Abstract
There is increasing interest in developing a reliable, affordable and accessible disease biomarker of Parkinson's disease (PD) to facilitate disease modifying PD-trials. Imaging biomarkers using magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) can describe parameters such as fractional anisotropy (FA), mean diffusivity (MD) or apparent diffusion coefficient (ADC). These parameters, when measured in the substantia nigra (SN), have not only shown promising but also varying and controversial results.
To clarify the potential diagnostic value of nigral DTI in PD and its dependency on selection of region-of-interest, we undertook a high resolution DTI study at 3 T. 59 subjects (32 PD patients, 27 age and sex matched healthy controls) were analysed using manual outlining of SN and substructures, and voxel-based analysis (VBA). We also performed a systematic literature review and meta-analysis to estimate the effect size (DES) of disease related nigral DTI changes.
We found a regional increase in nigral mean diffusivity in PD (mean ± SD, PD 0.80 ± 0.10 vs. controls 0.73 ± 0.06 · 10− 3 mm2/s, p = 0.002), but no difference using a voxel based approach. No significant disease effect was seen using meta-analysis of nigral MD changes (10 studies, DES = + 0.26, p = 0.17, I2 = 30%). None of the nigral regional or voxel based analyses of this study showed altered fractional anisotropy. Meta-analysis of 11 studies on nigral FA changes revealed a significant PD induced FA decrease. There was, however, a very large variation in results (I2 = 86%) comparing all studies. After exclusion of five studies with unusual high values of nigral FA in the control group, an acceptable heterogeneity was reached, but there was non-significant disease effect (DES = − 0.5, p = 0.22, I2 = 28%).
The small PD related nigral MD changes in conjunction with the negative findings on VBA and meta-analysis limit the usefulness of nigral MD measures as biomarker of Parkinson's disease. The negative results of nigral FA measurements at regional, sub-regional and voxel level in conjunction with the results of the meta-analysis of nigral FA changes question the stability and validity of this measure as a PD biomarker.
Abbreviations: PD, Parkinson's disease; MRI, Magnetic resonance imaging; DTI, Diffusion tensor imaging; MD, Mean diffusivity; ADC, Apparent diffusion coefficient; SN, Substantia nigra; SNc, Substantia nigra pars compacta; DES, Effect size of disease related nigral changes; VBA, Voxel based analysis; TCS, Transcranial sonography; ACE, Addenbrooke's cognitive examination test battery; UPDRS, Unified Parkinson's disease rating scale; EPI, Echo planar imaging; ROI, Region/regions of interest; ICC, Intraclass correlation coefficient
Keywords: Parkinson's disease, Parkinsonism, Diffusion weighted imaging, Magnetic resonance imaging, Substantia nigra, Fractional anisotropy
Highlights
-
•
Investigating diagnostic accuracy of nigral diffusion MRI to diagnose Parkinson's
-
•
There is small, inconsistent increase of mean diffusivity of the substantia nigra.
-
•
There is no change in nigral fractional anisotropy (FA) in the case–control study.
-
•
Meta-analysis revealed nigral FA change is dependent on high FA in controls.
-
•
This questions the usefulness of nigral diffusion MRI as biomarker in Parkinson's.
1. Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disease characterised by depletion of pigmented dopaminergic neurons of the substantia nigra pars compacta (SNc) (Tretiakoff, 1919). The correlation of the dopaminergic cell loss in SN with the progressive symptoms of PD (Ma et al., 1997) makes SN imaging promising for biomarker search in PD. The clinical diagnosis of PD is based on the application of the Queen Square Parkinson Brain Bank Criteria with accuracy rates varying from excellent in specialised centres (Hughes et al., 2002) to 15–25% false positive rate in some community studies (Meara et al., 1999; Schrag et al., 2002).
In the last two decades the search for imaging biomarkers of PD has revealed a variety of promising techniques including magnetic resonance imaging (MRI) and transcranial sonography (TCS). PD associated structural brain changes were reported for both modalities throughout many different brain regions with a wide variety of accuracy and repeatability (Auer, 2009; Berg et al., 2011). However, none of these techniques to date accomplished the required accuracy and reliability to justify introduction in standard clinical practice or as part of interventional clinical trials. Particularly promising, but equally controversial, are results obtained with diffusion tensor MR imaging (DTI) to probe SN diffusion characteristics.
MRI diffusion metrics assess the random movement of water protons in terms of overall extent (mean diffusivity, MD) and orientational dependence (fractional anisotropy, FA). The complex ultrastructure of the brain results in a distinctive pattern of diffusion restriction and anisotropy against which subtle alterations in intra- and extracellular diffusion barriers can be well described. Neurodegenerative cell loss removes diffusion barriers and their orientational dependence, which results in increased diffusivity and reduced anisotropy as demonstrated in a number of neurodegenerative diseases. In atypical parkinsonism, regional diffusional abnormalities enable the distinction of multi-system atrophy and progressive supranuclear palsy from PD and control patients (Blain et al., 2006; Schocke et al., 2002; Seppi et al., 2005).
In order to differentiate PD from healthy controls, several studies reported alterations of nigral FA, MD or the related apparent diffusion coefficient (ADC) using manual or ‘semi-manual’ delineation of the SN. Recently, a remarkable diagnostic accuracy was reported in a 3 T DTI study for a latero-dorsal sub-region of the SN (Vaillancourt et al., 2009). The authors suggested that their unique diagnostic accuracy resulted from the resolution and the location of the region of interest (ROI). The latter is plausible due to the known spatial heterogeneity of dopaminergic cell loss, predominantly affecting nigrosome 1 in the latero-dorsal aspect of the SNc (Damier et al., 1999). In addition, the borders of the SN are not well delineated and reported studies use different non-standardised landmarks.
In this study we aimed to evaluate the diagnostic accuracy of high resolution nigral DTI to distinguish PD from controls using a selection of ROI as well as voxel-based group analysis. To determine whether nigral diffusion metrics (FA and MD) qualify as candidate biomarkers we undertook a systematic review and meta-analysis of continuous data to estimate their ‘relative disease effect size’ (DES) for PD.
2. Material and methods
2.1. Diffusion tensor MRI case–control study
The study was approved by the local Ethics Committee and Research and Development department. All participants gave written informed consent prior to enrolment into the study. 67 participants were investigated, 8 were excluded from further analysis due to technical artefacts/factors or significant cognitive impairment. 32 patients fulfilling the UK Brain Bank criteria for PD were recruited from movement disorders clinics at two local NHS trusts. 27 age and gender matched controls were recruited from spouses, friends or within the university. The subjects underwent the Addenbrooke's cognitive examination (ACE) test battery (Bak and Mioshi, 2007) and were excluded if they had a history of cognitive impairment or an ACE score of < 80. Five controls did not complete the ACE test battery, however, these participants did not have any past cognitive problems nor a neurological, neurosurgical or psychiatric history. Parkinson's disease severity was scored using the UPDRS (Fahn et al., 1987) and Hoehn and Yahr staging (Hoehn and Yahr, 1967).
MR imaging was performed at 3 T (Achieva scanner, Philips Medical Systems, Best, Netherlands) with a standard eight-channel head coil. Axial diffusion tensor imaging was obtained using a single shot fat saturated spin echo EPI (TR/TE = 7415/60 ms, 52 slices, acquisition matrix of 112 × 112 with pixel size of 2 × 2 × 2 mm3, interpolated to 1 × 1 × 2 mm3, sense factor of 2, partial Fourier factor of 0.7, 32 diffusion gradient directions with b = 1000 s/mm2, and three repeats of b = 0). Patients were assessed and scanned whilst medicated.
2.1.1. Substantia nigra regional manual measurements
The image processing was completed using the diffusion tools in the FSL 4.1.0 software package (Jenkinson and Smith, 2001; Woolrich et al., 2009). DTI processing consisted of motion correction, affine registration to reduce the effects of eddy currents, and calculation of the diffusion tensor parametric maps of MD and FA.
Regions-of-interest were manually drawn by an experienced researcher with 5 years of experience in PD imaging research to outline the SN using JIM software (www.xinapse.com) or an in house developed image analysis software package NeuRoi (Dr C. Tench, University of Nottingham, UK). Anatomical landmarks were identified on the T2 weighted image for the red nucleus as hypointense structure, and on FA maps for the cerebral peduncle as hyperintense area and used to constrain the lateral border of the SN. Two types of SN and separate control ROI were used (Fig. 1): 1. Small SN ROI — three ROI measuring 2 × 2 × 2 mm3 (4 pixels in plane) placed over each of the T2 hypointense SN ventrally, medially and dorsally on two slices (lowest slice containing the red nucleus and one below) in accordance with anatomical landmarks and dimensions used in Vaillancourt et al. (2009), 2. Large SN ROI — Manual outlining of the hypointense region between the cerebral peduncles and posterior tegmentum on three slices (two at the lower level of the red nucleus and one below in accordance to landmarks and variable size in (Du et al., 2011)). 3. Control region — 2 × 2 × 2 mm3 ROI placed in the cerebral peduncles on the same slices as analysis 2.
Fig. 1.
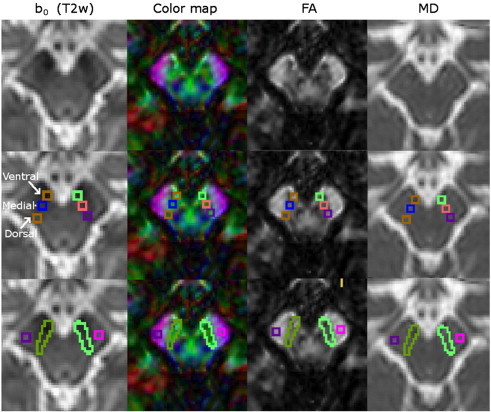
Example of a DTI scan of a healthy control and ROI positions in the SN and control regions.
Columns represent: 1st: T2 weighted b0 image; 2nd: colour coded map of the principle eigenvector of the diffusion matrix; 3rd: FA map; and 4th: MD maps. ROIs are demonstrated in the second row for small ROI (analysis 1) and in the third row for total SN and control ROI in the cerebral peduncle (analysis 2).
Reproducibility of manual SN ROI placement was assessed by calculation of intraclass correlation coefficients (ICC) for inter-rater (2nd researcher with 5 years experience in MRI research in PD) and intra-rater variability in 20 random subjects with a minimum of 12 weeks of interval between analyses. ‘Absolute agreement’ coefficients were calculated assuming a two-way random effect model with random people and measurement effects.
2.1.2. Voxel-based analysis
Individual FA images were aligned to FMRIB58_FA standard-space template and corresponding transformation matrixes were applied on MD images using FMRIB's Nonlinear Registration Tool as part of the FSL 4.1.0 software package (FMRIB's software library, http://www.fmrib.ox.ac.uk/fsl/ (Jenkinson and Smith, 2001; Woolrich et al., 2009)).
The spatially normalized MD and FA images were smoothed with a 8-mm isotropic Gaussian kernel to reduce the level of noise and correct the misalignment. Statistical comparisons were performed using statistical parametric mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm) by a two sample t-test between controls and patients with age as covariates of no interest. An uncorrected p < 0.001 at voxel level for multiple comparisons were considered as significant.
2.1.3. Statistics
Statistical tests were performed using IBM® SPSS® for windows (version 19.0). Demographics were compared between all patients and controls using analysis of variance or chi-square test. Non-parametric tests were used for group comparisons if the data was found to be not normally distributed. Results from right and left SN measures were averaged. Values are given as mean ± SD unless stated otherwise; significance was defined at p < 0.05.
2.2. Systematic review and meta-analysis
To identify relevant literature three databases and reference lists of articles were searched (PubMed, ScienceDirect and EMBASE 1980–2013) in accordance to the ‘preferred reporting items for systematic reviews and meta-analysis’ (PRISMA) statement (Liberati et al., 2009). The search was performed independently by two researchers using multiple Medical Subject Headings.
Out of 25 reviewed articles 14 were excluded for the following reasons: 10 publications because of missing description of regional SN DTI measures, 2 publications because of overlapping participant populations with studies included in the meta-analysis, 2 publications because of a missing healthy control arm.
Apart from FA, MD and ADC measures the following data was extracted from the publications if available: PD and control group age, measure of PD disease severity (HY-stage or UPDRS), Scanner make, scanner field strength, number of channels within the receiver head coil, number of diffusion gradient directions, voxel size, DTI post-processing software, motion and eddy current correction and method of ROI placement. In case of visual identification and manual outlining of the SN the method of ROI placement was classified as ‘manual’. In case of manual identification and automated digital boundary detection by changes in gradients (Rolheiser et al., 2011) or manual identification and SN sub-categorisation into pars compacta and pars reticulata by using connectivity measures (Menke et al., 2010) the ROI placement was classified as ‘semi-manual’.
Where available, mean and standard deviation measures of FA, MD or ADC were extracted. Results of two studies reporting nigral ADC values instead of MD values were also included into the meta-analysis. Even if the absolute values of ADC and MD differ, they are both descriptors of diffusivity with averaged out factors of directional diffusivity (anisotropy). For calculation of the effect size within the meta-analysis the relative difference of MD or ADC when comparing PD and controls was included. In one case only non-normal distribution measures such as median, range and quartiles were presented (Chan et al., 2007). To allow comparability within the meta-analysis, mean and standard deviation values were estimated by using previously published conversion techniques (Hozo et al., 2005).
In case of multiple publications on the same or overlapping study populations, the study describing results in the largest number of subjects was included in the meta-analysis. If values were only available as part of a diagram the values were extracted by manual measurement (using an image editing tool, GIMP, version 2.8, from www.gimp.org) on two separate occasions (4 weeks apart) and averaged. In two studies the SN was subdivided into three small regions of interest (rostral, middle and caudal) and only values of the sub-regions of the SN were described (Prakash et al., 2012; Vaillancourt et al., 2009). To allow comparability within the meta-analysis the mean FA over all three regions was calculated. Also for the purpose of the meta-analysis, the averaged results of subdivided ROI measures of this study were included (according to the data included from Vaillancourt et al. (2009)).
For further details see the supplementary material in Appendix A.
The disease effect size (DES) was defined as the standardised difference of nigral DTI measures between the PD patient group and healthy volunteer group using Cohen's d (Cohen, 1988). We used MetaAnalyst software (MetaAnalyst, version: Beta 3.13, Tufts Medical Centre (Wallace et al., 2009)) for continuous data using the DerSimonian and Laird random effect model and the assumption of unequal within study variances. I2 — values were computed as a measure of in between study heterogeneity (Higgins, 2003).
3. Results
3.1. Case–control DTI study
One participant had to be excluded because of significant cognitive impairment (ACE score 75), 7 participants were excluded because of usage of a previous DTI scanning protocol and/or scanning artefacts. The remainder 59 subjects did not show demographic differences (32 PD, age: 64.8 ± 11.8, 16 male and 27 controls, age 59.9 ± 10.5, 11 male). In 5 healthy controls the ACE was not obtained, however, these subjects had no past cognitive problems and no neurological, neurosurgical or psychiatric history. There was no difference in cognitive performance in the subjects assessed with ACE (n = 32, PD: 92.7 ± 4.8; n = 22 Controls 94.3 ± 6.4, n.s.). Patients had mild to moderate degrees of PD severity: mean UPDRS, 26.1 ± 13.9; mean HY score, 1.7 ± 0.9.
Independent of the type of ROI (small ROI of analysis 1 or large ROI of analysis 2, see Fig. 1) the intraclass correlation coefficients for intra-rater and inter-rater concordance were high with correlation coefficients ranging from 0.6 to 0.85 (all p ≤ 0.003). There were no differences in FA or MD in the control region between PD and controls excluding technical bias.
There were no differences in nigral FA between patients with PD and controls in the whole SN or the small dorsal SN known to be the most affected part. In contrast, a significant increase in nigral MD in PD was found independent of ROI used for analysis (Table 1).
Table 1.
Different types of SN ROI analysis in PD patients and controls.
| Analysis 1 (average of small SN ROI) |
Analysis 1b (small dorsal SN ROI) |
Analysis 2 (variable size whole SN ROI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PD | Controls | p | PD | Controls | p | PD | Controls | p | |
| FA | 0.44 ± 0.05 | 0.45 ± 0.05 | 0.39 | 0.45 ± 0.06 | 0.46 ± 0.05 | 0.46 | 0.44 ± 0.04 | 0.45 ± 0.05 | 0.43 |
| MD | 0.801 ± 0.102 | 0.728 ± 0.064 | 0.002 | 0.816 ± 0.097 | 0.748 ± 0.053 | 0.002 | 0.795 ± 0.099 | 0.732 ± 0.055 | 0.005 |
Voxel based analysis of co-registered FA and MD maps did not reveal any differences in nigral DTI measures when comparing patients with PD to controls (uncorrected p < 0.001).
3.2. Systematic review and meta-analysis
The search of three databases revealed 169 unique database entries. After applying in- and exclusion criteria 25 articles were reviewed out of which 11, including 10 studies for FA measures and 9 studies for MD or ADC measures were retained. The results presented above were added as a further study to the meta-analysis. Relevant publications and study intrinsic technical factors are listed in Table 2. Population details of the included studies are listed in the supplements (Inline Supplementary Table S1).
Table 2.
Overview of studies included in the meta-analysis.
| Study name | FA | MD (ADC) | Scanner make | Field-str. | Head coil | DTI dir. | Voxel size [mm] | DTI proc. | M-corr. | Eddy cur. | ROI placem. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prakash et al. 2012 | ✓ | (✓) | Philips Achieva | 3 T | ? | 16 | 0.9 x 0.9 x 3 | DTI studio V3.0.3 | ? | ? | Manual |
| Du et al. 2012 | ✓ | Siemens Magnetom Trio | 3 T | 8 | 42 | 2 x 2 x 2 | DTIPrep | ✓ | ✓ | Manual | |
| Skorpil et al. 2012 | ✓ | ✓ | Philips Intera | 1.5 T | 16 | 32 | 1.8 x 1.8 x 3 | FSL | ✓ | ✓ | Manual |
| Rolheiser et al. 2011 | ✓ | ✓ | General Electrics | 1.5 T | 8 | 31 | 2.03 x 2.03 x 3 | FSL V4.1 | ✓ | ✓ | Manual |
| Wang et al. 2011 | ✓ | ✓ | Siemens Magnetom Trio | 3 T | ? | 64 | 2 x 2 x 2 | Matlab V7.8 | ? | ? | Manual |
| Zhan et al. 2012 | ✓ | ✓ | Bruker/Siemens Medspec | 4 T | ? | 6 | 2 x 2 x 3 | FSL | ✓ | ✓ | Semi – Manual |
| Menke et al. 2010 | ✓ | ✓ | Siemens Trio | 3 T | 12 | 60 | 2 x 2 x 2 | FSL | ✓ | ✓ | Semi – Manual |
| Peran et al. 2010 | ✓ | ✓ | Siemens Allegro | 3 T | ? | 30 | 1.8 x 1.8 x 1.8 | FSL V4 | ✓ | ✓ | Manual |
| Gattellaro et al. 2009 | ✓ | Siemens Magnetom Avanto | 1.5 T | 4 | 12 | 1.9 x 1.9 x 2.5 | ? | ? | ? | Manual | |
| Vaillancourt et al. 2009 | ✓ | General Electrics, Signa | 3 T | 8 | 27 | 0.78 x 0.78 x 4 | DTI studio | ✓ | ✓ | Manual | |
| Chan et al. 2007 | ✓ | (✓) | Siemens Avanto | 1.5 T | ? | 12 | 1.2 x 1.2 x 4 | ? | ✓ | ✓ | Manual |
| this study | ✓ | ✓ | Philips Achieva | 3 T | 8 | 32 | 2 x 2 x 2 | FSL V4.1 | ✓ | ✓ | Manual |
FA = fractional anisotropy measures extracted from study, MD = mean diffusivity measures extracted from study, Field-str. = Field-strength of magnet, Headcoil = Number of channels within the receiver head coil, DTI dir. = Number of DTI diffusion directions for data acquisition, Voxel size [mm] = Size of voxels for acquisition, DTI proc. = Software used to process the DTI data prior to analysis, M-corr = Motion correction used, Eddy cur. = Eddy current correction used, ROI placem. = method of ROI placement. ‘✓’ is used as a positive indicator, ‘?’ is used in unclear cases.
Inline Supplementary Table S1.
Table S1.
Participant details of studies included in the meta-analysis.
| Study name | Controls |
PD patients |
Average age PD and control | ||||
|---|---|---|---|---|---|---|---|
| N | Age | N | Age | UPDRS | HY stage | ||
| Prakash et al. (2012) | 12 | 60.8 ± 8.5 | 11 | 60.4 ± 9.3 | 23.5 ± 9.5 | 1–3 | 60.6 |
| Du et al. (2012) | 28 | 59.8 ± 7.0 | 40 | 60.8 ± 8.2 | 23.5 ± 15.1 (UPDRS III) |
1.8 ± 0.6 | 60.4 |
| Skorpil et al. (2012) | 13 | 60 | 13 | 64 | 32.5 ± 6.1 | 1.76 ± 0.26 | 62 |
| Rolheiser et al. (2011) | 14 | 55.2 ± 6.2 | 14 | 56 ± 4.8 | ? | 1.3 ± 0.42 | 55.6 |
| Wang et al. (2011) | 30 | 65 ± 5.1 | 30 | 64.5 ± 3.4 | 33.6 ± 14.1 | ? | 64.8 |
| Zhan et al. (2012) | 20 | 67.2 ± 8 | 12 | 67.4 ± 8 | 26.3 ± 12.2 | ? | 67.3 |
| Menke et al. (2010) | 10 | 64.4 ± 9.9 | 10 | 63.7 ± 6.7 | ? | 2.3 ± 0.67 | 64.1 |
| Peran et al. (2010) | 22 | 57.4 ± 9.7 | 30 | 61.9 ± 11.1 | 12.0 ± 5.9 | 1.66 ± 0.48 | 59.7 |
| Gattellaro et al. (2009) | 10 | 58.1 ± 8 | 10 | 63.8 ± 15.7 | 20.5 ± 8.3 | ? | 61 |
| Vaillancourt et al. (2009) | 14 | 58 | 14 | 57.2 ± 9.6 | 18 ± 8.1 | 1.71 ± 0.46 | 57.6 |
| Chan et al. (2007) | 78 | 61.9 ± 9.3 | 73 | 63.6 ± 9.8 | ? | 2.4 | 62.8 |
| This data | 27 | 59.9 ± 10.5 | 32 | 64.8 ± 11.8 | 25.4 ± 13.7 | 1.7 ± 0.9 | 62.5 |
N = Number of participants, UPDRS = Unified Parkinson's disease rating scale, HY stage = Hoehn and Yahr stage, ? = data not demonstrated in publication.
The search of three databases revealed 169 unique database entries. After applying in- and exclusion criteria 25 articles were reviewed out of which 11, including 10 studies for FA measures and 9 studies for MD or ADC measures were retained. The results presented above were added as a further study to the meta-analysis. Relevant publications and study intrinsic technical factors are listed in Table 2. Population details of the included studies are listed in the supplements (Inline Supplementary Table S1).
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.006.
3.2.1. Meta-analysis of PD induced nigral FA changes
The initial meta-analysis included nigral FA measures in a population of 547 subjects (n = 268 controls, n = 279 PD). We found a highly significant PD induced nigral FA reduction with an estimated weighted pooled disease effect size of − 0.90 (p < 0.0001, Forrest plot see Fig. 2, values of individual studies see the supplements, Inline Supplementary Table S2). However, review of the heterogeneity measures revealed a very high level of in-between study result variation (I2 = 86%). To assess for potential causes of the observed high variability of study results an exploratory DES regression analysis with the categories of field strengths, DTI-directions, voxel-size, relative disease severity and mean age as independent variables was performed. This did not reveal any significant dependence of DES on these factors (smallest p = 0.43) but is of limited value due to the small number of included studies.
Fig. 2.
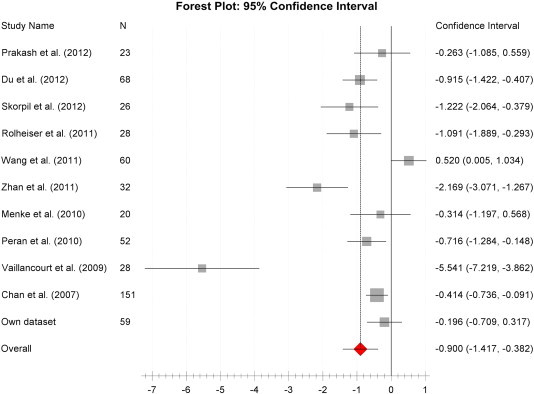
Fractional anisotropy disease effect size Forest plot.
Forest plot of the computed diseases effect sizes (DES, x-axis) of studies included into the initial meta-analysis on fractional anisotropy measures of the substantia nigra when comparing PD patients and controls. All but one included studies demonstrate a decrease of nigral FA in PD patients as indicated by a negative DES. The estimated pooled weighted DES was − 0.9 was highly significant (p < 0.0001), however, there was a large heterogeneity of study results (I2 = 86%).
Inline Supplementary Table S2.
Table S2.
FA DES of studies included into the initial meta-analysis of nigral FA changes in PD.
| Study name | PD patients |
Controls |
95% confidence interval |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean FA | SD | N | Mean FA | SD | Disease effect size (DES) | SE | Lower | Upper | |
| Prakash et al. (2012) | 11 | 0.43 | 0.04 | 12 | 0.44 | 0.03 | − 0.263 | 0.419 | − 1.085 | 0.559 |
| Du et al. (2012) | 40 | 0.49 | 0.05 | 28 | 0.53 | 0.04 | − 0.915 | 0.259 | − 1.422 | − 0.407 |
| Skorpil et al. (2012) | 13 | 0.37 | 0.04 | 13 | 0.41 | 0.02 | − 1.222 | 0.43 | − 2.064 | − 0.379 |
| Rolheiser et al. (2011) | 14 | 0.42 | 0.03 | 14 | 0.45 | 0.03 | − 1.091 | 0.407 | − 1.889 | − 0.293 |
| Wang et al. (2011) | 30 | 0.71 | 0.10 | 30 | 0.66 | 0.10 | 0.52 | 0.263 | 0.005 | 1.034 |
| Zhan et al. (2012) | 12 | 0.36 | 0.05 | 20 | 0.49 | 0.07 | − 2.169 | 0.46 | − 3.071 | − 1.267 |
| Menke et al. (2010) | 10 | 0.34 | 0.07 | 10 | 0.37 | 0.07 | − 0.314 | 0.45 | − 1.197 | 0.568 |
| Peran et al. (2010) | 30 | 0.50 | 0.04 | 22 | 0.53 | 0.03 | − 0.716 | 0.29 | − 1.284 | − 0.148 |
| Vaillancourt et al. (2009) | 14 | 0.44 | 0.02 | 14 | 0.54 | 0.02 | − 5.541 | 0.856 | − 7.219 | − 3.862 |
| Chan et al. (2007) | 73 | 0.40 | 0.03 | 78 | 0.42 | 0.03 | − 0.414 | 0.165 | − 0.736 | − 0.091 |
| This study | 32 | 0.44 | 0.05 | 27 | 0.45 | 0.05 | − 0.196 | 0.262 | − 0.709 | 0.317 |
N = Number of participants, SD = Standard deviation, SE = Standard error. Weight = weighting of study for calculation of the pooled weighted estimation of FA DES.
The initial meta-analysis included nigral FA measures in a population of 547 subjects (n = 268 controls, n = 279 PD). We found a highly significant PD induced nigral FA reduction with an estimated weighted pooled disease effect size of − 0.90 (p < 0.0001, Forrest plot see Fig. 2, values of individual studies see the supplements, Inline Supplementary Table S2). However, review of the heterogeneity measures revealed a very high level of in-between study result variation (I2 = 86%). To assess for potential causes of the observed high variability of study results an exploratory DES regression analysis with the categories of field strengths, DTI-directions, voxel-size, relative disease severity and mean age as independent variables was performed. This did not reveal any significant dependence of DES on these factors (smallest p = 0.43) but is of limited value due to the small number of included studies.
Inline Supplementary Table S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.006.
Interestingly, we found a strong variation of the mean nigral FA in the control populations between the different studies. Moreover, the strongest variation in DES was found in studies with the highest control FA values (Fig. 3). We therefore repeated the meta-analysis after exclusion of those studies with unexpected high FA value in SN based on a normative value derived from a freely available standard reference DTI atlas of 24 healthy adults (SRI24, Rohlfing et al., 2010). ROI were drawn as described above and averaged over duplicate measures two weeks apart which revealed a maximum mean nigral FA of 0.47. We hence chose to exclude studies reporting nigral FA ≥ 0.48 in controls. This reduced the between study heterogeneity to an acceptable level (I2 = 28%) for the 6 finally included studies (307 subjects, n = 154 controls and n = 153 PD) resulting in a pooled weighted disease effect size of − 0.5 (95% CI: − 0.8 to − 0.2, p = 0.22, Inline Supplementary Figure S1) which proved non-significant.
Fig. 3.
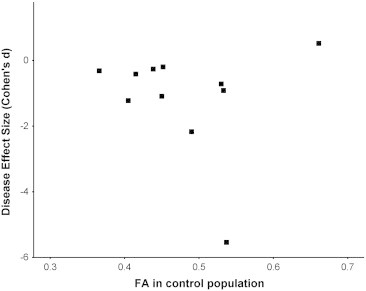
Scatter-plot of DES versus nigral FA in control arm of studies included in the initial meta-analysis.
The scatter-plot demonstrates that studies with a higher FA in the control arm show the largest variation in disease effect (DES) of PD induced nigral FA changes.
Inline Supplementary Figure S1.
Fig. S1.
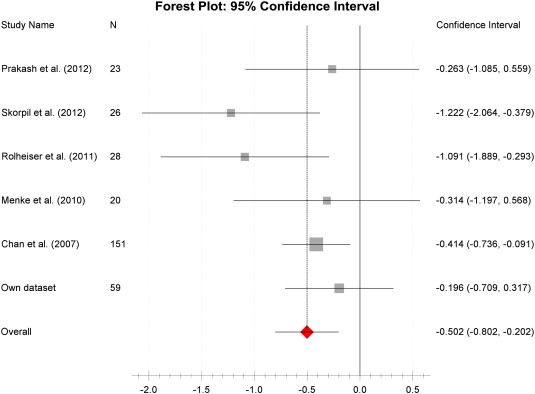
FA disease effect size Forest plot after excluding studies with an unexpected high FA measure in the control population.
Forest plot of the computed diseases effect sizes (DES, x-axis) of studies included into the repeated meta-analysis on fractional anisotropy measures of the SN when excluding 5 studies with unexpected high control group FA measures. All included studies demonstrate a decrease of FA in PD patients as indicated by a negative DES. The estimated pooled weighted DES was − 0.5 but did not reach the significance level (p = 0.22).
Interestingly, we found a strong variation of the mean nigral FA in the control populations between the different studies. Moreover, the strongest variation in DES was found in studies with the highest control FA values (Fig. 3). We therefore repeated the meta-analysis after exclusion of those studies with unexpected high FA value in SN based on a normative value derived from a freely available standard reference DTI atlas of 24 healthy adults (SRI24, Rohlfing et al., 2010). ROI were drawn as described above and averaged over duplicate measures two weeks apart which revealed a maximum mean nigral FA of 0.47. We hence chose to exclude studies reporting nigral FA ≥ 0.48 in controls. This reduced the between study heterogeneity to an acceptable level (I2 = 28%) for the 6 finally included studies (307 subjects, n = 154 controls and n = 153 PD) resulting in a pooled weighted disease effect size of − 0.5 (95% CI: − 0.8 to − 0.2, p = 0.22, Inline Supplementary Figure S1) which proved non-significant.
Inline Supplementary Fig. S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.006.
3.2.2. Meta-analysis of PD induced nigral MD changes
The meta-analysis of MD or ADC — SN measures included a population of 471 subjects (n = 236 controls, n = 235 PD). Over all studies there was a non-significant increase in MD/ADC of the SN when comparing PD patients to controls (see also Forest plot: Fig. 4). The pooled weighted disease effect size over all studies was estimated to be + 0.26 (95% CI: 0.028–0.497, p = 0.17). Computation of heterogeneity measures revealed an acceptable level of in between study heterogeneity (I2 = 30%, Inline Supplementary Table S3).
Fig. 4.
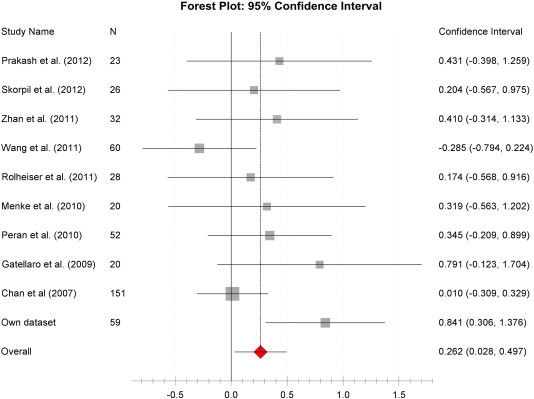
Mean diffusivity disease effect size Forest plot.
Forest plot of the computed diseases effect sizes (DES, x-axis) of different studies on mean diffusivity measures of the substantia nigra when comparing PD patients and controls. In the majority of studies a small increase of MD in PD patients can be observed as indicated by a positive DES.
Inline Supplementary Table S3.
Table S3.
MD DES of studies included into the meta-analysis of PD induced nigral MD changes.
| Study name | PD patients |
Control |
95% confidence interval |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean MD or (ADC) | SD | N | Mean MD or (ADC) | SD | Disease effect size (DES) | SE | Lower | Upper | Weight | |
| Prakash et al. (2012) | 11 | (2.41) | 0.1715 | 12 | (2.345) | 0.1295 | 0.431 | 0.423 | − 0.398 | 1.259 | 0.049 |
| Skorpil et al. (2012) | 13 | 0.814 | 0.106 | 13 | 0.796 | 0.066 | 0.204 | 0.393 | − 0.567 | 0.975 | 0.057 |
| Zhan et al. (2012)⁎ | 12 | 9.945 | 0.615 | 20 | 9.49 | 1.315 | 0.41 | 0.369 | − 0.314 | 1.133 | 0.064 |
| Wang et al. (2011) | 30 | 0.68 | 0.15 | 30 | 0.72 | 0.13 | − 0.285 | 0.26 | − 0.794 | 0.224 | 0.13 |
| Rolheiser et al. (2011) | 14 | 0.84 | 0.048 | 14 | 0.793 | 0.379 | 0.174 | 0.379 | − 0.568 | 0.916 | 0.061 |
| Menke et al. (2010) | 10 | 0.98 | 0.139 | 10 | 0.9311 | 0.166 | 0.319 | 0.45 | − 0.563 | 1.202 | 0.043 |
| Peran et al. (2010) | 30 | 0.778 | 0.0805 | 22 | 0.754 | 0.0508 | 0.345 | 0.283 | − 0.209 | 0.899 | 0.109 |
| Gattellaro et al. (2009) | 10 | 0.79 | 0.08 | 10 | 0.74 | 0.04 | 0.791 | 0.466 | − 0.123 | 1.704 | 0.04 |
| Chan et al. (2007) | 73 | (7.3273) | 0.795 | 78 | (7.32) | 0.651 | 0.01 | 0.163 | − 0.309 | 0.329 | 0.33 |
| Own dataset | 32 | 0.801 | 0.102 | 27 | 0.728 | 0.064 | 0.841 | 0.273 | 0.306 | 1.376 | 0.117 |
MD unit = 10− 3 mm2/s. The study indicated by * reported the MD unit = 10− 6 mm2/s. Studies reporting ADC indicated with brackets. N = Number of participants, SD = Standard deviation, SE = Standard error. Weight = weighting of study for calculation of the pooled weighted estimation of MD DES.
The meta-analysis of MD or ADC — SN measures included a population of 471 subjects (n = 236 controls, n = 235 PD). Over all studies there was a non-significant increase in MD/ADC of the SN when comparing PD patients to controls (see also Forest plot: Fig. 4). The pooled weighted disease effect size over all studies was estimated to be + 0.26 (95% CI: 0.028–0.497, p = 0.17). Computation of heterogeneity measures revealed an acceptable level of in between study heterogeneity (I2 = 30%, Inline Supplementary Table S3).
Inline Supplementary Table S3 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.006.
4. Discussion
4.1. PD induced nigral MD increase
In our prospective case–control study we found a significant increase in nigral MD in patients with PD compared to matched controls when using ROI to outline the SN. There were no significant MD differences when using a voxel-based analysis approach. The meta-analysis of PD induced nigral MD changes did also not confirm a significant disease effect.
Regional increases in diffusivity metrics like ADC or MD, are useful markers of neurodegeneration that can help to distinguish the parkinsonian variant of MSA from PD (Blain et al., 2006; Schocke et al., 2002; Seppi et al., 2005). It is less clear whether increased diffusivity can also index PD induced nigral pathology. Strong nigral MD increases were found in one animal model of PD (Boska et al., 2007), while other studies found less or no disease effect (Soria et al., 2011; van Camp et al., 2009). This is largely in line with our findings of a small increase in nigral MD, but no effect in the meta-analysis or VBA. A tendency of increased MD or ADC can be seen in most studies, and the lack of observable pooled disease effect may be a type II error. Alternatively, these inconsistencies may reflect differences in patient characteristics or ROI selection. Nevertheless, against the criteria of identifying promising biomarkers, nigral MD measurements have limited value to reliably differentiate PD pathology from the healthy brain on a case by case basis.
4.2. PD induced nigral FA change
We did not observe a PD induced reduction of nigral FA in our studied population. This observation is in contrast with some recent publications claiming very high diagnostic accuracy (Vaillancourt et al., 2009), but well in line with other reports showing small or no PD induced nigral FA decrease (Chan et al., 2007; Focke et al., 2011; Menke et al., 2010). A single study even reported an increase of nigral FA in PD patients (Wang et al., 2011). Interestingly in animal models of PD findings also range from increasing (van Camp et al., 2009) to decreasing nigral FA (Boska et al., 2007; Soria et al., 2011). The research synthesis of the published literature assessing PD induced nigral FA changes revealed a significant FA reduction in the first instance (weighted pooled disease effect size of − 0.9, p < 0.0001), however, we found a very high variation of results of the included studies which is reflected in an exceptionally large I2 of 86%. Evaluation of DES of individual studies demonstrate that our study findings of FA changes are well within the range of the majority of studies with overlapping confidence intervals between our and the pooled DES. In contrast, the reported study by Vaillancourt et al. (2009) and Wang et al. (2011) need to be considered as outliers with DES and CI outside the range of results from the other 10 studies, and from each other.
A recently published meta-analysis by Cochrane and Ebmeier describes DTI changes in parkinsonian syndromes and found similar highly significant PD induced nigral FA reduction (DES = − 0.64, p < 0.0001) but a much smaller variation in results comparing the different studies (I2 = 9.5%) (Cochrane and Ebmeier, 2013). The reason for the difference in results of the two meta-analyses is likely due to two main reasons: The 1st reason is a difference in studies included in the two meta-analyses. Cochrane and Ebmeier included two studies not included in this meta-analysis, one by Yoshikawa et al. (2004) which describes changes in peri-nigral FA rather than changes of the SN and a second one by Focke et al. (2011) which did not find any PD induced nigral FA changes nor reported any relevant FA values (and was therefore excluded from this meta-analysis). Cochrane and Ebmeier for unknown reasons did not include the only study reporting a PD induced nigral FA increase by Wang et al. (2011) which contributes largely to the variation of results we identified when comparing all studies. Further newer studies which were unpublished at the time of Cochrane's meta-analysis (Prakash et al., 2012; Skorpil et al., 2012) and this study are included additionally in this meta-analysis. The 2nd reason for the observed differences is likely due to a variation of extracted values from included studies. When directly comparing the extracted values and Forest plots of the two meta-analyses study by study, there is a good correlation of effect sizes and CI for most of the studies. However, two studies with the largest effect sizes (Vaillancourt et al., 2009; Zhan et al., 2012) differ in reported DES and CI. Vaillancourt et al. (2009) used sub-regional SN analysis with values demonstrated in diagrams only which could explain a variation in value extraction for both analyses. Zhan et al. (2012), however, illustrated absolute SN FA measures including SD and p-values which seem to be underestimated in Cochrane and Ebmeier's meta-analysis. Therefore methodical differences are likely contributing to the dissimilar results of heterogeneity measures, however, by including new studies and solely focusing on nigral FA changes this meta-analysis provides new insights, specifically the large variation of observed nigral FA DES throughout the literature.
A potential factor contributing to the in this meta-analysis observed large in between study heterogeneity could be technical aspects resulting in limited quality of FA measurements. Indirect assessment of technical quality of published DTI studies is challenging. However, a preliminary exploratory regression analysis did not show an obvious association between field strength, number of diffusion directions and nigral FA in the control population of the studies. Further potential contributing factors are study population characteristics such as disease severity conceivably causing difference in DES. However, a recent study demonstrating nigral FA changes in PD did not find any correlation of disease severity with FA changes (Du et al., 2012) nor did we find an indication of disease severity related nigral FA changes on the exploratory regression analysis. We also investigated the influence of choice of ROI on nigral FA changes. The pars compacta of the substantia nigra (SNc) contains the cells that progressively deplete in PD. Delineation of the SNc on MRI in vivo is not standardised and varies from study to study using indirect landmark based techniques to outline the SN or SNc. This is causing considerable anatomical variability with some studies using small sub regions within the iron rich SN pars reticulate as ROI (Chan et al., 2007; Vaillancourt et al., 2009) whilst other studies outline the whole of the SN as ROI (Du et al., 2012). Interestingly, Vaillancourt et al. (2009) described the by far the largest DES when choosing a small ROI in the dorso-caudal region of the SN. Even when replicating these ROI we could not reproduce their finding nor could we or others (Scherfler et al., 2006) identify altered SN substructures when using an operator independent voxel-based analysis.
There was considerable variation in reported nigral FA of the control group across studies ranging from 0.37 to 0.7 which covered the entire range of reported nigral FA in patients. This highlights a larger between study heterogeneity in nigral control FA than potential disease effect pointing to either grossly different choice of region-of-interest or substantial technical differences. The SN is a grey matter nucleus, for which FA values as a measure of orientation dependence are expectedly small. FA values closer to 1 are expected to be found in highly organised white matter structures rather than poorly organised grey matter structures. The FA values of the hippocampal region in control populations in Alzheimer's disease studies for example range from 0.1 to 0.2 (den Heijer et al., 2012; Hong et al., 2010; Palesi et al., 2012). The FA in the region of the basal ganglia like the globus pallidus, thalamus, caudate and putamen are reported in the range from 0.19 to 0.41 (Pfefferbaum et al., 2010) which is also lower than most of the reported nigral control FA values. Iron deposition in the basal ganglia, specifically in the Substantia nigra increases with age (Daugherty and Raz, 2013) and this process is accelerated in PD (Lotfipour et al., 2012) with some evidence that iron deposition even correlates with disease stage in PD(Martin et al., 2008). The effect of iron increase on DTI measures like FA are poorly understood, however, age-dependent increase in putaminal iron deposition was found to significantly correlate to an increase of FA in the same region raising the possibility of iron deposition induced FA alterations. It is conceivable that accelerated nigral iron deposition in PD (Lotfipour et al., 2012; Martin et al., 2008) affects FA measures and decreases observable differences when comparing PD patients and controls.
The bulk of between study heterogeneity in this meta-analysis of nigral FA changes was observed in studies reporting higher nigral FA values in healthy controls including the two studies with the largest effect size. This raises the possibility of the inclusion of peri-nigral fibre tracts into ‘SN’ ROI especially in studies with relative thick slices (4 mm in 11). In fact, FA changes of the white-matter closely related to the SN and within the basal ganglia have been well described (Yoshikawa et al., 2004). Also, the study using the most advanced segmentation of the SN reported the lowest FA in controls and did not find a significant disease effect (Menke et al., 2010). Therefore variation in size and placement of the ROI with resulting inclusion of adjacent white matter is likely to contribute to the large observed heterogeneity of FA-DES. Exclusion of studies with unexpectedly high nigral FA in controls in our meta-analysis confirmed a non-significant PD induced nigral FA reduction.
This case–control study and meta-analysis of nigral DTI measures demonstrates that usage of nigral FA changes as biomarker of PD is neither reliable nor useful at this point in time. Even if there is some evidence of nigral FA decrease in some published studies, it is unclear if this is a true PD induced effect or rather due to other factors like inclusion of peri-nigral white matter or differences in iron deposition in the area of the SN. To clarify the diagnostic value of PD induced nigral FA alterations and development of this technique as PD biomarker future studies would need to address a range of issues:
-
1.
Confirmation and standardisation of anatomical position of SN/SNc ROI using magnetic resonance imaging. MR sequences demonstrating neuromelanin (black pigment of the SN) dependent contrast might be helpful to confirm the definite localisation of the SN pars compacta (Sasaki et al., 2006; Schwarz et al., 2011) and could be used to improve and/or standardize SN ROI placement.
-
2.
Assessment of influence of nigral iron deposition on FA changes and correlation to age related physiological SN changes.
-
3.
Assessment of validity and repeatability of nigral FA measures by assessing volunteers on varying scanner platforms.
4.3. Limitations of this study
In common with all literature searches and meta-analyses, publication practices may bias the results. Hence the reported mean DES is probably overestimated as unpublished negative study results are probably under-represented.
Due to the large heterogeneity of the results of studies included in the FA meta-analysis we decided to introduce a relative quality measure (atlas derived upper threshold for nigral FA value in the healthy population arm) to identify studies for inclusion in the final FA meta-analysis. This meta-analysis resulted in an acceptable distribution of study DES, however, with the limitation that it is not entirely representative of all published studies in this field.
5. Conclusion
In conclusion, this DTI study in 59 subjects together with the systematic review and meta-analysis of available published reports do not support nigral DTI metrics as useful diagnostic marker of PD at this point in time.
Acknowledgement
We would like acknowledge Dr. Graham Warren (Fellow of the Royal Statistical Society, University of Nottingham) for help with the analysis and interpretation of the meta-analysis results, Pragya Tomar (‘Translational Neuroimaging’ Masters student, University of Nottingham) for help with/and re-testing of the literature search results. We would also like to thank PD research study nurses of the Royal Derby Hospital (Nicola Watson and Lindsey Kimber) for support with participant recruitment and assessment, Sharon Foreman for continuous administrative help and all study volunteers for their participation. We would like to thank Dr Chris Tench for providing the NeuRoi software.
Funding
The study was funded by Special Trustees for Nottingham University Hospital (Grant Ref: STR 82/04/N), by the Sarah Matheson Trust (now Multiple System Atrophy Trust founded by Sarah Matheson) and by the Division of Radiological and Imaging Sciences of the University of Nottingham. The author S.T. Schwarz is funded by a National Institute for Health Research UK training-grant.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Stefan T. Schwarz, Email: stefan.schwarz@nottingham.ac.uk.
Maryam Abaei, Email: maryam.abaei@gmail.com.
Vamsi Gontu, Email: Vamsi.Gontu@nuh.nhs.uk.
Paul S. Morgan, Email: Paul.Morgan@nottingham.ac.uk.
Nin Bajaj, Email: Nin.Bajaj@nuh.nhs.uk.
Dorothee P. Auer, Email: dorothee.auer@nottingham.ac.uk.
Appendix A. Supplementary data
Supplementary material.
References
- Auer D.P. In vivo imaging markers of neurodegeneration of the substantia nigra. Exp. Gerontol. 2009;44:4–9. doi: 10.1016/j.exger.2008.08.051. [DOI] [PubMed] [Google Scholar]
- Bak T.H., Mioshi E. A cognitive bedside assessment beyond the MMSE: the Addenbrooke's Cognitive Examination. Pract. Neurol. 2007;7:245–249. [PubMed] [Google Scholar]
- Berg D., Steinberger J.D., Warren Olanow C., Naidich T.P., Yousry T.A. Milestones in magnetic resonance imaging and transcranial sonography of movement disorders. Mov. Disord. 2011;26:979–992. doi: 10.1002/mds.23766. [DOI] [PubMed] [Google Scholar]
- Blain C.R.V., Barker G.J., Jarosz J.M., Coyle N.A., Landau S., Brown R.G., Chaudhuri K.R., Simmons A., Jones D.K., Williams S.C.R., Leigh P.N. Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology. 2006;67:2199–2205. doi: 10.1212/01.wnl.0000249307.59950.f8. [DOI] [PubMed] [Google Scholar]
- Boska M.D., Hasan K.M., Kibuule D., Banerjee R., McIntyre E., Nelson J.A., Hahn T., Gendelman H.E., Mosley R.L. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson's disease. Neurobiol. Dis. 2007;26:590–596. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.-L., Rumpel H., Yap K., Lee E., Loo H.-V., Ho G.-L., Fook-Chong S., Yuen Y., Tan E.-K. Case control study of diffusion tensor imaging in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2007;78:1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C.J., Ebmeier K.P. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80:857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 2nd ed. Routledge Academic; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Raz N. Age-related differences in iron content of subcortical nuclei observed in vivo: a meta-analysis. NeuroImage. 2013;70:113–121. doi: 10.1016/j.neuroimage.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Heijer T., van der Lijn, F., Vernooij M.W., de Groot M., Koudstaal P.J., van der Lugt A., Krestin G.P., Hofman A., Niessen W.J., Breteler M.M.B. Structural and diffusion MRI measures of the hippocampus and memory performance. NeuroImage. 2012;63:1782–1789. doi: 10.1016/j.neuroimage.2012.08.067. [DOI] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Styner M., Shaffer M.L., Sen S., Yang Q.X., Huang X. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov. Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Sen S., Wang J., Shaffer M.L., Styner M., Yang Q.X., Huang X. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov. Disord. 2012;27:1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Jenner P., Marsden C.D., Teychenne P. Fahn S, Elton RI, and members of the UPDRS Development Committee. The Unified Parkinson's Disease Rating Scale. In: Fahn S., Marsden C.D., Calne D.B., editors. Recent Developments in Parkinson's Disease. Macmillan Healthcare Information; Florham Park, NJ: 1987. [Google Scholar]
- Focke N.K., Helms G., Pantel P.M., Scheewe S., Knauth M., Bachmann C.G., Ebentheuer J., Dechent P., Paulus W., Trenkwalder C. Differentiation of typical and atypical Parkinson syndromes by quantitative MR imaging. AJNR Am. J. Neuroradiol. 2011;32:2087–2092. doi: 10.3174/ajnr.A2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattellaro G., Minati L., Grisoli M., Mariani C., Carella F., Osio M., Ciceri E., Albanese A., Bruzzone M.G. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2009;30:1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hong Y.J., Yoon B., Shim Y.S., Cho A.H., Lim S.C., Ahn K.J., Yang D.W. Differences in microstructural alterations of the hippocampus in Alzheimer disease and idiopathic normal pressure hydrocephalus: a diffusion tensor imaging study. Am. J. Neuroradiol. 2010;31:1867–1872. doi: 10.3174/ajnr.A2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A.J., Daniel S.E., Ben-Shlomo Y., Lees A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour A.K., Wharton S., Schwarz S.T., Gontu V., Schäfer A., Peters A.M., Bowtell R.W., Auer D.P., Gowland P.A., Bajaj N.P.S. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson's disease. J. Magn. Reson. Imaging. 2012;35:48–55. doi: 10.1002/jmri.22752. [DOI] [PubMed] [Google Scholar]
- Ma S.Y., Röyttä M., Rinne J.O., Collan Y., Rinne U.K. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J. Neurol. Sci. 1997;151:83–87. doi: 10.1016/s0022-510x(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Martin W.R.W., Wieler M., Gee M. Midbrain iron content in early Parkinson disease — a potential biomarker of disease status. Neurology. 2008;70:1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5. [DOI] [PubMed] [Google Scholar]
- Meara J., Bhowmick B.K., Hobson P. Accuracy of diagnosis in patients with presumed Parkinson's disease. Age Ageing. 1999;28:99–102. doi: 10.1093/ageing/28.2.99. [DOI] [PubMed] [Google Scholar]
- Menke R.A., Jbabdi S., Miller K.L., Matthews P.M., Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. NeuroImage. 2010;52:1175–1180. doi: 10.1016/j.neuroimage.2010.05.086. [DOI] [PubMed] [Google Scholar]
- Palesi F., Vitali P., Chiarati P., Castellazzi G., Caverzasi E., Pichiecchio A., Colli-Tibaldi E., D'Amore F., D'Errico I., Sinforiani E., Bastianello S. DTI and MR volumetry of hippocampus-PC/PCC circuit: in search of early micro- and macrostructural signs of Alzheimers's disease. Neurol. Res. Int. 2012;2012:1–9. doi: 10.1155/2012/517876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., Celsis P., Rascol O., Demonet J.-F., Stefani A., Pierantozzi M., Pontieri F.E., Caltagirone C., Spalletta G., Sabatini U. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain. 2010;133:3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Adalsteinsson E., Rohlfing T., Sullivan E.V. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol. Aging. 2010;31:482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B.D., Sitoh Y.-Y., Tan L.C.S., Au W.L. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:1029–1033. doi: 10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Rohlfing T., Zahr N.M., Sullivan E.V., Pfefferbaum A. The SRI24 multi-channel atlas of normal adult human brain structure. Hum. Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolheiser T.M., Fulton H.G., Good K.P., Fisk J.D., McKelvey J.R., Scherfler C., Khan N.M., Leslie R.A., Robertson H.A. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson's disease. J. Neurol. 2011;258:1254–1260. doi: 10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Shibata E., Tohyama K., Takahashi J., Otsuka K., Tsuchiya K., Takahashi S., Ehara S., Terayama Y., Sakai A. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006;17:1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7. [DOI] [PubMed] [Google Scholar]
- Scherfler C., Schocke M.F., Seppi K., Esterhammer R., Brenneis C., Jaschke W., Wenning G.K., Poewe W. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson's disease. Brain. 2006;129:538–542. doi: 10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- Schocke M.F.H., Seppi K., Esterhammer R., Kremser C., Jaschke W., Poewe W., Wenning G.K. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58:575–580. doi: 10.1212/wnl.58.4.575. [DOI] [PubMed] [Google Scholar]
- Schrag A., Ben-Shlomo Y., Quinn N. How valid is the clinical diagnosis of Parkinson's disease in the community? J. Neurol. Neurosurg. Psychiatry. 2002;73:529–534. doi: 10.1136/jnnp.73.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S.T., Rittman T., Gontu V., Morgan P.S., Bajaj N., Auer D.P. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson's disease. Mov. Disord. 2011;26:1633–1638. doi: 10.1002/mds.23722. [DOI] [PubMed] [Google Scholar]
- Seppi K., Schocke M.F.H., Wenning G.K., Poewe W. How to diagnose MSA early: the role of magnetic resonance imaging. J. Neural Transm. 2005;112:1625–1634. doi: 10.1007/s00702-005-0332-2. [DOI] [PubMed] [Google Scholar]
- Skorpil M., Soderlund V., Sundin A., Svenningsson P. MRI diffusion in Parkinson's disease: using the technique's inherent directional information to study the olfactory bulb and substantia nigra. J. Park. Dis. 2012;2:171–180. doi: 10.3233/JPD-2012-12091. [DOI] [PubMed] [Google Scholar]
- Soria G., Aguilar E., Tudela R., Mullol J., Planas A.M., Marin C. In vivo magnetic resonance imaging characterization of bilateral structural changes in experimental Parkinson's disease: a T2 relaxometry study combined with longitudinal diffusion tensor imaging and manganese-enhanced magnetic resonance imaging in the 6-hydroxydopamine rat model. Eur. J. Neurosci. 2011;33:1551–1560. doi: 10.1111/j.1460-9568.2011.07639.x. [DOI] [PubMed] [Google Scholar]
- Tretiakoff C. 1919. Contribution a l'etude de I'anatomie pathologique du locus nigra de Soemmering avec quelques de'ductions relatives a la pathogenie des troubles de musculaire et de la maladie de Parkinson. ((Thesis, no 293, Paris). Paris, No 293) [Google Scholar]
- Vaillancourt D.E., Spraker M.B., Prodoehl J., Abraham I., Corcos D.M., Zhou X.J., Comella C.L., Little D.M. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp N., Blockx I., Verhoye M., Casteels C., Coun F., Leemans A., Sijbers J., Baekelandt V., van Laere K., van der Linden A. Diffusion tensor imaging in a rat model of Parkinson's disease after lesioning of the nigrostriatal tract. NMR Biomed. 2009;22:697–706. doi: 10.1002/nbm.1381. [DOI] [PubMed] [Google Scholar]
- Wallace B.C., Schmid C.H., Lau J., Trikalinos T.A. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med. Res. Methodol. 2009;9 doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-J., Lin W.-Y., Lu C.-S., Weng Y.-H., Ng S.-H., Wang C.-H., Liu H.-L., Hsieh R.-H., Wan Y.-L., Wai Y.-Y. Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology. 2011;261:210–217. doi: 10.1148/radiol.11102277. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Nakata Y., Yamada K., Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J. Neurol. Neurosurg. Psychiatry. 2004;75:481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W., Kang G.A., Glass G.A., Zhang Y., Shirley C., Millin R., Possin K.L., Nezamzadeh M., Weiner M.W., Marks W.J., Jr., Schuff N. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Mov. Disord. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


