tumors of the spine or spine metastases were surgically treated with the use of computer-assisted navigation system from March to October 2011.
Results
At 18 months mean F.U. (min 15–max 23), no LR were observed. Revision surgery was necessary only in one case for C1 pedicle screw malpositioning.
Conclusions
Navigation system can improve surgical accuracy in screws placement and tumor localization and excision. Learning curve and technical aspects must be considered to avoid potential serious mistakes.
Keywords: Navigation, Computer-assisted spine surgery, Oncologic surgery, Spine surgery, Tumors
Introduction
Spine surgery is a high-risk surgery carrying the potential of injury to the spinal cord, nerves and important vascular structures [4]. After many years of practice, experience, development of new surgical instrumentation and variety of surgical procedures requirements for surgical accuracy, safety and good results are also growing. Spine surgery requires knowledge of spinal anatomy and biomechanics and depends on proprioceptive feedback during surgery. Effectiveness and safety depends on accuracy of surgery.
There is general agreement among surgeons that imaging techniques are essential for safe and accurate placement of spinal instrumentation regardless of the complexity of the operation, the anatomical region, and the level of training and comfort level of the individual surgeon. Traditionally imaging techniques involve the use of intraoperative fluoroscopy [14] for active guidance throughout surgery [11]. Unfortunately the use of intraoperative fluoroscopy has significant drawbacks. The entire surgical team (surgeon, assistant surgeon and scrub nurse) has to stay at the surgical field, directly adjacent to the image intensifier. Occupational radiation exposure leads to a high morbidity and mortality risk concerning the development of a thyroid cancer. Radiation-reducing procedures and shielding devices such as a whole-body apron, lead-collar and goggles lead to a significant decrease of mortality and morbidity of radiation-sensitive tissues. This gear is extremely uncomfortable, especially when forces the surgical team to work in awkward positions.
Surgical navigation system is a promising technique that addresses many of these concerns [17, 18]. Computer-guided navigation techniques in spinal instrumentation improve screw placement accuracy [7, 20, 25], potentiate the ability to maximize the screw diameter relative to the pedicle, and reduce potential injury to critical neurovascular structures [1, 21]. Navigation systems are already widely used mainly for pedicle screws placement [10, 23, 24].
In oncologic spine surgery the navigation system could be very helpful for tumor identification and resection, for specific device placement (electroporation’s electrodes) and for the execution of mini-invasive surgical procedure (vertebroplasty). The aim of this study is to evaluate prospectively the results obtained using the navigation system in oncologic spine surgery in a series of seven selected patients.
Materials and methods
From March to October 2011, seven selected patients, five females and two males, mean age 44 years (min 17–max 62) affected by primary or metastatic tumors of the spine have been surgically treated using computer-assisted navigation system.
Pre-and post-operative X-ray and CT scan were performed in all cases.
Indications for surgery were: C2 aggressive angioma; T9 osteoid osteoma; T2 osteoblastoma; C2 pathological fracture due to plasmocytoma; L2 uterus metastasis; T3 pancreas metastasis; L5 melanoma metastasis;
Intralesional excision without instrumentation was performed in case of osteoid osteoma and osteoblastoma; C2 vertebroplasty in case of aggressive angioma; C1–C4 posterior instrumentation in case of C2 plasmocytoma; palliative posterior decompression and instrumentation for L2 metastasis; palliative posterior decompression, instrumentation and T3 vertebroplasty for T3 metastasis; palliative posterior decompression and electroporation in case of L5 melanoma metastasis.
In all cases Medtronic (StealthStation® Navigation System) or Brainlab (VectorVision Spine®) navigation system was used.
The navigation system comprised a computer workstation, reference frame with passive markers, standard probe, and electro-optical camera connected to the computer workstation, which served as a position sensor. The basic data used for navigation included the preoperative computed tomography (CT) imaging data (slice thickness, 2.5 mm; slice range 2 mm) and were matched with intraoperative fluoroscopy images. The data were transferred and recorded on the system computer and reconstructed into 3D images.
After exposing the posterior element, the reference frame of the navigation system was mounted on the exposed spinous process or, in the cervical cases, on the Mayfield support.
Under the navigation system assistance pedicle screws were placed and precise real-time tumor localization was performed.
The accuracy of computer-assisted pedicle screw positioning was evaluated in terms of cortical perforations graded by 2-mm increments: Grade I (good, no cortical perforation), Grade II (screw outside the pedicle less than 2 mm), and Grade III (screw outside the pedicle larger than 2 mm).
From an oncologic point of view all the patients were classified as NED (no evidence of disease), CDF (continuos disease free) AWD (alive with disease) or DOD (dead of disease).
Results
Mean F.U. is 18 months (min 15–max 23).
No complications were recorded intraoperatively.
A total number of 20 pedicle screws were placed.
With regards to their placement accuracy, 14 screws (70 %) were graded I, 5 screws (25 %) graded II and 1 screw (5 %) graded III (C1 right pedicle screw).
In this last case (graded III), C1 pedicle screw removal was needed and new instrumentation was performed (C0–C4).
From an oncological point of view at the last F.U. no LR were recorded.
The three patients affected by benign primary tumors, after 18 months mean F.U. (min 15–max 23), were classified as NED and CDF.
The patient affected by C2 plasmocytoma after 16 months F.U. is actually classified as NED (Hematologic chemotherapy after surgery was performed).
The three patients affected by spinal metastases after 17 months mean F.U. (min 15–max 20) were classified as locally NED.
Discussion
The state of the art of image-guided spine surgery is heterogeneous in the different spine surgery branches.
The most common application of image-guided spine surgery is nowadays pedicle screws placement [8, 15]; the clinical benefits in terms of increased accuracy and reduced exposure [6, 9] to ionizing radiation are well documented.
The application of this technology for spinal tumor surgery is being developed.
Only few publications report on image-guided spine tumor surgery.
Two papers report on a total of five patients with benign tumors [12]. Rajasekaran et al. [16] report a series of four patients treated for osteoid osteoma in the cervical, thoracic and lumbar spine. Van Royen et al. [22] report on osteoid osteoma in the thoracic and lumbar spine using an image-guided high-speed drill in five patients. Image-guided surgery for dorsal instrumentation in eight patients with metastatic disease of the thoracic spine was analyzed by Arand et al. [2] and Gebhard et al. [5] who report on 12 patients. Smitherman et al. [19] report on a case of Giant Cell Tumor (GCT) in the thoracic spine, Nagashima et al. [13] described a case of C2 osteoid osteoma curetted using a navigation system.
In March 2011 for the first time this technology was applied to oncologic spine surgery in our unit.
The patient was a 52-year-old female presenting low back pain (VAS = 8/10) resistant to medications due to L2 uterus metastasis (WWB: sec. 6–1; lay. B–C) [3] previously treated with local radiotherapy.
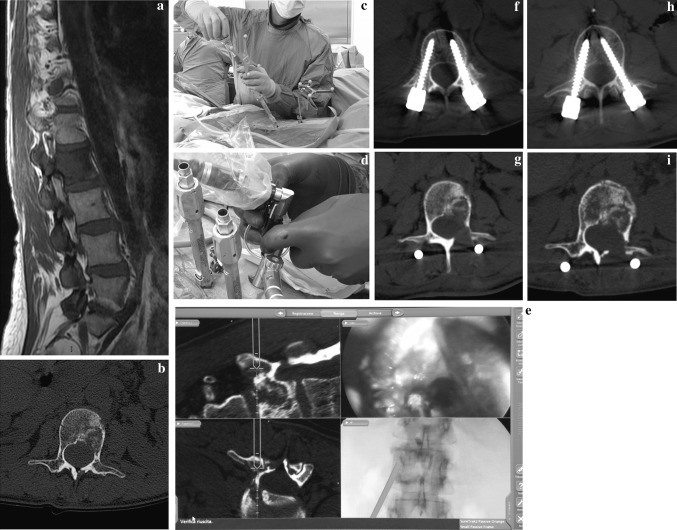
No neurological deficits were present before surgery (Frankel E). Videoendoscopic L2 debulking and percutaneous screws fixation L1–L3 under navigation system control were performed. From the day after surgery the patient was able to stand and walk and a pain reduction was recorded (VAS 3/10). Post-operative CT scan has shown well-positioned screws (2 grade I and 2 grade II) (Fig. 1 a–i).
Fig. 1.
C.C. 52 years, L2 uterus metastases (a, b). Using navigator system, the patient was subjected to minimally invasive L1–L3 instrumentation (c). On the site of the tumor a working camera was positioned (d) whereby, by a navigated high-speed burr, the excision of the tumor was performed through the lamina and the pedicle (e). Post-op CT scan shows the good position of the minimally invasive screws and the amount of the decompression (f, g, h). At 6 months from the surgery, CT scan shows a good local control of the disease (i)
The second case of this series was a 61-year-old female presenting low back pain and left sciatic pain due to L5 melanoma metastases (WBB: sec. 5–6; lay. B–C) and L4–L5 left herniated disc.
Surgical planning was mini-invasive L4–L5 left herniectomy after L4 laminectomy and local mini-invasive treatment of the metastasis using electroporation. Using a real-time 3D imaging reconstruction of the anatomy of the vertebral body it was possible to clearly identify the tumor mass and place electroporation (local electrochemotherapy) electrodes around the metastases.
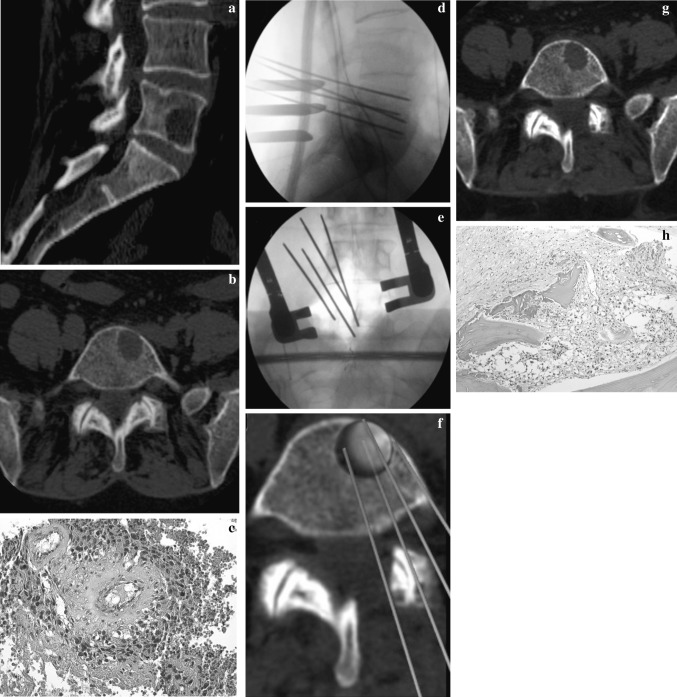
The 6 months F.U. CT scan underlined a small osteolytic area in the same position previously treated. In order to exclude a local recurrence PET-CT and CT scan trocar biopsy were executed. The last PET-CT final result was negative and pathologist’s final report referred the presence of necrotic tissue, as the consequence of local chemotherapy (Fig. 2 a–h). After 17 month F.U. the patient is NED.
Fig. 2.
M.A. 61 years, L5 melanoma metastases (a, b). A histological slide obtained by a CT-trocar biopsy confirmed the diagnosis (tissue positive to MART 1) (c). After an open laminectomy, using navigator system, four needles were positioned around the lesion and electroporation was performed (d, e, f). A good local control of the disease was confirmed by a CT-trocar biopsy performed 6 months after surgery (necrotic tissue) (g, h)
The first results obtained were considered positive and the application of a navigation system useful. In particular, the possibility to match pre-operative imaging (i.e. CT scan) with real-time 3D anatomy reconstruction during the surgical procedure was considered interesting for the treatment of the tumor of the spine not only to detect neurovascular structures, but also to define in real time the tumor mass margin and check step by step the removal.
Moreover, new technologies developed for spinal navigation system, permit to upload pre-operative patient’s MRI and match it with intraoperative images, giving the surgeons the possibility to better define tumor’s pseudocapsule or capsule, neurovascular structures and soft-tissue surrounding the lesion.
For these reasons the use of navigation system was extended to primary tumors.
The first case treated was a 17-year-old female presenting scoliosis and thoracic spine pain due to T9 Osteoid Osteoma (WBB: sec. 10–7; lay. B–C).
Right thoracoscopy was performed first. Under fluoroscopic control T9–T10 vertebral bodies were identified. Carbon fiber marker was placed on T9 spinous process, navigation system was set and once the lesion was identified it was completely removed using a high-speed drill connected to the navigation system. In this way it was possible to check moment by moment every drill movement and observe in real time the osteoid osteoma removal.
After surgery the patient was strictly followed up by CT scan and 20 months after surgery she is CDF and NED.
At the beginning of our experience the use of navigation system was limited to lumbar and thoracic spine.
The use was extended for the first time to the cervical spine in case of C2 aggressive angioma (WBB: sec. 11–5; lay. B–C) in a 37-year-old female.
The patient was previously treated using a Halo Vest for 2 months then, under computer-assisted navigation system, C2 vertebroplasty was performed as augmentation. Postoperatively a Philadelphia collar was placed for 2 months.
Vertebroplasty of the cervical spine is normally a high demanding procedure, but with the use of the navigation system it was executed in an easier and safer way.
Immediate post-operative pain relief was obtained and after 18 months F.U. the patient was still pain free with a normal range of motion.
The same system was then used to treat C2 pathological fracture due to plasmocytoma (WBB: sec. 8–5; lay. B–C) in a 62-year-old male.
The patient was neurologically intact (Frankel E).
The patient was placed prone and the use of Mayfield 360° support was necessary. The planned surgery was C1–C4 posterior instrumentation and six pedicle screws were placed under navigation system control. No problems, technical difficulties or complications were recorded during the surgical procedure.
Surprisingly, post-operative CT scan revealed the malpositioning of C1 screws (Grade II the left one, Grade III the right one) that were immediately removed and a new C0–C4 fusion without navigation system was performed. Final Frankel score was E. After surgery specific hematologic chemotherapy was necessary to treat the systemic disease. After 16 month F.U. the patient was locally NED (Fig. 3).
Fig. 3.
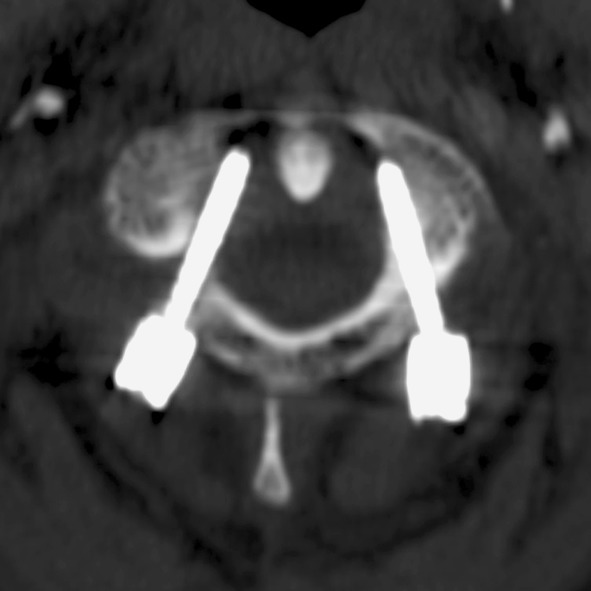
R.R. 62 years, C2 pathological fracture due to plasmocytoma. Post-operative CT scan shows C1 screws malpositioning
The reason of C1 screws malpositioning is unclear.
Probably there was a mismatch between pre-operative CT scan uploaded in the system and the real-time imaging in the OR due to the different position of the patient during pre-operative CT scan execution (patient supine), and the intraoperative position (prone with a traditional Mayfield 360° support).
In our opinion the use of intraoperative CT scan could help in avoiding these extremely dangerous mistakes in difficult cases.
Following a chronologic order other two cases complete our series.
The first case is a 61-year-old female presenting progressive paraplegia (Frankel C) due to T3 pancreas metastasis (WBB: sec.11–2; lay. B–E). Under navigation system control a complete, precise and rapid T3 debulking and vertebroplasty was performed, ten pedicle screws were placed and T1–T8 instrumentation was achieved. A complete recovery of neurological function was recorded after surgery (Frankel E). Standard post-operative CT scan showed the correct positioning of the cement and screws (8 graded I and 2 graded II). 17 months after surgery the patient is locally NED.
The last case is a T2 osteoblastoma (WBB: sec. 11–10; lay. B–C) in a 23-year-old male that was completely identified and removed with the use of navigation system. After surgery the patient was strictly followed up by CT scan and 16 months after surgery he was CDF and NED.
Conclusions
The use of navigation system in oncologic spine surgery could be particularly interesting and helpful.
Clinical benefit in terms of increased accuracy in pedicle screws placement and reduction of the exposure to ionizing radiation are documented.
In oncologic spine surgery the use can be extended to the identification of tumor mass and definition of its margins, giving the surgeon the possibility to control the excisional procedure and protect the neurovascular surrounding structures step by step and in real time, with a 3D point of view.
Considering that image-guided surgery is technically demanding and a learning curve has to be completed, not only “particular” or “demanding” cases should be navigated to establish a proper routine. Moreover, the presence of carbon fiber devices (i.e. surgical bed and Mayfield support) and intraoperative CT scan could be useful to avoid imaging distortions and increase accuracy of navigation system imaging capture, giving the surgeons the possibility to repair possible mistakes due to mismatch between pre-and intraoperative imaging.
In conclusion, it is relevant that assisted navigation system spine surgery or mini-invasive spine surgery could be applied with good results also in spine tumor field, but these new techniques must be used absolutely according to and respecting the oncological criteria of the treatment.
Acknowledgments
The authors thank Dr. C. Griffoni and C. Piovani at the Oncological and Degenerative Spine Unit at Orthopedic Rizzoli Institute, for their cooperation for this study.
Conflict of interest
None.
References
- 1.Amiot LP, Lang K, Putzier M, Zippel H, Labelle H. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine. 2000;25:606–614. doi: 10.1097/00007632-200003010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Arand M, Hartwig E, Kinzl L, Gebhard F. Spinal navigation in tumor surgery of the thoracic spine: first clinical results. Clin Orthop Relat Res. 2002;399:211–218. doi: 10.1097/00003086-200206000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 4.Boriani S, Bandiera S, Donthineni R, Amendola L, Cappuccio M, De Iure F, Gasbarrini A. Morbidity of en bloc resections in the spine. Eur Spine J. 2010;19:231–241. doi: 10.1007/s00586-009-1137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gebhard F, Kinzl L, Hartwig E, Arand M. Navigation of tumors and metastases in the area of the thoraco-lumbar spine. Unfallchirurg. 2003;106(11):949–955. doi: 10.1007/s00113-003-0684-8. [DOI] [PubMed] [Google Scholar]
- 6.Geerling J, Gösling T, Gösling A, Ortega G, Kendoff D, Citak M, Krettek C, Hüfner T. Navigated pedicle screw placement: experimental comparison between CT- and 3D fluoroscopy-based techniques. Comput Aided Surg. 2008;13:157–166. doi: 10.3109/10929080802102110. [DOI] [PubMed] [Google Scholar]
- 7. Han W, Gao ZL, Wang JC, Li YP, Peng X, Rui J, Jun W (2010) Pedicle screw placement in the thoracic spine: a comparison study of computer-assisted navigation and conventional techniques. Orthopedics 33(8). doi:10.3928/01477447-20100625-14 [DOI] [PubMed]
- 8.Jarvers JS, Katscher S, Franck A, Glasmacher S, Schmidt C, Blattert T, Josten C. 3D-based navigation in posterior stabilisations of the cervical and thoracic spine: problems and benefits. Results of 451 screws. Eur J Trauma Emerg Surg. 2011;37:109–119. doi: 10.1007/s00068-011-0098-1. [DOI] [PubMed] [Google Scholar]
- 9.Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J. 2000;9(3):235–240. doi: 10.1007/s005860000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig SC, Kowalski JM, Edwards CC, 2nd, Heller JG. Cervical pedicle screws: comparative accuracy of two insertion techniques. Spine. 2000;25:2675–2681. doi: 10.1097/00007632-200010150-00022. [DOI] [PubMed] [Google Scholar]
- 11.Mirza SK, Wiggins GC, Kuntz IVCh, York JE, Bellabarba C, Knonodi MA, Chapman JR, Shaffrey CI. Accuracy of thoracic vertebral body screw placement using standard fluoroscopy, fluoroscopic image guidance, and computed tomographic image guidance. Spine. 2003;28:402–413. doi: 10.1097/01.BRS.0000048461.51308.CD. [DOI] [PubMed] [Google Scholar]
- 12.Moore T, McLain RF. Image-guided surgery in resection of benign cervicothoracic spinal tumors: a report of two cases. Spine J. 2005;5:109–114. doi: 10.1016/j.spinee.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Nagashima H, Nishi T, Yamane K, Tanida A. Case report: osteoid osteoma of the C2 pedicle: surgical technique using a navigation system. Clin Orthop Relat Res. 2010;468(1):283–288. doi: 10.1007/s11999-009-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rampersaud YR, Foley KT, Shen AC, Williams S, Solomita M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine. 2000;25:2637–2645. doi: 10.1097/00007632-200010150-00016. [DOI] [PubMed] [Google Scholar]
- 15.Rampersaud YR, Pik JH, Salonen D, Farooq S. Clinical accuracy of fluoroscopic computer-assisted pedicle screw fixation: a CT analysis. Spine. 2005;30:183–190. doi: 10.1097/01.brs.0000157490.65706.38. [DOI] [PubMed] [Google Scholar]
- 16.Rajasekaran S, Kamath V, Shetty AP. Intraoperative Iso-C three-dimensional navigation in excision of spinal osteoid osteomas. Spine. 2008;33:E25–E29. doi: 10.1097/BRS.0b013e31815e6308. [DOI] [PubMed] [Google Scholar]
- 17.Slomczykowski M, Roberto M, Schneeberger P. Radiation dose for pedicle screw insertion. Fluoroscopic method versus computer-assisted surgery. Spine. 1999;24:975–982. doi: 10.1097/00007632-199905150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Smith HE, Welsch MD, Sasso RC, Vaccaro AR. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med. 2008;31:532–537. doi: 10.1080/10790268.2008.11753648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smitherman SM, Tatsui CE, Rao G, Walsh G, Rhines LD. Image guided multilevel vertebral osteotomies for en bloc resection of giant cell tumor of the thoracic spine: case report and description of operative technique. Eur Spine J. 2010;19:1021–1028. doi: 10.1007/s00586-009-1273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjardes T, Shafizadeh S, Rixen D, Paffrath T, Bouillon B, Steinhausen ES, Baethis H. Image-guided spine surgery: state of the art and future directions. Eur Spine J. 2010;19:25–45. doi: 10.1007/s00586-009-1091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian NF, Xu HZ. Image-guided pedicle screw insertion accuracy: a meta-analysis international orthopaedics (SICOT) 2009;33:895–903. doi: 10.1007/s00264-009-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Royen BJ, Baayen JC, Pijpers R, Noske DP, Schakenraad D, Wuisman PI. Osteoid osteoma of the spine: a novel technique using combined computer-assisted and gamma probe- guided high-speed intralesional drill excision. Spine. 2005;30:369–373. doi: 10.1097/01.brs.0000152531.49095.34. [DOI] [PubMed] [Google Scholar]
- 23.Vougioukas VI, Hubbe U, Schipper J, Spetzger U. Navigated transoral approach to the cranial base and the craniocervical junction: technical note. Neurosurgery. 2003;52:247–250. doi: 10.1097/00006123-200301000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Wendl K, von Recum J, Wentzensen A, Grützner PA. Iso-C (3D0-assisted) navigated implantation of pedicle screws in thoracic lumbar vertebrae. Unfallchirurg. 2003;106:907–913. doi: 10.1007/s00113-003-0683-9. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Gao ZL, Wang JC, Li YP, Xia P, Jiang R. Pedicle screw placement in the thoracic spine: a randomized comparison study of computer-assisted navigation and conventional techniques. Chin J Traumatol. 2010;13(4):201–205. [PubMed] [Google Scholar]