Abstract
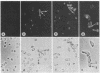
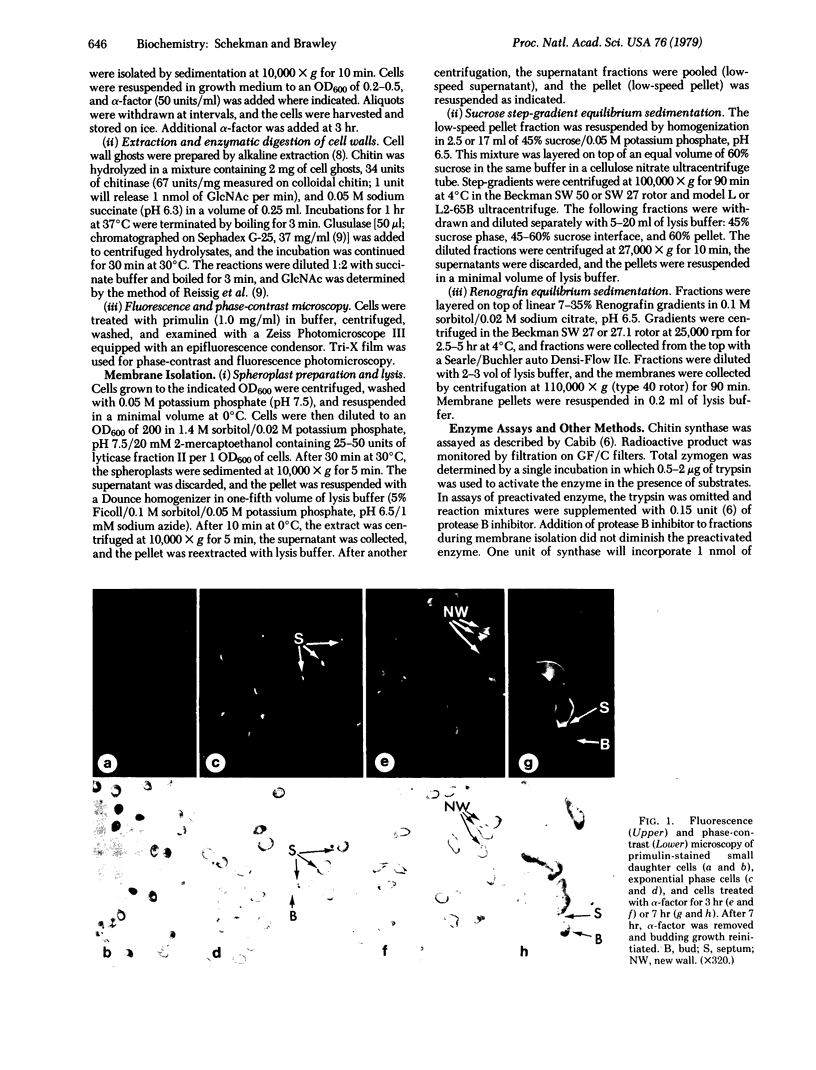
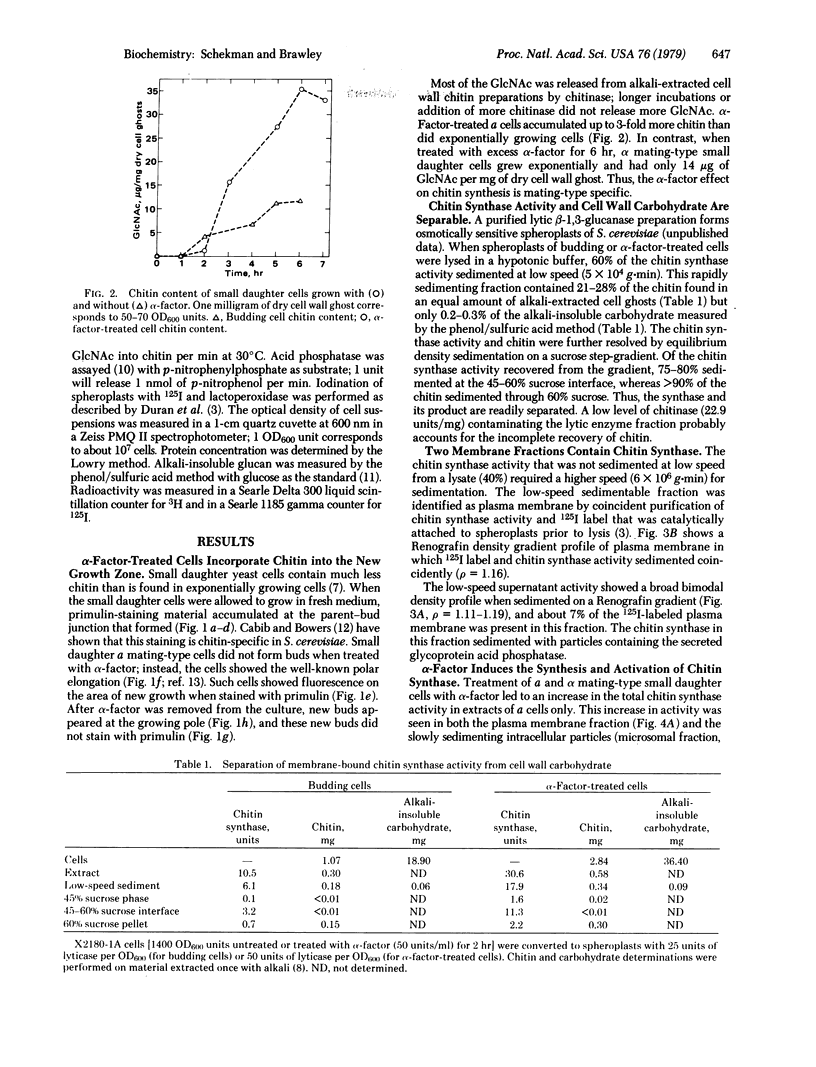
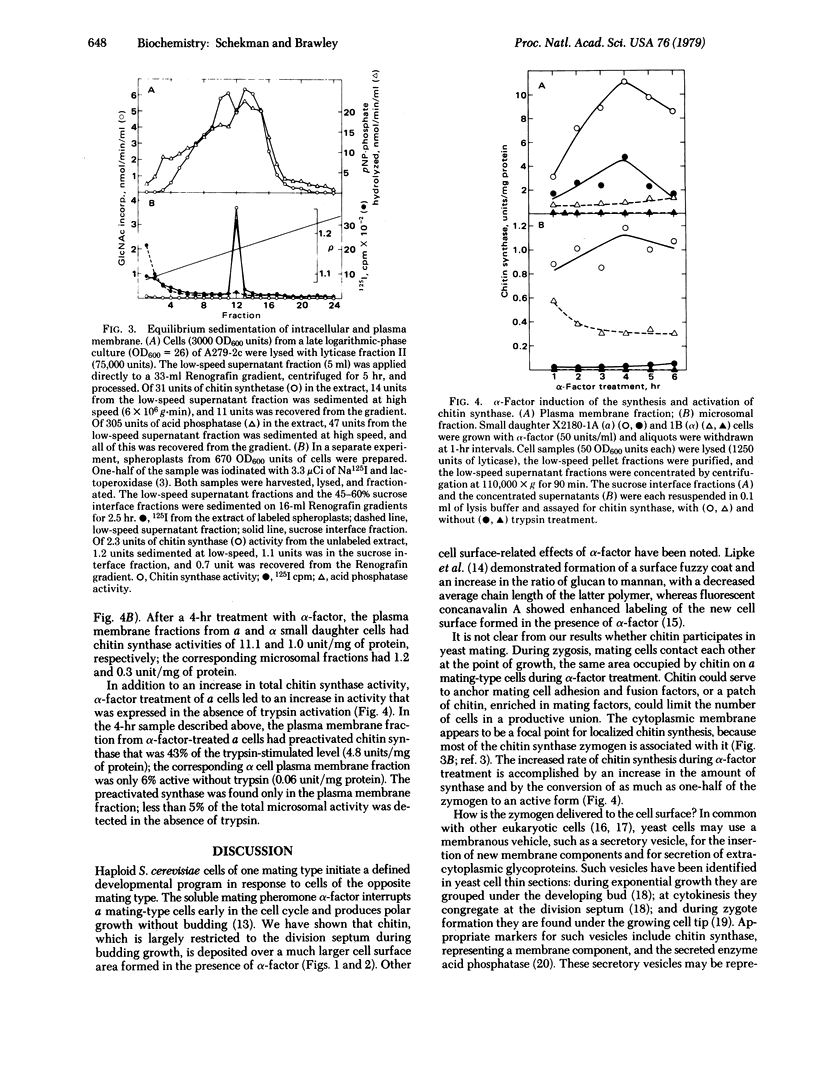
Treatment of a mating-type Saccharomyces cerevisiae cells with the pheromone α-factor (secreted by α mating-type cells) induces the synthesis of chitin. Small daughter cells, which start with no detectable chitin, make 3 times more chitin when grown in the presence of α-factor than do untreated exponentially growing cells. Budding cells accumulate chitin in the nascent division septum [Cabib, E. & Bowers, B. (1975) J. Bacteriol. 124, 1586), as detected by staining with the fluorescent dye primulin. In the absence of a division septum, α-factor-treated cells accumulate chitin in the area of pheromone-stimulated growth. Enzymatic lysis of budding and pheromone-treated cells allows the separation of membrane-bound chitin synthase (UDP-2-acetamido-2-deoxy-D-glucose: chitin 4-β-acetamidodeoxyglucosyltransferase, EC 2.4.1.16) activity from a dense particulate fraction containing chitin. Chitin synthase activity is associated with both the plasma membrane and small intracellular particles. During pheromone treatment, the levels of chitin synthase in the plasma membrane and in intracellular particle fractions increase 11- and 4-fold, respectively. Although chitin synthase is made as zymogen that requires proteolytic activation, the plasma membrane of pheromone-treated cells shows a significant fraction of preactivated enzyme; intracellular membrane-bound synthase is found exclusively in the zymogen form.
Keywords: chitin synthase, zymogen activation, asymmetric membrane assembly
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bracker C. E., Ruiz-Herrera J., Bartnicki-Garcia S. Structure and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4570–4574. doi: 10.1073/pnas.73.12.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun P. C., Calderone R. A. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978 Mar;133(3):1472–1477. doi: 10.1128/jb.133.3.1472-1477.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976 Jun;69(3):717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Bowers B. Timing and function of chitin synthesis in yeast. J Bacteriol. 1975 Dec;124(3):1586–1593. doi: 10.1128/jb.124.3.1586-1593.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Cartledge T. G., Rose A. H., Belk D. M., Goodall A. A. Isolation and properties of two classes of low-density vesicles from Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):426–433. doi: 10.1128/jb.132.2.426-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E., Thorner J., Geier M. Solid phase peptide synthesis of alpha-factor, a yeast mating pheromone. Biochem Biophys Res Commun. 1977 Oct 10;78(3):952–961. doi: 10.1016/0006-291x(77)90514-9. [DOI] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hirano H., Parkhouse B., Nicolson G. L., Lennox E. S., Singer S. J. Distribution of saccharide residues on membrane fragments from a myeloma-cell homogenate: its implications for membrane biogenesis. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2945–2949. doi: 10.1073/pnas.69.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. A., Kidby D. K. Role of vacuoles and vesicles in extracellular enzyme secretion from yeast. Can J Microbiol. 1973 Jan;19(1):113–117. doi: 10.1139/m73-017. [DOI] [PubMed] [Google Scholar]
- Linnemans W. A., Boer P., Elbers P. F. Localization of acid phosphatase in Saccharomyces cerevisiae: a clue to cell wall formation. J Bacteriol. 1977 Aug;131(2):638–644. doi: 10.1128/jb.131.2.638-644.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J Bacteriol. 1976 Jul;127(1):610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Ruiz-Herrera J., Lopez-Romero E., Bartnicki-Garcia S. Properties of chitin synthetase in isolated chitosomes from yeast cells of Mucor rouxii. J Biol Chem. 1977 May 25;252(10):3338–3343. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Sloat B. F., Pringle J. R. A mutant of yeast defective in cellular morphogenesis. Science. 1978 Jun 9;200(4346):1171–1173. doi: 10.1126/science.349694. [DOI] [PubMed] [Google Scholar]
- Van Rijn H. J., Boer P., Steyn-Parvé E. P. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim Biophys Acta. 1972 May 12;268(2):431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]