Abstract
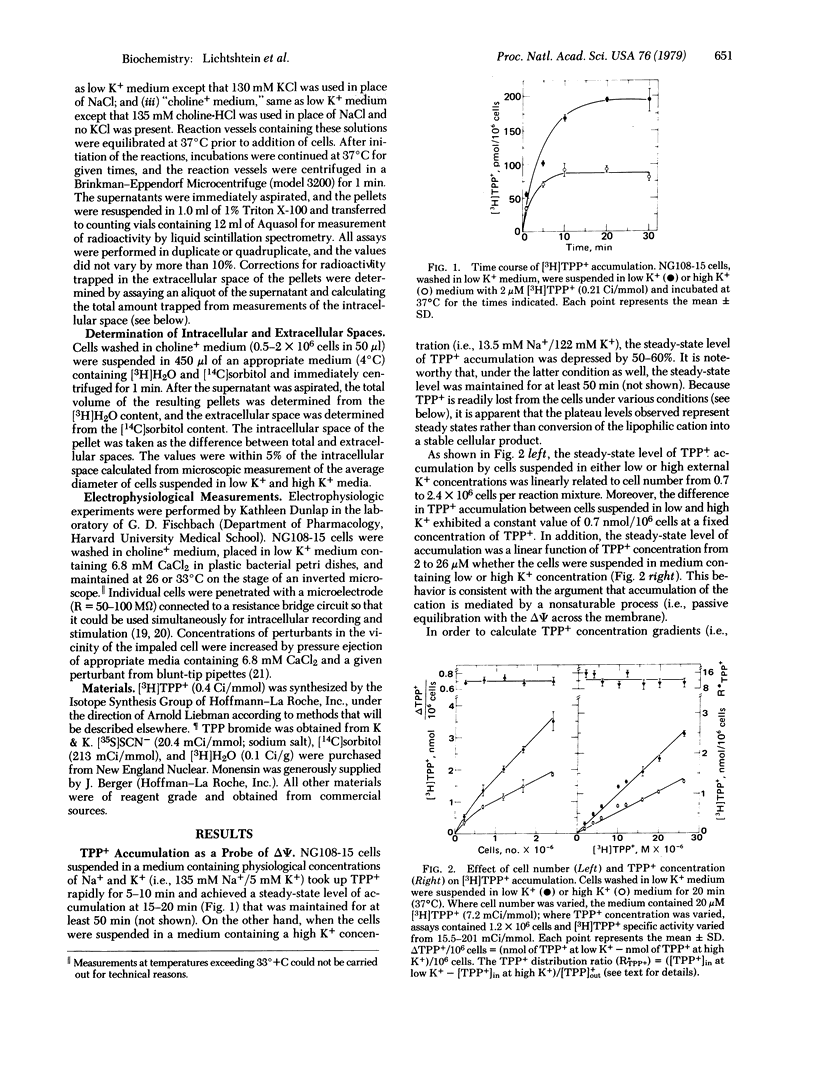
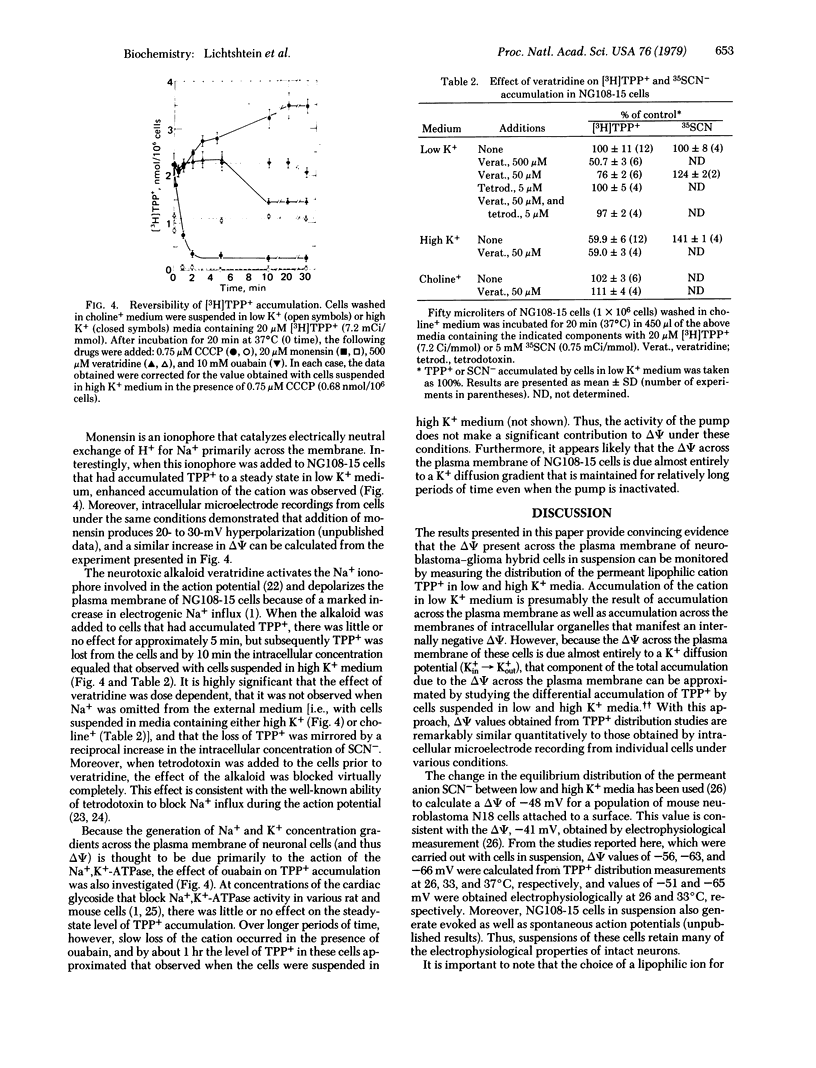
Neuroblastoma-glioma hybrid cells (NG108-15) in suspension accumulate the permeant lipophilic cation [3H]tetraphenylphosphonium (TPP+) against a concentration gradient. The steady-state level of TPP+ accumulation is about twice as great in physiological media of low K+ concentration (i.e., 5 mM K+/135 mM Na+) than in a medium of high K+ concentration (i.e., 121 mM K+/13.5 mM Na+). The latter manipulation depolarizes the NG108-15 plasma membrane and indicates that the resting membrane potential (ΔΨ) is due primarily to a K+ diffusion gradient (Kin+ → Kout+). TPP+ accumulation is time and temperature dependent, achieving a steady state in 15-20 min at 37°C, and is a linear function of cell number and TPP+ concentration (i.e., the concentration gradient is constant). The difference in TPP+ accumulation in low and high K+ media under various conditions has been used to calculate mean (±SD) ΔΨ values of -56 ± 3, -63 ± 4, and -66 ± 5 mV at 26, 33, and 37°C, respectively. Importantly, these values are virtually identical to those obtained by direct electrophysiological measurements made under the same conditions. TPP+ accumulation is abolished by the protonophore carbonylcyanide-m-chlorophenylhydrazone, whereas the neurotoxic alkaloid veratridine diminishes uptake to the same level as that observed in high K+ media. In addition, the effect of veratridine is dependent upon the presence of external Na+ and is blocked by tetrodotoxin. The steady-state level of TPP+ accumulation is enhanced by monensin, indicating that this ionophore induces hyperpolarization under appropriate conditions. Finally, ouabain has essentially no effect on the steady-state level of TPP+ accumulation in short-term experiments, suggesting that Na+,K+-ATPase activity makes little contribution to the resting potential in these cells. Because many of these observations are corroborated by intracellular recording techniques, it is concluded that TPP+ distribution measurements can provide a biochemical method for determining membrane potentials in populations of cultured neuronal cells.
Keywords: [3H]tetraphenylphosphonium+, veratridine, protonophore, monensin, ouabain
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Catterall W. A., Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Ray R., Morrow C. S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977 Sep 22;269(5626):342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- Daniels M. P., Hamprecht B. The ultrastructure of neuroblastoma glioma somatic cell hybrids. Expression of neuronal characteristics stimulated by dibutyryl adenosine 3',5' cyclic monophosphate. J Cell Biol. 1974 Nov;63(2 Pt 1):691–699. doi: 10.1083/jcb.63.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cespedes C., Christensen H. N. Complexity in valinomycin effects on amino acid transport. Biochim Biophys Acta. 1974 Feb 26;339(1):139–145. doi: 10.1016/0005-2736(74)90339-3. [DOI] [PubMed] [Google Scholar]
- Evans M. H. Tetrodotoxin, saxitoxin, and related substances: their applications in neurobiology. Int Rev Neurobiol. 1972;15:83–166. doi: 10.1016/s0074-7742(08)60329-3. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Dichter M. A. Electrophysiologic and morphologic properties of neurons in dissociated chick spinal cord cell cultures. Dev Biol. 1974 Mar;37(1):100–116. doi: 10.1016/0012-1606(74)90172-9. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Grinius L. L., Jasaitis A. A., Kadziauskas Y. P., Liberman E. A., Skulachev V. P., Topali V. P., Tsofina L. M., Vladimirova M. A. Conversion of biomembrane-produced energy into electric form. I. Submitochondrial particles. Biochim Biophys Acta. 1970 Aug 4;216(1):1–12. doi: 10.1016/0005-2728(70)90153-2. [DOI] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ambesi-Impiombato F. S., Meldolesi M. F., Aloj S. M., Coon H. G., Kaback H. R., Kohn L. D. Effects of thyrotropin on the thyroid cell membrane: hyperpolarization induced by hormone-receptor interaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2352–2356. doi: 10.1073/pnas.74.6.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Heinz E., Geck P., Pietrzyk C. Driving forces of amino acid transport in animal cells. Ann N Y Acad Sci. 1975 Dec 30;264:428–441. doi: 10.1111/j.1749-6632.1975.tb31501.x. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P., Christian C., Nirenberg M. Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):123–127. doi: 10.1073/pnas.73.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Repke K., Est M., Portius H. J. Uber die Ursache der Speciesunterschiede in der Digitalisempfindlichkeit. Biochem Pharmacol. 1965 Dec;14(12):1785–1802. doi: 10.1016/0006-2952(65)90269-8. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Traber J., Fischer K., Buchen C., Hamprecht B. Muscarinic response to acetylcholine in neuroblastoma times glioma hybrid cells. Nature. 1975 Jun 12;255(5509):558–560. doi: 10.1038/255558a0. [DOI] [PubMed] [Google Scholar]
- Traber J., Fischer K., Latzin S., Hamprecht B. Morphine antagonises action of prostaglandin in neuroblastoma and neuroblastoma times glioma hybrid cells. Nature. 1975 Jan 10;253(5487):120–122. doi: 10.1038/253120a0. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]


