Abstract
Multifaceted and idiosyncratic aberrancies in social cognition characterize autism spectrum disorders (ASDs). To advance understanding of underlying neural mechanisms, we measured brain hemodynamic activity with functional magnetic resonance imaging (fMRI) in individuals with ASD and matched-pair neurotypical (NT) controls while they were viewing a feature film portraying social interactions. Pearson's correlation coefficient was used as a measure of voxelwise similarity of brain activity (InterSubject Correlations—ISCs). Individuals with ASD showed lower ISC than NT controls in brain regions implicated in processing social information including the insula, posterior and anterior cingulate cortex, caudate nucleus, precuneus, lateral occipital cortex, and supramarginal gyrus. Curiously, also within NT group, autism-quotient scores predicted ISC in overlapping areas, including, e.g., supramarginal gyrus and precuneus. In ASD participants, functional connectivity was decreased between the frontal pole and the superior frontal gyrus, angular gyrus, superior parietal lobule, precentral gyrus, precuneus, and anterior/posterior cingulate gyrus. Taken together these results suggest that ISC and functional connectivity measure distinct features of atypical brain function in high-functioning autistic individuals during free viewing of acted social interactions. Our ISC results suggest that the minds of ASD individuals do not ‘tick together’ with others while perceiving identical dynamic social interactions.
Keywords: Asperger syndrome, fMRI, Intersubject correlation, Movie, Social brain
Highlights
-
•
We studied brain function in autism during free viewing of social interactions.
-
•
The brains of individuals with autism do not ‘tick together’ with others.
-
•
Long-range functional connectivity is altered in individuals with autism.
-
•
Link between autistic traits and social brain synchrony extends to normal population.
1. Introduction
Autism spectrum disorders (ASD), affecting about 1% of adult populations (Brugha et al., 2011), are characterized by abnormal social interaction, communication, restricted interests, and repetitive behavior (Baron-Cohen and Belmonte, 2005; Baskin et al., 2006; Woodbury-Smith and Volkmar, 2009). Individual ASD phenotypes evolve in complex nature–nurture interactions (Jones and Klin, 2009; Pelphrey et al., 2011) and are difficult to characterize. Widely used tests measuring specific aspects of social cognition such as facial expression recognition (Falck-Ytter and von Hofsten, 2011), mentalizing of others' thoughts (Happe, 1993; Ziatas et al., 2003), and understanding or imitating others' actions (Hamilton, 2009), each capture some aspects of the multifaceted social cognition impairments. With such tasks it has been challenging to characterize especially high-functioning ASD individuals who often compensate their poor performance in tasks probing isolated social functions by adopting alternative strategies (Frith, 2004). For instance, images of facial expression of happiness can be recognized by analyzing facial features around mouth and eyes, while in real-life recognition of other person’s happiness requires, in addition to fast detection of facial expression, an ability to interpret contextual cues and goals of behavior. Therefore, performance in typical behavioral tests does not predict how patients with ASD guide their social interactions in complex natural environments. Brain imaging studies probing the neural basis of ASDs using similar tasks as in behavioral studies (Behrmann et al., 2006; Iacoboni and Dapretto, 2006; Zilbovicius et al., 2006) naturally share these limitations.
Challenges in measuring autistic traits and underlying brain functions have required development of novel paradigms that enable characterization of behavior in complex, dynamic social conditions that better imitate real life. Such paradigms, when they are used to measure spontaneous recognition of social cues (Golan et al., 2006; Heavey et al., 2000; Klin et al., 2002; Loveland et al., 1997; Speer et al., 2007) or interpretation of social interaction (Barnes et al., 2009; Dziobek et al., 2006) portrayed in movies, have indeed turned out to be successful in characterizing social-cognitive impairments in ASDs. Importantly, novel brain imaging methods allow investigation of hemodynamic activity associated with viewing social interactions portrayed in a movie (Bartels and Zeki, 2005; Hasson et al., 2004, 2010; Jääskeläinen et al., 2008; Lahnakoski et al., 2012a,b; Nummenmaa et al., 2012a). In a pioneering study, Hasson et al. (2004) used spatiotemporal activity patterns of one brain to predict activity in another brains, and found a strong voxel-by-voxel synchronization in several cortical areas. It seems that naturalistic stimuli are very efficient in eliciting reliable responses in the human brain (Hasson et al., 2010). Hasson et al. (2009) also demonstrated that in autistic participants regional temporal synchronization of fMRI signals, intersubject correlation (ISC), was decreased during free viewing of a movie excerpt in multiple brain areas, including visual and auditory cortices, suggesting that autistic persons respond to dynamic naturalistic stimulation in more individualistic ways than neurotypical (NT) controls.
Experiments using simple stimuli and isolated behavioral tasks and those using very rich naturalistic free viewing conditions may offer complementary insight into brain basis of ASD. Traditional experiments are tuned to carefully tease apart specific aspects of stimulus processing and task demands. However, it may be difficult to predict how such findings generalize to more complex ecological stimulus conditions. For instance, even responses of early sensory neurons to complex naturalistic stimuli are difficult to predict based on their responses to simple static stimuli (Touryan et al., 2005; Yao et al., 2007). Studying brain activity of ASD versus control subjects in more naturalistic settings, such as while viewing complex social interactions depicted in a movie, may enhance understanding how the brain is functioning in real life. Nevertheless, the obvious drawback is that in such experiments it may be very difficult to determine specific associations between stimulus features and corresponding brain activity.
Recent functional brain imaging studies on ASDs, measuring the functional connectivity among brain areas, have characterized distributed brain networks participating in social cognition (for reviews see (Just et al., 2012; Müller et al., 2011; Schipul et al., 2011)). Several studies report decreased frontal-posterior connectivity in ASD participants during simple behavioral tasks (Courchesne and Pierce, 2005; Just et al., 2004, 2007; Kleinhans et al., 2008; Koshino et al., 2005; Monk et al., 2010; Mostofsky et al., 2009; Solomon et al., 2009) and during resting state (Kennedy et al., 2006; Monk et al., 2009; Weng et al., 2010). Although the validity of these findings has recently been questioned by studies demonstrating that the methods that were used are sensitive to spurious effects caused by movement of the participants during scanning (Power et al., 2012; Van Dijk et al., 2012), these studies have significantly shaped views of autism-related brain functions. Instead of local amplitude changes in brain responses, several studies provided evidence of atypical large-scale brain network structure in ASDs, such as increase of randomness in local brain activity (Dinstein et al., 2012) or brain network structure (Lai et al., 2010). Theories of autism are therefore now accounting for findings related to distributed brain networks, typically relating autistic traits to delays in fast interactions among brain areas which characterize most of the social brain functions (Gepner and Féron, 2009). Brain imaging studies using complex dynamic stimuli such as movies that portray human social interactions may thus be well suited for addressing brain connectivity in ASD, as they provide optimal, large and time-variable dataset for functional connectivity analyses.
In this study, we examined using ISC and functional connectivity measures the neural basis of social impairments in ASD during naturalistic stimulation. We measured brain activity of 13 carefully diagnosed and characterized ASD participants and 13 matched-pair NT controls with fMRI while they were viewing a film depicting core aspects of social cognition (social interaction, goal-directed action, and facial and bodily emotional expressions). This movie reliably activates brain networks involved in social information processing in NT participants (Lahnakoski et al., 2012a). We included only high-functioning participants with ASD diagnosis that matched the NT controls in other domains of intellectual performance excluding social cognition, and restricted and/or stereotyped behavior. We also studied the link between the severity of the autistic traits and synchronization of brain activity. Whole brain functional connectivity analyses were performed using fourteen regions of interest (ROIs) as seeds. The selection of ROIs was based on our recent study localizing key areas involved in perception of dynamic social events containing faces, bodies, biological motion, goal-oriented action, emotions, social interaction, pain, or speech (Lahnakoski et al., 2012b). We predicted finding group differences in ISC especially in brain areas that have a key role in social perception and cognition, including the occipito-temporal fusiform cortex (Kanwisher et al., 1997), the inferior frontal gyrus (Dapretto et al., 2006), the superior temporal sulcus (Koldewyn et al., 2011; Pelphrey and Carter, 2008), and medial prefrontal cortex (Spengler et al., 2010). Furthermore, encouraged by our recent study demonstrating a link between similarity of brain activity during movie viewing and similarity of participants emotional experiences (Nummenmaa et al., 2012a), we expected that the synchronization of brain activity in the social brain areas is associated with social skills measured by the autism quotient (AQ) also in the NT group (Nummenmaa et al., 2012b; von dem Hagen et al., 2011). Finally, we expected to find decreased functional connectivity between the frontal and posterior brain areas in ASD participants, previously reported during simple behavioral tasks and resting state.
2. Material and methods
2.1. Participants
We studied 13 adult ASD males (mean age = 29 years, S.E.M = 1.7 years, age range = 20–41 years) and 13 NT adult male matched-pair control subjects (mean age = 29 years, S.E.M = 2.1 years, age range 19–47 years). The individuals with ASD filled the criteria for Asperger syndrome based on ICD-10 criteria. The diagnostic process included a detailed developmental history. Current symptoms were assessed with a review of diagnostic criteria and participants filled in the autism-spectrum quotient (AQ) questionnaire (Baron-Cohen et al., 2001a). AQ is a self-rating scale developed for assessment of the degree to which an individual with normal intelligence has the traits associated with the autism spectrum, validated in several studies (Allison et al., 2012; Hoekstra et al., 2011; Woodbury-Smith et al., 2005). Five additional participants were scanned for both groups, however, two ASD participants were excluded for not meeting all the required criteria (due to the usage of medication, remission of particular symptoms, and additional diagnosis) and three ASD participants were excluded from the analysis due to excessive head movements (> 3 mm absolute movement, i.e. more than one voxel in any direction) during scanning. One of the included ASD participants diagnosed in childhood had a remission of excessive routines and rituals and the concurrent symptoms were therefore more accurately characterized by the diagnosis of PDD-NOS. The matched-pair (NT) controls of the excluded ASD participants were also excluded. All participants had normal or corrected-to-normal vision, and normal hearing and spoke Finnish as their native language. Moreover, they had no other neurological or psychiatric diagnoses, and none of them were currently receiving medication affecting the central nervous system. Exclusion of psychiatric symptoms for the NT controls was confirmed with Structured Clinical Interview for DSM-IV. Groups were matched by age, and on each individual test on the Wechsler Adult Intelligence Scale III (Table 1). AQ, (Baron-Cohen et al., 2001a), Empathizing and Systemizing Quotients (EQ/SQ, (Baron-Cohen et al., 2003), Reading the Mind in the Eyes Test (Baron-Cohen et al., 2001b), and Benton Face Recognition Test (Benton et al., 1983) were also administered to the participants (Table 1). AQ, EQ, SQ, and Reading the Mind in the Eyes Test were translated into Finnish for the purpose of the study. Each participant gave a written informed consent prior to the testing as a part of the study protocol approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa. The study was conducted in accordance with the Helsinki Declaration. The NT participants were paid and the ASD participants were financially compensated for the loss of income and for travel expenses, as required by the local ethics committee.
Table 1.
Participant IQ and social cognition characteristics.
| NT/ASD min–max | NT (n = 13) mean (S.E.M.) | ASD (n = 13) mean (S.E.M) |
P | |
|---|---|---|---|---|
| Age | 19–47/20–41 | 29 (2.1) | 29 (1.7) | 0.84 |
| WAIS-III | ||||
| Full-scale IQ | 114–140/110–146 | 129 (2.0) | 127 (3.4) | 0.58 |
| Verbal IQ | 112–139/105–145 | 128 (2.2) | 127 (3.5) | 0.73 |
| Performance IQ | 115–144/102–143 | 127 (2.2) | 123 (3.4) | 0.37 |
| Verbal comprehension | 109–141/115–139 | 129 (2.6) | 127 (2.4) | 0.73 |
| Perceptual organization | 112–144/108–144 | 127 (2.5) | 127 (3.2) | 0.97 |
| Working memory | 93–142/85–139 | 120 (4.3) | 116 (4.0) | 0.46 |
| Processing speed | 93–142/72–136 | 116 (3.6) | 103 (4.8) | 0.09 |
| Other tests and scales | ||||
| Eyes test | 23–30/16–33 | 26 (0.6) | 25 (1.4) | 0.56 |
| Benton face recognition | 36–54/42–51 | 48 (1.6) | 47 (0.7) | 0.46 |
| Autism quotient | 6–35/17–43 | 12.5 (2.1) | 30.5 (2.1) | 0.00001 |
| Empathy quotient | 18–58/3–62 | 41.5 (3.2) | 25.9 (4.5) | 0.009 |
| Systemizing quotient | 18–58/26–67 | 33.4 (2.9) | 43.1 (3.8) | 0.051 |
2.2. The movie stimulus
The stimulus was a Finnish movie “The Match Factory Girl” (Aki Kaurismäki, 1990) that was shown as a full-length original version (67 min). Long stimulus duration served to obtain good signal-to-noise ratio in extrasensory brain areas, where the ISC signals develop gradually during movie stimulus (Jääskeläinen et al., 2008; Kauppi et al., 2010; Lerner et al., 2011). The selected film describes a challenging period in a life of a young woman. She tries to find a boyfriend, but is rejected when she finally meets a proper candidate and gets pregnant. Simultaneously she struggles with her relationship with her parents.
Subjective evaluations of the film (Table 3) were obtained from a sample that includes 9 ASD and 11 NT participants. In the beginning of the study we had a more comprehensive web-based questionnaire. However, since four of the first participated ASD individuals did not complete the web-questionnaire (two match-pair controls were also collected by that time), we changed to a more focused questionnaire reported here for the rest of the participants.
Table 3.
Participants' subjective evaluations of the film (1–5 scale, excluding familiarity).
| NT/ASD min–max | NT mean (S.E.M.) | ASD mean (S.E.M) |
P | |
|---|---|---|---|---|
| Familiar (1)/non-familiar (2) | – | 1.73 (0.14) | 1.8 (0.13) | 1.0 |
| Overall rating of the movie | 0–5/0–3 | 3.1 (0.31) | 2.4 (0.26) | 0.22 |
| Visual environment | ||||
| Pleasant/unpleasant | 2–5/2–4 | 3.13 | 3.38 | 0.55 |
| Riveting/revolting | 2–4/1–5 | 2.88 | 2.88 | 0.84 |
| Foreseeable/unexpected | 1–4/1–5 | 3.00 | 2.75 | 0.40 |
| Complex/simple | 3–5/3–5 | 4.00 | 4.25 | 0.51 |
| Auditory environment | ||||
| Pleasant/unpleasant | 1–4/1–4 | 2.5 | 2.63 | 0.59 |
| Riveting/revolting | 2–4/2–4 | 2.88 | 3.00 | 0.80 |
| Foreseeable/unexpected | 1–4/1–5 | 2.63 | 2.38 | 0.70 |
| Complex/simple | 2–5/2–5 | 4.25 | 3.75 | 1.0 |
| Social behavior | ||||
| Pleasant/unpleasant | 3–5/3–5 | 4.25 | 4.13 | 0.78 |
| Riveting/revolting | 1–5/3–5 | 3.00 | 3.88 | 0.14 |
| Foreseeable/unexpected | 1–5/2–5 | 3.00 | 3.00 | 0.70 |
| Complex/simple | 2–5/2–5 | 3.63 | 4.00 | 0.24 |
2.3. MRI data acquisition and preprocessing
The participants were instructed to watch the movie during the fMRI scanning as they normally would but with the instruction of trying to avoid any movements (of especially their heads) during the experiment. The movie was projected on a semi-transparent screen behind the participant's head using a 3-micromirror data projector (Christie X3, Christie Digital Systems Ltd., Mönchengladbach, Germany). The distance to the screen was 34 cm from a mirror located above their eyes (image width 28 cm). The audio track of the movie was played to the subjects with a UNIDES ADU2a audio system (Unides Design, Helsinki, Finland) via plastic tubes through porous EAR-tip (Etymotic Research, ER3, IL, USA) earplugs. The movie was delivered using Presentation software (Neurobehavioral Systems Inc., Albany, California, USA). The loudness of the sound was adjusted to a comfortable level that could be clearly heard on top of the scanner noise. It was confirmed that each participant could well hear the sounds and the same loudness was used for all participants.
MR imaging was carried out with a Signa VH/I 3.0 T scanner with HDxt upgrade (GE Medical Systems, USA) using a quadrature 8-channel head coil. The imaging area consisted of 29 functional gradient-echo planar axial slices (thickness 4 mm, 1 mm gap between slices, in-plane resolution 3.4 mm × 3.4 mm, voxel matrix 64 × 64, TE 32 ms, TR 2000 ms, flip angle 90°). A total of 1980 functional images were acquired continuously during the experiment. T1-weighted anatomical 3D images were acquired for anatomical alignment with inversion recovery—prepared spoiled gradient echo sequence (TE 2.988 ms, TR 10.02 ms, flip angle 15°). The T1 image acquisition used the same slice prescription as the functional image acquisition, except for a denser in-plane resolution (in-plane resolution 1 mm × 1 mm, matrix 256 × 256) and thinner slices (1 mm, no gap).
The data were preprocessed with the tools implemented in the Functional Magnetic Resonance Imaging of the Brain Centre (FMRIB) software library (FSL, release 4.1.6 www.fmrib.ox.ac.uk/fsl; (Smith et al., 2004)). To allow for the initial stabilization of the fMRI signal, the first 29 volumes of the session were excluded from the analysis; during this time the front titles of the movie were presented. The data were motion corrected (McFlirt) and non-brain matter was removed (BET); there were no group differences (P < 0.05) in the 6 motion direction parameters. Framewise displacement was less than 0.5 mm for at least 95% of the samples in the participants who were included in the data analysis (Power et al., 2012). The data were spatially smoothed using a Gaussian kernel with 6 mm FWHM, and high-pass filtered with a 150 s cutoff. The functional data were first co-registered (with FLIRT) to each participant's anatomical image allowing 7 DOF and then another registration was conducted from the anatomical space further to MNI152 standard space allowing 12 DOF. Manual correction of the registration (mainly rotation in the x-axis and minor scaling in z-axis) was conducted with Nudge.
2.4. Data analysis
Intersubject correlation analysis (Hasson et al., 2004) was performed using the ISC toolbox (Kauppi et al., 2010). We calculated voxel-wise temporal correlations using Pearson's correlation coefficient between every pair of subjects across the whole full-band time series (1951 volumes) after regressing out the subject motion. To examine the source of group differences in ISC and to link the present findings with recent studies suggesting that the brain activity in ASD participants is associated with ‘random noise’ (Dinstein et al., 2012; Lai et al., 2010) we also computed randomness of the spectrum in the time series signal. This was estimated as the slope of a straight line fitted (in the least squares sense) to the power spectrum of each voxel time series on a log–log scale. Thus, the closer the slope is to zero the more the spectrum resembles white noise, and more random is the signal.
To test the statistical significance of the ISC maps, we performed a fully nonparametric voxelwise permutation test for the r statistic (Kauppi et al., 2010). From the correlation matrix, we computed mean ISC maps separately within the ASD and NT groups. A between-groups comparison for the subject-wise ISCs that were relative to other participants within the same group was conducted with independent samples t-test of the pairwise Fisher Z-transformed correlation coefficients with the null hypothesis of no difference in the mean ISC between the groups. The significance of the t-statistics was estimated by randomly re-labeling the groups so that at least 4 participants in each group were changed and recalculating the t-values using 10,000 permutations. We then calculated the 95th percentile of t-values for each permutation and selected the maximum value over the permutations as the threshold of significance thereby controlling the false discovery rate (FDR). The association of ISC and other measures was estimated by constructing matrices of average pairwise scores of each subject pair and correlating the upper triangle entries of these matrices with the pairwise ISC matrices. Thus, the average score of each pair was used as a predictor of the ISC strength of that pair. Furthermore, we correlated AQ scores with brain responses, because prior studies have found AQ scores to correlate with autistic traits (Allison et al., 2012; Baron-Cohen et al., 2001a; Hoekstra et al., 2011; Woodbury-Smith et al., 2005) as well as brain structure and brain responses in simple social tasks and during rest (Baron-Cohen et al., 2001b, 2003).
The significance of the association of ISC with the randomness of the fMRI signal spectra, as well as the age, and behavioral indices was estimated using permutation testing. This was done across both groups including ASD and NT participants, and separately for the NT subjects. The testing was performed by randomly shuffling the order of the scores and calculating the percentiles of the observed correlations over the brain and taking the maximum of the observed percentiles over 1,000,000 repetitions thereby controlling the FDR.
Functional connectivity among brain areas during movie watching was examined using seed-voxel correlation analysis. The analysis was performed in the following steps: 1) Seed-regions were based on our previous study using fMRI during perception of dynamic social events (Lahnakoski et al., 2012b) and defined as local maxima in the FDR corrected brain map of areas that were activated [cerebellum crus I (37, 26, 16, the MNI x, y and z coordinates, respectively); middle temporal gyrus—MTG (70, 50, 33; caudate nucleus—Cau bilaterally (16, 10, 10 and − 16, 4, 10); superior temporal gyrus—STG (− 58, − 16, − 2); superior frontal gyrus—SFG (two seeds: 6, 54, 24, and 10, 10, 66); precentral gyrus—PreCG (46, 0, 50)] or deactivated [occipital fusiform cortex—OFC (32, − 46, − 8); frontal pole—FP bilaterally (20, 56, − 6 and − 20, 68, 12); paracingulate gyrus/anterior cingulate cortex—ACC (− 4, 28, 32); lateral occipital cortex—LOC/angular gyrus—AG (− 42, − 60, 40); postcentral gyrus—PostCG (− 38, − 26, 50)] during perception of dynamic social events in our previous study (Hasson et al., 2010). 2) The data were band-pass filtered (0.01 to 0.08 Hz) to avoid artifacts 3) Seed-voxel correlations were computed in each individual participant between the timeseries of 5-mm spherical ROIs surrounding the seed voxels and timeseries of all the other voxels in the brain for each seed individually. 4) Group differences were tested with t-test. 5) Significance of the t-statistics was estimated by generating random data and recalculating the t-values using 5000 permutations. Using framewise displacement value as a covariate in the second level analysis (Yan et al., 2013) had a negligible effect on the results. This is probably due to lack of statistically significant differences in the mean framewise displacement values between the two groups in the present study (p = 0.6825).
2.5. Eye movement recordings and analysis
The subjects' eye movements were recorded with SMI MEye Track long-range eye tracking system (Sensomotoric Instruments GmbH, Germany), based on video-oculography and the dark pupil–corneal reflection method. Calibration of the eye-tracking camera was done using five validated reference points and the data was collected with a 60-Hz sampling rate. Data from two ASD participants could not be collected due to interrupted access to the eye (the body of the participant partially occluded the camera view). Data from four additional ASD participants had to be rejected due to the poor quality of the recording (weak signal from the corneal reflection, movement of the camera during the scan, or occasional occlusion of the camera view) retaining 7 ASD participants for the final analysis. The criterion for rejection was that fixations were located outside of the calibration window in more than 10% of the time windows. The same number of NT participants was included to group comparison (dropping the subjects that had the highest number of fixations outside the calibration window). Similarity of fixation locations was calculated by creating a heat map of fixation locations for each participant in 2-s windows and calculating the pairwise spatial correlation of the heat maps between the participants. Heat maps were created by setting a 2-dimensional Gaussian kernel (sigma ~ 1°) at each fixation location within the time window.
3. Results
ISCs of NT participants were stronger than those of ASD participants in multiple cortical and subcortical areas (Fig. 1). Table 2 lists the anatomical labels, t-scores and MNI coordinates of the maxima of these areas. There were no brain areas showing significantly higher ISC in the ASD than the NT group. Age correlated with ISC in precuneus (PC) and LOC, in areas partially overlapping with those showing group differences, and in some other areas, including right temporoparietal junction and superior parietal lobule (Supplementary Fig. 1). IQ was not significantly associated with ISC in any of the brain areas showing ISC group differences (Supplementary Fig. 1). Because two recent studies have shown evidence that brain activity of ASD individuals is more random than in NT participants (Dinstein et al., 2012; Lai et al., 2010), we inspected whether our ISC results are associated with increased randomness of the fMRI signals, using Mantel tests. Indeed, our results suggest that the randomness of the hemodynamic responses of subject pairs, estimated as the mean randomness score of the pair, predict decreased pairwise ISC values in the areas where the ISC is greatest (Fig. 2). However, we failed to find any significant between-group differences in the randomness of signals per se.
Fig. 1.
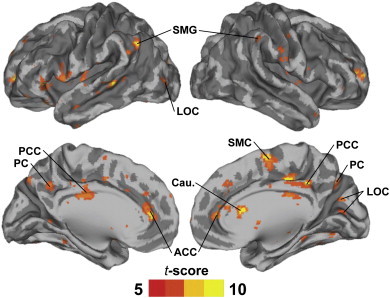
Volume renders of the brain showing regions where ISCs were larger (P < 0.05 FDR corrected) in NT than ASD group. SMG, supramarginal gyrus; LOC, lateral occipital cortex; PCC, posterior cingulate cortex; PC, precuneus; ACC, anterior cingulate cortex; Cau, caudate nucleus; SMC, supplementary motor cortex.
Table 2.
Anatomical labels, MNI-coordinates, and t-scores of local maxima in brain areas showing significant (P < 0.05 FDR corrected) ISC group differences (NT > ASD). Anatomical labels are based on Harvard–Oxford cortical atlas.
| Brain region | X | Y | Z | t-score |
|---|---|---|---|---|
| Supramarginal gyrus, posterior division | 34 | − 48 | 20 | 12.4 |
| Lateral occipital cortex, superior division | − 26 | − 62 | 42 | 11.7 |
| Lateral occipital cortex, inferior division | 32 | − 76 | 10 | 11.2 |
| Caudate nucleus | 18 | 26 | 0 | 11.0 |
| Posterior cingulate gyrus | 10 | − 26 | 40 | 10.6 |
| Precuneus cortex | − 28 | − 54 | 18 | 10.1 |
| Anterior cingulate gyrus | − 4 | 44 | 6 | 9.8 |
| Supplementary motor cortex | 4 | − 2 | 62 | 9.4 |
| Nucleus accumbens | − 12 | 14 | − 6 | 9.2 |
| Central opercular cortex/Insula | − 38 | 0 | 14 | 8.8 |
| Supramarginal gyrus, anterior division | 68 | − 20 | 28 | 8.7 |
Supplementary Fig. 1.
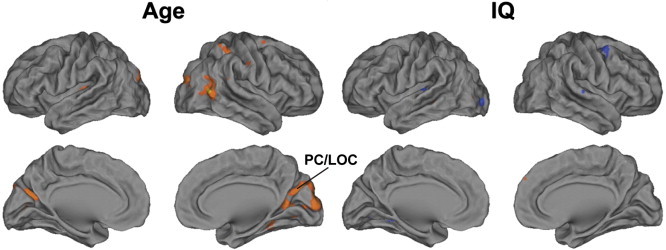
Volume renders of the brain areas where age and IQ correlated significantly (P < 0.05, FDR corrected) with ISC.
Fig. 2.
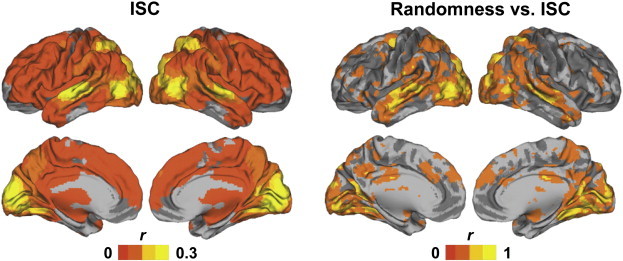
ISC across all participants (both NT and ASD; left) and the correlation between ISC and randomness of the power spectrum for the time series data (right). Correlation between ISC and randomness is strong mostly in the same regions in which ISC effect is largest. Both are thresholded at P < 0.005, FDR corrected.
ISCs of NT participants were stronger than those of ASD participants in multiple cortical and subcortical areas (Fig. 1). Table 2 lists the anatomical labels, t-scores and MNI coordinates of the maxima of these areas. There were no brain areas showing significantly higher ISC in the ASD than the NT group. Age correlated with ISC in precuneus (PC) and LOC, in areas partially overlapping with those showing group differences, and in some other areas, including right temporoparietal junction and superior parietal lobule (Supplementary Fig. 1). IQ was not significantly associated with ISC in any of the brain areas showing ISC group differences (Supplementary Fig. 1). Because two recent studies have shown evidence that brain activity of ASD individuals is more random than in NT participants (Dinstein et al., 2012; Lai et al., 2010), we inspected whether our ISC results are associated with increased randomness of the fMRI signals, using Mantel tests. Indeed, our results suggest that the randomness of the hemodynamic responses of subject pairs, estimated as the mean randomness score of the pair, predict decreased pairwise ISC values in the areas where the ISC is greatest (Fig. 2). However, we failed to find any significant between-group differences in the randomness of signals per se.
Associations between autistic traits, measured with the AQ, and ISC magnitude within NT group was studied using the Mantel test (Kriegeskorte et al., 2008). Fig. 3 shows the brain areas in which the Mantel test revealed significant effects (P < 0.05 FDR corrected). These areas included frontal orbital cortex, PreCG, occipital pole, and PC extending to several other areas. Clusters showing significant association between AQ and ISC overlap with the areas showing ISC group difference (Fig. 1) in the inferior frontal gyrus (IFG), MTG, supramarginal gyrus (SMG), PC, and OFC.
Fig. 3.
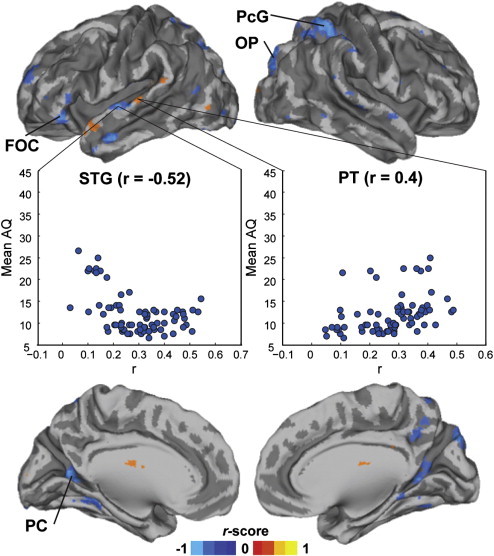
Volume renders of the brain showing areas with significant (P < 0.05 FDR corrected) correlation between ISC and AQ (Mantel test) across NT participants. Scatter plots are for visualization only and this data were not subjected to statistical testing. PcG, precentral gyrus; OP, occipital pole; FOC, frontal orbital cortex; STG, superior temporal gyrus; PT, planum temprorale; PC, precuneus cortex.
We conducted seed-voxel correlation analysis, using seeds in the brain regions that were significantly modulated by viewing dynamic social events in our previous study [Fig. 4a, ref. (Lahnakoski et al., 2012b), to examine group differences in large-scale brain connectivity. One of the examined networks with a seed in the frontal pole showed significant NT > ASD between-group differences. This network, shown in Fig. 4b included areas in the SFG, AG, SPL, PreCG, PC, PCC, and ACC. We failed to see significant group differences in any of the other networks that were tested (see Materials and methods section).
Fig. 4.
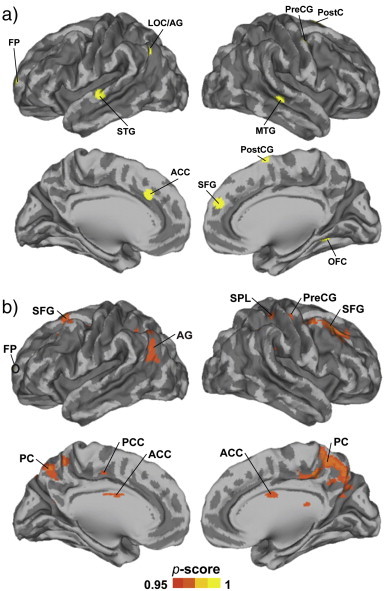
Results of the functional connectivity analysis. a) Seeds that were at the cortical surface are in yellow. b) A network of areas that were significantly more strongly connected in NT than in ASD subjects (FDR corrected P < 0.05). Superior frontal gyrus, SFG; angular gyrus, AG.
Eye movements calculated in 2-s samples showed between-group difference across the whole experiment and in 0.5% of the individual samples (corrected P < 0.05). Hence, eye movements were different between the two groups across the long stimulus, but the differences were not reliably associated with specific parts of the film. Since the between-group ISC differences were not focused on frontal and parietal eye fields involved in controlling eye movements but rather observed in other areas often in the literature implicated in social perception, we decided not to perform further analyses accounting for the possible effects of eye movements on brain activity.
The subjective evaluations of the film, obtained after the fMRI experiment from 9 ASD and 11 NT individuals, suggested that the groups did not differ in their interest towards the stimulus and had quite similar subjective experiences of the visual aspects and sounds of the movie, as well as of social behavior of the actors (Table 3).
4. Discussion
Our results show that ASD is associated with decreased ISC in brain hemodynamic activity time courses during viewing a feature movie that depicts various social interactions. There were significant ISC differences between the ASD and NT groups in several brain areas. These areas responded in more individualistic fashion in ASD subjects and thus lacked the group-level synchrony seen in NT subjects. Furthermore, the magnitude of ISC among the NT subjects was associated with their AQ scores, even though their AQ scores were in the normative range. In other words, the better the social brain of an NT individual ‘ticks together’ with others during viewing of naturalistic social interactions, the lower the AQ scores. Finally, atypical long-range functional connectivity was observed in ASD, using a seed-voxel based correlation analysis, in a cerebral network connecting the frontal pole with cingulate, superior frontal, and posterior parietal cortices.
Model-free ISC analysis method, introduced by Hasson et al. (2004), makes it possible to study altered brain activity in ASD during complex stimulation resembling real life. In ISC analysis the similarity or reliability of brain responses is quantified instead of mean response amplitudes. This analysis captures timing of the brain responses that is particularly important during complex stimulation (Hasson et al., 2004). Furthermore, this approach allows testing the relationship between intersubject synchronization of brain activity and the mental states or behavioral traits of the participants (Nummenmaa et al., 2012a).
Recent studies have demonstrated that autism is associated with decreased regional response reliability (Dinstein et al., 2012) as well as inter-regional randomness of the responses (Lai et al., 2010). Our findings are in concordance with these results. Our study demonstrates that decreased ISC and increased randomness of responses measure largely the same thing. However, the group differences in the ISC were more sensitive predictors of autistic traits than the randomness of the spectrum, as we failed to see statistically significant differences in the mean randomness of the groups. These distributed changes in regional and network activity in the brain provide new information about a difference in brain function between high-functioning autistic individuals and NT controls.
Our results suggest that the strength of ISC in IFG, MTG, SMG, precuneus, and OFC during natural viewing of social information correlates with autistic traits, as measured with AQ, also within the NT participants (Fig. 3). This finding corroborates previous studies suggesting that social cognitive impairments found with ASDs form a continuum where also NT subjects are located according to their social skills (Iidaka et al., 2012; Nummenmaa et al., 2012b; Suda et al., 2011; von dem Hagen et al., 2011) and that these skills are largely connected to the same brain regions in both ASD and NT subjects.
The ISC differences were observed in multiple brain areas known to be involved in social perception/cognition. The caudate nucleus has been implicated in emotion and subjective experience (Aron et al., 2005; Ishizu and Zeki, 2011), the SMG in human action perception (Caspers et al., 2010; Culham and Valyear, 2006), the LOC in perception of faces, bodies, and objects (Pitcher et al., 2009; Taylor and Downing, 2011), the ACC and PCC in mentalizing and self-monitoring (Amodio and Frith, 2006; Mar, 2011). The insula has been proposed to have a role in the perception of negative emotions such as disgust and regulation of related autonomic nervous system responses (Wicker et al., 2003), which is yet another domain showing consistently abnormal functions in ASDs.
In their pioneering study utilizing a naturalistic stimulus, Hasson et al. (2009) demonstrated decreased ISCs in autistic participants with an average IQ in visual cortical areas and also in the superior temporal cortex areas associated with auditory processing. The more widespread ISC differences in the present study might be due to different types of stimuli (i.e. different duration and content of the movie), distinct clinical populations (i.e., autistic participants with average IQ in (Hasson et al., 2009) and high-IQ individuals with Asperger syndrome in the present study), or both. Crucially, possibly due to the relatively short duration of the stimulus and selection of fictional western movie, ISC was focused on the occipito-parietal and temporal cortex, thus limiting the areas where the group differences in ISC could be observed. Moreover, Hasson et al. (2009) examined ASD participants that may have had other intellectual deficits than those limited to the social cognitive domain, perhaps observed also in the relatively low within-group correlation of the eye movement patterns (r ≈ 0.1). Nonetheless, our findings extend those of Hasson et al. (2009) by showing decreased ISC in high-functioning autistic subjects in brain areas important for social cognition.
Our results also show that decreased functional connectivity in ASD participants, as previously reported during simple behavioral tasks and at resting state, is also evident during the viewing of a complex real-life stimulus. Connectivity differences between the ASD and NT groups were not, however, as widespread as ISC group differences. Despite some overlap between areas showing between-group ISC differences and those revealed by seed-voxel functional connectivity analyses, only one network manifested statistically significant between-group differences in functional connectivity. On a cautionary note, the group differences were observed in long-range prefrontal-posterior connections, which could be prone to spurious effects caused by movement of the participants (Power et al., 2012; Van Dijk et al., 2012) and, therefore, these results should be regarded as tentative.
Despite the encouraging results, there are limitations in the present study that should be considered in future studies utilizing naturalistic stimulation. We were not able to obtain the Autism Diagnostic Interview, Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS), which are standard instruments in the diagnostics of ASD in many countries. In Finland, they were not in standard use at the time of diagnosis of the AS participants of this study. However, AQ, EQ and SQ have been especially designed for high-functioning individuals with ASD. We were not able to obtain subjective evaluations of the film and eye tracking during movie viewing from all ASD participants. In future studies, it would be useful to collect for instance dynamic ratings of emotional arousal and valence (Nummenmaa et al., 2012a) and use such information in modeling the brain imaging data collected during natural viewing. Further, we focused to a selected and highly specific subpopulation of the autism-spectrum disorders, high-functioning autistic subjects with Asperger syndrome diagnosis. Therefore, it remains unclear whether these findings generalize to broader sample of autistic individuals. Moreover, we used slightly different temporal filtering in ISC and SVC analysis, because SVC analysis with standard preprocessing showed artifactual correlations that extended to white matter and ventricles. Finally, we observed slightly lower synchronization of eye movements in ASD participants. It is possible that this affected ISC analysis.
5. Conclusions
Our results showed that when high-functioning ASD participants view naturalistic complex social encounters, their multiple brain areas respond in individualistic ways resulting in lower ISC of hemodynamic activity compared with NT controls. Using seed-voxel based correlation analysis, we further identified a functional network of brain areas comprising the FP, SFG, AG, SPL, PreCG, PC, ACC, and PCC, that functions abnormally in ASD subjects. The associations between the strength of the ISC in multiple brain areas contributing to social functions and the severity of the autistic traits, which was observed even within the NT group, further suggests that the better the social brain of an individual ‘ticks together’ with others during viewing of naturalistic social interactions, the less likely it is that a given individual has autistic features. Our findings provide evidence that the increased randomness of regional brain activity and decreased inter-regional connectivity, previously demonstrated during resting state and non-naturalistic behavioral tasks, is evident in autistic participants also during a condition approaching the complexity of real-life. Our results encourage one to the use of ISC as a tool in further studies examining the neural basis of complex behavioral traits associated with high-functioning autistic disorders.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.011.
Acknowledgments
The study was supported by the Academy of Finland (National Centres of Excellence program 2006–2011, grants #129670, #130412, #138145, #259752), the aivoAALTO project grant from the Aalto University, Päivikki and Sakari Sohlberg Foundation. We thank Ms. Marita Kattelus for her help in collecting the MRI data.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
J. Salmi, Email: juha.salmitaival@gmail.com.
M. Sams, Email: mikko.sams@aalto.fi.
References
- Allison C., Auyeung B., Baron-Cohen S. Toward brief “Red Flags” for autism screening: the Short Autism Spectrum Quotient and the Short Quantitative Checklist for Autism in toddlers in 1,000 cases and 3,000 controls [corrected] J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:202–212. doi: 10.1016/j.jaac.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A., Fisher H., Mashek D.J., Strong G., Li H., Brown L.L. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Barnes J.L., Lombardo M.V., Wheelwright S., Baron-Cohen S. Moral dilemmas film task: a study of spontaneous narratives by individuals with autism spectrum conditions. Autism Res. 2009;2:148–156. doi: 10.1002/aur.79. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Belmonte M.K. Autism: a window onto the development of the social and the analytic brain. Annu. Rev. Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Baron-Cohen S., Richler J., Bisarya D., Gurunathan N., Wheelwright S. The systemizing quotient: an investigation of adults with asperger syndrome or high-functioning autism, and normal sex differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:361–374. doi: 10.1098/rstb.2002.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Baskin J.H., Sperber M., Price B.H. Asperger syndrome revisited. Rev. Neurol. Dis. 2006;3:1–7. [PubMed] [Google Scholar]
- Behrmann M., Thomas C., Humphreys K. Seeing it differently: visual processing in autism. Trends Cogn. Sci. 2006;10:258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Sivan A.B., Hamsher K., Vareny N.R., Spreen O. Facial recognition: stimulus and multiple choice pictures. In: Benton A.L., Sivan A.B., Hamsher K., Vareny N.R., Spreen O., editors. Contributions to Neuropsychological Assessment. Oxford University Press; NewYork: 1983. pp. 30–40. [Google Scholar]
- Brugha T.S., McManus S., Bankart J., Scott F., Purdon S., Smith J., Bebbington P., Jenkins R., Meltzer H. Epidemiology of autism spectrum disorders in adults in the community in England. Arch. Gen. Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- Caspers S., Zilles K., Laird A.R., Eickhoff S.B. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Culham J.C., Valyear K.F. Human parietal cortex in action. Curr. Opin. Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I., Heeger D.J., Lorenzi L., Minshew N.J., Malach R., Behrmann M. Unreliable evoked responses in autism. Neuron. 2012;75:981–991. doi: 10.1016/j.neuron.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I., Fleck S., Kalbe E., Rogers K., Hassenstab J., Brand M. Introducing MASC: a movie for the assessment of social cognition. J. Autism Dev. Disord. 2006;36:623–636. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Falck-Ytter T., von Hofsten C. How special is social looking in ASD: a review. Prog. Brain Res. 2011;189:209–222. doi: 10.1016/B978-0-444-53884-0.00026-9. [DOI] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: confusions and controversies about Asperger syndrome. J. Child Psychol. Psychiatry. 2004;45:672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Gepner B., Féron F. Autism: a world changing too fast for a mis-wired brain? Neurosci. Biobehav. Rev. 2009;33:1227–1242. doi: 10.1016/j.neubiorev.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Golan O., Baron-Cohen S., Hill J.J., Golan Y. The “reading the mind in films” task: complex emotion recognition in adults with and without autism spectrum conditions. Soc. Neurosci. 2006;1:111–123. doi: 10.1080/17470910600980986. [DOI] [PubMed] [Google Scholar]
- Hamilton A.F. Goals, intentions and mental states: challenges for theories of autism. J. Child Psychol. Psychiatry. 2009;50:881–892. doi: 10.1111/j.1469-7610.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- Happe F.G. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition. 1993;48:101–119. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U., Avidan G., Gelbard H., Vallines I., Harel M., Minshew N. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2:220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Malach R., Heeger D.J. Reliability of cortical activity during natural stimulation. Trends Cogn. Sci. 2010;14:40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heavey L., Phillips W., Baron-Cohen S. M Rutter M, The awkward moments test: a naturalistic measure of social understanding in autism. J. Autism Dev. Disord. 2000;30:225–236. doi: 10.1023/a:1005544518785. [DOI] [PubMed] [Google Scholar]
- Hoekstra R.A., Vinkhuyzen A.A., Wheelwright S., Bartels M., Boomsma D.I., Baron-Cohen S., Posthuma D., van der Sluis S. The construction and validation of an abridged version of the autism-spectrum quotient (AQ-Short) J. Autism Dev. Disord. 2011;41:589–596. doi: 10.1007/s10803-010-1073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat. Rev. Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iidaka T., Miyakoshi M., Harada T., Nakai T. White matter connectivity between superior temporal sulcus and amygdala is associated with autistic trait in healthy humans. Neurosci. Lett. 2012;29:154–158. doi: 10.1016/j.neulet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Ishizu T., Zeki S. Toward a brain-based theory of beauty. PLoS One. 2011;6:e21852. doi: 10.1371/journal.pone.0021852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen I.P., Koskentalo K., Balk M.H., Autti T., Kauramäki J., Pomren C. Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. Open Neuroimaging J. 2008;2:14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W., Klin A. Heterogeneity and homogeneity across the autism spectrum: the role of development. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Keller T.A., Malave V.L., Kana R.K., Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi J.P., Jääskeläinen I.P., Sams M., Tohka J. Inter-subject correlation of brain hemodynamic responses during watching a movie: localization in space and frequency. Front. Neuroinforma. 2010;4:5. doi: 10.3389/fninf.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Redcay E., Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Klin A., Jones W., Schultz R., Volkmar F., Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Koldewyn K., Whitney D., Rivera S.M. Neural correlates of coherent and biological motion perception in autism. Dev. Sci. 2011;14:1075–1088. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. Representational similarity analysis—connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski J.M., Salmi J., Jääskeläinen I.P., Lampinen J., Glerean E., Tikka P., Sams M. Stimulus-related independent component and voxel-wise analysis of human brain activity during free viewing of a feature film. PLoS One. 2012;7:e35215. doi: 10.1371/journal.pone.0035215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahnakoski J.M., Glerean E., Salmi J., Jääskeläinen I.P., Sams M., Hari R., Nummenmaa L. Naturalistic FMRI mapping reveals superior temporal sulcus as the hub for the distributed brain network for social perception. Front. Hum. Neurosci. 2012;6:233. doi: 10.3389/fnhum.2012.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Chakrabarti B., Sadek S.A., Pasco G., Wheelwright S.J., Bullmore E.T., Baron-Cohen S., Suckling J., MRC AIMS Consortium A shift to randomness of brain oscillations in people with autism. Biol. Psychiatry. 2010;68:1092–1099. doi: 10.1016/j.biopsych.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Lerner Y., Honey C.J., Silbert L.J., Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 2011;31:2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland K.A., Tunali-Kotoski B., Chen Y.R., Ortegon J., Pearson D.A., Brelsford K.A. Emotion recognition in autism: verbal and nonverbal information. Dev. Psychopathol. 1997;9:579–593. doi: 10.1017/s0954579497001351. [DOI] [PubMed] [Google Scholar]
- Mar R.A. The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Peltier S.J., Wiggins J.L., Weng S.J., Carrasco M., Risi S., Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Weng S.J., Wiggins J.L., Kurapati N., Louro H.M., Carrasco M., Maslowsky J., Risi S., Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. J. Psychiatry Neurosci. 2010;35:105–114. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S.H., Powell S.K., Simmonds D.J., Goldberg M.C., Caffo B., Pekar J.J. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–2425. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.A., Shih P., Keehn B., Deyoe J.R., Leyden K.M., Shukla D.K. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex. 2011;21:2233–2243. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Glerean E., Viinikainen M., Jääskeläinen I.P., Hari R., Sams M. Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl. Acad. Sci. U. S. A. 2012;12:9599–9604. doi: 10.1073/pnas.1206095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Engell A.D., von dem Hagen E., Henson R.N., Calder A.J. Autism spectrum traits predict the neural response to eye gaze in typical individuals. Neuroimage. 2012;15:3356–3363. doi: 10.1016/j.neuroimage.2011.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Carter E.J. Brain mechanisms for social perception: lessons from autism and typical development. Ann. N. Y. Acad. Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B.C. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Charles L., Devlin J.T., Walsh V., Duchaine B. Triple dissociation of faces, bodies, and objects in extrastriate cortex. Curr. Biol. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipul S.E., Keller T.A., Just M.A. Inter-regional brain communication and its disturbance in autism. Front. Syst. Neurosci. 2011;22:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. (Suppl.) [DOI] [PubMed] [Google Scholar]
- Solomon M., Ozonoff S.J., Ursu S., Ravizza S., Cummings N., Ly S., Carter C.S. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47:2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer L.L., Cook A.E., McMahon W.M., Clark E. Face processing in children with autism: effects of stimulus contents and type. Autism. 2007;11:265–277. doi: 10.1177/1362361307076925. [DOI] [PubMed] [Google Scholar]
- Spengler S., Bird G., Brass M. Hyperimitation of actions is related to reduced understanding of others' minds in autism spectrum conditions. Biol. Psychiatry. 2010;68:1148–1155. doi: 10.1016/j.biopsych.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Suda M., Takei Y., Aoyama Y., Narita K., Sakurai N., Fukuda M., Mikuni M. Autistic traits and brain activation during face-to-face conversations in typically developed adults. PLoS One. 2011;6:e20021. doi: 10.1371/journal.pone.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.C., Downing P.E. Division of labor between lateral and ventral extrastriate representations of faces, bodies, and objects. J. Cogn. Neurosci. 2011;23:4122–4137. doi: 10.1162/jocn_a_00091. [DOI] [PubMed] [Google Scholar]
- Touryan J., Felsen G., Dan Y. Spatial structure of complex cell receptive fields measured with natural images. Neuron. 2005;45:781–791. doi: 10.1016/j.neuron.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen E.A., Nummenmaa L., Yu R., Engell A.D., Ewbank M.P., Calder A.J. Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb. Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S.J., Wiggins J.L., Peltier S.J., Carrasco M., Risi S., Lord C., Monk C.S. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith M.R., Volkmar F.R. Asperger syndrome. Eur. Child Adolesc. Psychiatry. 2009;18:2–11. doi: 10.1007/s00787-008-0701-0. [DOI] [PubMed] [Google Scholar]
- Woodbury-Smith M.R., Robinson J., Wheelwright S., Baron-Cohen S. Screening adults for Asperger Syndrome using the AQ: a preliminary study of its diagnostic validity in clinical practice. J. Autism Dev. Disord. 2005;35:331–335. doi: 10.1007/s10803-005-3300-7. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Shi L., Han F., Gao H., Dan Y. Rapid learning in cortical coding of visual scenes. Nat. Neurosci. 2007;10:772–778. doi: 10.1038/nn1895. [DOI] [PubMed] [Google Scholar]
- Ziatas K., Durkin K., Pratt C. Differences in assertive speech acts produced by children with autism, Asperger syndrome, specific language impairment, and normal development. Dev. Psychopathol. 2003;15:73–94. doi: 10.1017/s0954579403000051. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M., Meresse I., Chabane N., Brunelle F., Samson Y., Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29:359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]


