Abstract
Brain imaging in Klinefelter syndrome (47, XXY) (KS), a genetic disorder characterized by the presence of an extra X chromosome, may contribute to understanding the relationship between gene expression, brain structure, and subsequent cognitive disabilities and psychiatric disorders.
We conducted the largest to date voxel-based morphometry study of 65 KS subjects and 65 controls matched for age and education and correlated these data to neuropsychological test scores. The KS patients had significantly smaller total brain volume (TBV), total gray matter volume (GMV) and total white matter volume (WMV) compared to controls, whereas no volumetric difference in cerebral spinal fluid (CSF) was found. There were no differences in TBV, GMV, WMV or CSF between testosterone treated KS (T-KS) and untreated KS (U-KS) patients. Compared to controls, KS patients had significantly decreased GMV bilaterally in insula, putamen, caudate, hippocampus, amygdala, temporal pole and frontal inferior orbita. Additionally, the right parahippocampal region and cerebellar volumes were reduced in KS patients. KS patients had significantly larger volumes in right postcentral gyrus, precuneus and parietal regions. Multivariate classification analysis discriminated KS patients from controls with 96.9% (p < 0.001) accuracy. Regression analyses, however, revealed no significant association between GMV differences and cognitive and psychological factors within the KS patients and controls or the groups combined. These results show that although gene dosage effect of having and extra X-chromosome may lead to large scale alterations of brain morphometry and extended cognitive disabilities no simple correspondence links these measures.
Keywords: Klinefelter syndrome, MRI, Cognition, Personality, Mental disorders
Highlights
-
•
KS is associated with reduced global and regional brain volumes.
-
•
Based on the brain scans the KS diagnosis could be predicted with 96.9% accuracy.
-
•
No correlations were found between brain volumes and neuropsychological measures.
-
•
No effect of testosterone treatment on brain volumes was seen in the KS patients.
1. Introduction
Klinefelter syndrome (KS) (47, XXY) affects 1 out of 660 men, making it the most common sex-chromosome aneuploidy in males (Bojesen et al., 2003). KS is associated with verbal learning disabilities (Ratcliffe, 1999), impairments in memory (Fales et al., 2003) and executive functions (Kompus et al., 2011) and the general intelligence has been found to range between sub-average and average levels (Barker and Black, 1976; Bender et al., 1999; Fales et al., 2003; Geschwind et al., 1998; Giedd et al., 2007; Graham et al., 1988; Itti et al., 2006; Kompus et al., 2011; Lee et al., 2011; Nielsen and Pelsen, 1987; Stemkens et al., 2006; Tartaglia et al., 2012; van Rijn et al., 2006, 2008). A vulnerability is also present in KS towards psychiatric diseases such as depression, anxiety, schizophrenia, attention deficit hyperactivity disorder and autism spectrum diseases (Bruining et al., 2009). The most prominent hormonal abnormality in KS is hypergonadotropic hypogonadism. Brain imaging studies of KS may contribute not only to the understanding of the neurobiology underlying the cognitive disabilities and the increased risk of psychiatric diseases that have not been delineated yet in KS (Bryant et al., 2011), but also to an understanding of X-chromosome linked mechanisms underlying cognitive disabilities and psychiatric diseases in general.
Studies using brain imaging techniques in boys and adults with KS have found significant volume differences in multiple brain regions, but the results are rather discordant. Total brain volume (TBV) was reported by some studies to be reduced (DeLisi et al., 2005; Giedd et al., 2007; Shen et al., 2004; Warwick et al., 1999) while others report no difference (Bryant et al., 2011; Patwardhan et al., 2000). Regarding brain ventricles, these have been reported to be enlarged in some studies (Giedd et al., 2007; Itti et al., 2006; Shen et al., 2004; Warwick et al., 1999) while others have found no volumetric differences (DeLisi et al., 2005; Patwardhan et al., 2000). Furthermore, discrepant data also exist regarding lobar volumes including the temporal lobes (Bryant et al., 2011; DeLisi et al., 2005; Giedd et al., 2007; Itti et al., 2006; Patwardhan et al., 2000; Shen et al., 2004; Warwick et al., 1999), frontal lobes (Bryant et al., 2011; DeLisi et al., 2005; Giedd et al., 2007; Itti et al., 2006; Warwick et al., 1999) and parietal lobes (Giedd et al., 2007; Itti et al., 2006; Shen et al., 2004; Warwick et al., 1999) and the volumes of more specific brain structures such as amygdala and hippocampus (Bryant et al., 2011; DeLisi et al., 2005; Itti et al., 2006; Patwardhan et al., 2000; Rose et al., 2004; Shen et al., 2004; Warwick et al., 1999) and the caudate nuclei (Bryant et al., 2011; Giedd et al., 2007; Warwick et al., 1999) with some studies reporting smaller volumes for these structures while other studies report these to be of normal size. All studies that have been published so far had small sample sizes. Moreover, investigators used different volumetric neuroimaging techniques and there was considerable variability in studied sample age as well as the frequency of testosterone treatment (Steinman et al., 2009).
As KS is associated with hypergonadotropic hypogonadism, many of the KS patients start testosterone therapy around puberty or later in life when the syndrome is diagnosed, often with a substantial delay and a resulting long period of exposure to hypogonadism. Sex hormones and especially testosterone may have both an ‘organizational effect’ and an ‘activational effect’ on neural pathways (Cooke et al., 1998; Phoenix et al., 2013), which have raised the question whether testosterone therapy in KS patients could have a modulating effect on brain volume. In one study (Patwardhan et al., 2000) KS patients not receiving testosterone were found to have smaller temporal volumes than controls, while KS patients receiving testosterone treatment did not. Other investigators (Itti et al., 2006) found no significant effect of testosterone treatment on brain volumes. Another area of uncertainty is the possible correlation between the neuropsychological profile in KS and results of structural brain imaging. The few studies that attempted to investigate this either had very small sample sizes (DeLisi et al., 2005; Itti et al., 2006; Patwardhan et al., 2000; Warwick et al., 1999) and/or involved very few neuropsychological tests (Bryant et al., 2011; Patwardhan et al., 2000; Warwick et al., 1999).
In the present study, we studied brain volumes in a large sample of 65 KS patients using voxel-based morphometry (VBM), and correlated these data to scores on a comprehensive neuropsychological test battery. The presented data do not support a correlation between morphometric indices and neuropsychological parameters or testosterone treatment.
2. Materials and methods
2.1. Participants
Seventy-nine patients with verified KS recruited from endocrinology, genetics and fertility clinics in Denmark agreed to participate in the study. Seventy-seven of seventy-nine KS patients were registered by the Danish Cytogenetic Central Register in Denmark and the karyotype was confirmed by the register. Two of the KS patients were not registered in register and were karyotyped using standard techniques to verify their KS diagnose. Inclusion criteria were ages between 18 and 60 years and signed informed consent. Exclusion criteria were i) a history of neurological disease or head injury more severe than simple concussion, ii) current substance abuse, iii) color blindness or iiii) metal implants not suitable for magnetic resonance (MR) imaging. Subsequently, 14 of the KS patients were excluded from the study due to either anatomical scans with motion artifacts (n = 1), missing scan images (n = 1), developing claustrophobia inside the scanner (n = 3), too large body-proportion to enter the scanner (n = 2), unknown metal splinter in finger (n = 1), chronic solvent-induced encephalopathy diagnosis (n = 1) and use of cannabis within the last month before examination (n = 5), thus resulting in 65 patients with KS eligible for the study (mean age 36.8 ± 10.5 (SD) years). Sixty two (95%) of the KS patients had 47,XXY karyotype and 3 (5%) had chromosomal mosaicism (1 patient had 88% 47,XXY/10% 46,XY/2% 48,XXXY; 1 patient with 90% 47,XXY/10% 46,XXY; 1 patient with 40–60% 47,XXY/40–60% 46, XY). At the time of participation, 44 (68%) of the KS patients received testosterone treatment (intramuscular testosterone injections (n = 38), oral testosterone (n = 1), cutaneous testosterone gel (n = 5)). Fourteen of the 21 KS patients (67%) who did not receive testosterone treatment had never received treatment with testosterone, while six had received testosterone therapy in the past for an average time period of 35.3 months (range: 10 month–7 years) and one KS patients had received testosterone for an unspecified period when he was younger. Sixty of the KS patients were right-handed, 3 were left-handed and 2 were ambidextrous.
Sixty five male controls matched for age and education were recruited through advertisement in local hospitals, in local newspapers, at local work services, among volunteer fire fighters, at citizen service offices and at local libraries. Karyotyping on the 73 controls was not performed, but none of these showed signs of more than one X-chromosome in androgen receptor polymorphism or X-chromosome microsatellite analysis. None of the controls received or had received treatment with testosterone. Fifty-seven of the controls were right-handed and 8 were left-handed. Inclusion and exclusion criteria were the same as the criteria for KS patients mentioned above.
All participants received oral and written information about the study before giving their written consent. The study was approved by the Danish Data Protection Agency and local ethics committee (Region Midtjylland, Denmark number M-20080238) and registered at ClinicalTrials.gov (Clinical trial NCT00999310).
2.2. Cognitive assessment
Participants were administered a 3-h battery of standardized cognitive tests designed to assess major cognitive functions. Administration and scoring of the tests were done by trained research assistants under supervision of a psychologist and a senior specialist in clinical neuropsychology. The test battery consisted of the following cognitive domains and tests:
-
▪
Processing speed; tests included Trail Making A and B (Reitan, 1958) and the Coding subtest of the Wechsler Adult Intelligence Scale — third edition (WAIS-III) (Wechsler, 1997).
-
▪
Working memory; tests included Digit Span (DS) and Letter–Number Sequencing (LN) subtests of WAIS-III (Wechsler, 1997).
-
▪
Visual/spatial construction and performance; tests included the Copy subtest of the Rey Complex Figure Test and Recognition Trial (RCFT) (Meyers and Meyers, 1995) and the Block Design (BD) and the Matrix Reasoning (MR) subtests of WAIS-III (Wechsler, 1997).
-
▪
Visual memory and learning; tests included the Immediate Recall and Delayed Recall subtests of RCFT (Meyers and Meyers, 1995).
-
▪
Verbal memory and learning; tests included Rey Auditory Verbal Learning Test (RAVL) (Nielsen et al., 1989).
-
▪
Verbal fluency and comprehension; tests included the Similarities (S) and the Vocabulary (V) subtests of WAIS-III (Wechsler, 1997), Sentence Repetition (Spreen and Benton, 1969), and Verbal fluency tests (animals and words by initial letter N, S and F. Score was sum of all 4 categories) (Lezak et al., 2004).
-
▪
Response inhibition; test included the Stroop Color and Word Test (SCWT) (Golden and Freshwater, 2002).
-
▪
Executive function; test included Tower of London (TOL) (Culbertson and Zillmer, 2005) and Wisconsin Card Sorting tests (WCST) (Heaton et al., 1993).
-
▪
Intelligence; Full scale Intelligence Quotient (FSIQ), Verbal Intelligence Quotient (VIQ) and Performance Intelligence Quotient (PIQ) were determined by the following regression equation derived from summary statistics data e.g. mean, standard deviation, correlations of the subtest from the WAIS-III Danish reference material (Wechsler, 1997): FSIQ = 40.21 + (1.13 × S) + (1.38 × MR) + (2.10 × V) + (1.35 × BD); VIQ = 50.91 + (2.14 × S) + (2.76 × V); PIQ = 49.86 + (2.57 × MR) + (2.47 × BD).
2.3. Psychological questionnaires
A week prior to the study participants received three self-administered psychological questionnaires to be completed before the day of participation. These included questions concerning three psychological domains:
-
▪
Psychological distress; SCL-8 (mental illness) (Fink et al., 1999; Fink et al., 2004a; Fink et al., 2004b), SCL-DEP6 (depression) (Christensen et al., 2005; Christensen et al., 2010) and SCL-ANX4 (anxiety) (Christensen et al., 2005; Christensen et al., 2010) derived from the Symptom Checklist (SCL-90) (Bech, 2005; Derogatis et al., 1973) used to measure psychological distress.
-
▪
Autism traits; Autism Spectrum Quotient (AQ) (Baron-Cohen et al., 2001) measuring autistic traits.
-
▪
Personality; Revised NEO Personality Inventory (NEO PI-R), short form (Costa and McCrae, 1992). A 60-item measure of the Big Five personality traits — neuroticism, extraversion, openness to experience, agreeableness and conscientiousness.
2.4. Magnetic resonance imaging (MRI)
2.4.1. MRI acquisition
A 3-T General Electrics Medical systems (Milwaukee, WI USA) MR system with a standard headcoil was used to acquire high-resolution 3D GR contiguous T1-weighted anatomical scans, consisting of 256 × 256 × 134 voxels with a 0.94 × 0.94 × 1.2 mm3 voxel size, obtained with a TR of 6.552 ms, a 2.824 ms TE and a 14° flip angle.
2.4.2. MRI data pre-processing
Voxel-based morphometry (VBM) analysis was performed using Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/). T1-weighted scan images were converted from DICOM format to NIfTI format and these images were manually inspected to rule out large scale and focal brain abnormalities as well as acquisition artifacts. In addition, images were manually co-registered to the anterior commissure–posterior commissure axis in order to provide optimal conditions for automated pre-processing and analysis. Images were classified into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using the unified segmentation tool in SPM8 (Ashburner and Friston, 2005) with very light bias regularization (0.0001, 60 mm FWHM cutoff) and an affine transformation to the ICBM space template (European brains). For analysis of global brain volumes, we considered the preprocessed tissue classification probability in each voxel to be an estimate of regional GM/WM/CSF volume. Global differences in GM, WM, CFS and total brain volumes between KS patients and controls were analyzed using two-sample t-tests on the sum of tissue classification probabilities across all voxels for a given structure type. Age effect on global brain volumes was analyzed by Spearmann correlation within and between groups. We did not find any significant effect of age on global brain volumes (all p-values > 0.05). To enable voxel-wise comparisons, GM images were further normalized to the templates generated from Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) (Ashburner, 2007). A study specific template was generated using DARTEL to minimize between scan variations (Klein et al., 2009). The template was made using the complete set (n = 130) of segmented GM and WM scans and linear elastic energy regularization together with all DARTEL default settings. Finally, the segmented and normalized images were smoothed with a default 8 mm FWHM Gaussian filter.
2.4.3. Classification analysis
Classification analysis was based on the same images as those used in the SPM analysis. Additionally, each subject's image was scaled by the global brain volume (GM + WM) in order to parallel the scaling in the univariate SPM analysis. The classification analysis was performed as a whole brain analysis, using the same masking as in the SPM analysis. A binary classification task was set up to classify KS patients vs. controls. For classification we used Fisher's linear discriminant analysis (FDA) which is a well known method that considers dimensionality reduction and classification jointly. FDA seeks to find an optimal subspace where the projected class means are separated the most in terms of variance (Hastie et al., 1995; Hastie et al., 2009). We used a version of FDA also referred to as regularized/penalized discriminant analysis (Hastie et al., 2009) in order to control for model over-fitting and to ensure model uniqueness. On top of the FDA basis we implemented a simple nearest mean classifier to evaluate the prediction accuracy. Leave-one-out (LOO) cross validation was used to evaluate the prediction accuracy: One subject was routinely held out from the training sample, and the FDA model was trained on the remaining 129 subjects. The trained model was then used to predict the category of the out-of-sample subject. This was repeated for all subjects. Selection of the regularization parameter was based only on training subjects in a nested LOO cross validation loop. To interpret/visualize the FDA model we use the model weight vector (Kjems et al., 2002). To estimate a stable representation of the underlying spatial brain pattern we used the NPAIRS resampling framework (Strother et al., 2002). This resampling analysis provides a reproducible brain map (rSPM) that is on an approximate Z-score scale. Finally, the rSPM was thresholded according to correction for multiple comparisons by the false discovery rate (FDR) procedure (Benjamini and Hochberg, 1995). For further details on model visualization and the NPAIRS resampling analysis see Strother et al. (2002) and Rasmussen et al. (2012).
2.5. Statistical analysis
Voxel-based statistical analyses were conducted using a general linear model approach. Correction for global brain volume (GM + WM) was done using proportional scaling. Initial analyses included age as a covariate, however no significant voxel-wise effect was seen (all p-values > 0.7 FWE corrected) and the age covariate was removed from the final analysis. Analysis of GM was performed using an explicit mask, including all voxels with a probability above 5% for the given tissue class. Two between-group contrasts were defined for GM: KS > C and C > KS and tested with two-sample t-test. P < 0.05 was used as significance threshold, whole brain corrected for family-wise error and cluster size > 20 voxels. Anatomical regions were located using WFU (Wake Forest University School of Medicine) Pickatlas referencing the AAL atlas (Tzourio-Mazoyer et al., 2002).
To test the relationship between regional brain volumes and psychological and cognitive variables, we first applied principal component analyses (PCA) (Mardia et al., 1979) to the tests in each individual psychological and cognitive domain. This was done to reduce the dimensionality of the psychological and cognitive variables. The PCA analysis was performed by STATA (StataCorp, College Station, TX). The first component extracted from each domain was included as a covariate in the general linear model in SPM8 (see Supplementary material for details about the covariates). Multiple regressions were used to assess the variation in regional brain volume attributable to the three psychological and ten cognitive domains. P < 0.05 was used as significant threshold, corrected for family-wise error. Spearman's rank correlation coefficient was used to test the relationship between global brain volumes and the three psychological and the ten cognitive domains, employing Bonferroni's adjustment for multiple testing. Between-group differences in psychological and cognitive test scores and demographic variables were analyzed in SPSS (SPSS Inc., Chicago, IL, USA) using independent two-sample t-tests if data met the criteria for normal distribution; otherwise data were analyzed with non-parametric Mann Whitney test. Chi-square tests were used for nominal variables.
To test the relationship between regional brain volumes and psychological and cognitive variables, we first applied principal component analyses (PCA) (Mardia et al., 1979) to the tests in each individual psychological and cognitive domain. This was done to reduce the dimensionality of the psychological and cognitive variables. The PCA analysis was performed by STATA (StataCorp, College Station, TX). The first component extracted from each domain was included as a covariate in the general linear model in SPM8 (see Supplementary material for details about the covariates). Multiple regressions were used to assess the variation in regional brain volume attributable to the three psychological and ten cognitive domains. P < 0.05 was used as significant threshold, corrected for family-wise error. Spearman's rank correlation coefficient was used to test the relationship between global brain volumes and the three psychological and the ten cognitive domains, employing Bonferroni's adjustment for multiple testing. Between-group differences in psychological and cognitive test scores and demographic variables were analyzed in SPSS (SPSS Inc., Chicago, IL, USA) using independent two-sample t-tests if data met the criteria for normal distribution; otherwise data were analyzed with non-parametric Mann Whitney test. Chi-square tests were used for nominal variables.
3. Results
3.1. Demographic and psychological characteristics
Demographic and psychological characteristics of participants are presented in Table 1. There was no difference with respect to age and educational level between KS patients and controls. KS patients reported significantly more symptoms of psychological distress. Furthermore, KS patients had a different personality profile with a significantly higher level of neuroticism, and a significantly lower level of extraversion, agreeableness and conscientiousness. The KS patients also scored significantly higher on all subscales of the Autism Spectrum Quotient test, except the attention to detail subscale.
Table 1.
Age, education and psychological data. Data are median (total range) or mean ± SD. SCL, Symptoms Check List; NEO PI-R, Revised NEO Personality Inventory; AQ, Autism Spectrum Quotient.
| KS (n = 65) | Controls (n = 65) | p-Value KS vs. controls | |
|---|---|---|---|
| Age (yr) | 36.8 ± 10.5 | 36.8 ± 10.3 | 0.96 |
| Education (yr) | 13 (7–18) | 13 (8–18) | 0.94 |
| SCL | |||
| DEP4 (depression) | 3 (0–20) | 1 (0–14) | 0.01 |
| ANX6 (anxiety) | 2 (0–15) | 2 (0–8) | 0.006 |
| SCL-8 (mental disorder) | 6 (0–28) | 2.5 (0–19) | 0.001 |
| NEO PI-R | |||
| Neuroticism | 63 (42–78) | 50 (31–70) | < 0.001 |
| Extraversion | 44 (22–69) | 55 (22–78) | < 0.001 |
| Openness | 51 (28–71) | 46 (22–70) | 0.23 |
| Agreeableness | 51 (22–67) | 56 (32–74) | 0.002 |
| Conscientiousness | 45 (22–71) | 51 (22–71) | 0.02 |
| AQ | |||
| Total | 19 (9–41) | 14 (7–28) | < 0.001 |
| Communication skills | 3 (0–10) | 2 (0–6) | 0.01 |
| Social skills | 3 (0–10) | 1 (0–9) | < 0.001 |
| Imagination | 4 (0–10) | 3 (0–8) | < 0.001 |
| Attention to detail | 4 (0–9) | 4 (0–8) | 0.42 |
| Attention switching | 4 (0–10) | 3 (0–9) | 0.007 |
3.2. Cognitive test results
The cognitive test scores (Table 2) showed that the KS patients had significantly lower full scale IQ, verbal IQ and performance IQ. Furthermore, KS patients had significantly slower processing speed, lower working memory capacity, significantly decreased verbal abilities and poorer response inhibition compared to controls. No differences in visual abilities were found between the two groups, except for the two tests influenced by intelligence (WAIS-BD, WAIS-MR). As for executive functions data from the TOL test indicated poorer planning ability among KS patients (significantly higher total move score and a significantly lower correct score), and reduced mental flexibility as measured with the WCST (KS patients used significantly more cards, completed significantly fewer categories with more errors and perseverative responses).
Table 2.
Neurocognitive ability in patients with Klinefelter syndrome and controls. IQ, intelligence quotient; WAIS-III, Wechsler Adult Intelligence Scale — third edition; WAIS-III C, WAIS-III Coding; TMA, Trail Making A; TMB, Trail Making B; WAIS-III DS, WAIS-III Digit Span; WAIS-III LN, WAIS-III Letter-Number Sequencing; RCFT, Rey Complex Figure Test and Recognition Trail; WAIS-III BD, WAIS-III Block Design; WAIS-III MR, WAIS-III Matrix Reasoning; RAVL, Rey Auditory Verbal Learning Test; WAIS-III V, WAIS-III Vocabulary; WAIS-III S, WAIS-III Similarities; SCWT diff, Stroop Color and Word Test Difference Score; TOL, Tower of London; WCST, Wisconsin Card Sorting Test; Data are medians (total range) or means ± SD.
| KS (n = 65) | Controls (n = 65) | p-Value KS vs. controls | |
|---|---|---|---|
| Intelligence | |||
| Full scale IQ | 87.8 ± 12.0 | 103.7 ± 11.3 | < 0.001 |
| Verbal IQ | 84.7 ± 12.3 | 100.8 ± 11.7 | < 0.001 |
| Performance IQ | 96.7 ± 13.2 | 106.2 ± 10.9 | < 0.001 |
| Processing speed | |||
| WAIS-III C | 57 (31–91) | 71 (39–112) | < 0.001 |
| TMA (sec.)a | 29 (26–80) | 23 (12–64) | < 0.001 |
| TMB (sec.)a | 77 (25–205) | 62 (32–208) | 0.002 |
| Working memory | |||
| WAIS-III DS | 13 (8–22) | 15 (10–24) | < 0.001 |
| WAIS-III LN | 9 (4–18) | 11 (7–18) | 0.005 |
| Visual performance | |||
| RCFT Copy | 33 (22–36) | 33 (22–36) | 0.86 |
| WAIS-III BD | 43 (19–66) | 55 (12–66) | < 0.001 |
| WAIS-III MR | 18 (5–25) | 21 (7–25) | < 0.001 |
| Visual memory and learning | |||
| RCFT immediate recall | 22.5 (9.5–33.0) | 22.5 (9.0–34.0) | 0.49 |
| RCFT delayed recall | 21.5 (11.5–33.0) | 21.5 (7.5–34.0) | 0.84 |
| Verbal memory and learning | |||
| RAVLT 1.trail | 5 (2–11) | 6 (2–12) | 0.003 |
| RAVLT total | 43 (21–66) | 51 (29–70) | < 0.001 |
| RAVL delayed recall | 10 (0–15) | 11 (5–15) | 0.13 |
| Verbal performance | |||
| Verbal fluency | 51 (21–94) | 67 (35–107) | < 0.001 |
| WAIS-III S | 17.5 (8.0–26.0) | 23 (11–31) | < 0.001 |
| WAIS-III V | 24.5 (8.0–49.0) | 37 (12–57) | < 0.001 |
| Sentence span | 15 (10–21) | 17.5 (13.0–22.0) | < 0.001 |
| Response inhibition | |||
| SCWT diff | 1.72 ± 7.48 | 3.28 ± 8.3 | < 0.001 |
| Executive function | |||
| WCST cards administereda | 113 (70–128) | 83 (70–128) | < 0.001 |
| WCST errors (%)a | 26 (11–62) | 18(11–58) | < 0.001 |
| WCST persev. responses (%)a | 15 (5–40) | 9 (5–34) | < 0.001 |
| WCST categories completed | 6 (1–6) | 6 (1–6) | 0.007 |
| WCST failure to maintain seta | 1 (0–4) | 0 (0–5) | 0.01 |
| TOL total move scorea | 26 (0–67) | 16 (0–55) | 0.003 |
| TOL total correct score | 5 (0–10) | 6 (1–10) | 0.005 |
Lower score means better performance.
3.3. MRI data
Significantly smaller total brain volume (TBV), total gray matter volume (GMV) and total white matter volume (WMV) were found in KS patients compared to controls, whereas there was no significant difference in CSF volume (Table 3). Asymmetries in left and right GM volume were similar between the two groups (GMV_difKS = − 11.2 ± 4.2 mL; GMV_difc = − 11.6 ± 4.1; p = 0.625). There were no significant differences in TBV, GMV, WMV or CSF between testosterone treated KS (T-KS) and untreated KS (U-KS) patients. VBM-analysis (Table 4, Fig. 1) showed that KS patients had significantly decreased GMV bilaterally in insula, putamen, caudate, hippocampus, amygdala, temporal pole and frontal inferior orbita compared to controls. Additionally, the right cerebellum and parahippocampal gyrus was reduced in KS patients. KS patients had significantly larger volumes in regions of the postcentral gyrus, inferior and superior parietal lobules and precuneus, bilaterally, compared to controls (Table 4, Fig. 1). We did not find any significant difference in regional GMV between untreated and treated KS patients (data not shown). The classification analysis correctly discriminated KS patients from controls with 96.9% accuracy (p < 0.001 using nonparametric permutation) (four controls were misclassified). Fig. 2 shows the reproducible brain map (rSPM) extracted within the NPAIRS resampling framework. The rSPM represents a stable pattern of brain locations supporting discriminative information to the classifier. The rSPM was thresholded according to p < 0.05 FDR correction for multiple testing. Hot and cold colors represent positive and negative Z-scores respectively. The interpretation of the map is as follows: Increasing the signal locally in cold areas will make a particular brain scan more likely to be classified as a KS patients, these area include the same brain regions and lobes as found we found by VBM analysis. Conversely, increasing the signal locally in warm areas will make a brain scan more likely to be classified as a control, these areas include the same brain areas as found by primary VBM analysis.
Table 3.
Brain volumetric measures in KS patients and controls. Data are mean ± SD (p < 0.05, uncorrected). GMV, gray matter volume; WMV, white matter volume; CSF, Cerebrospinal fluid; U-KS, untreated KS males; T-KS, testosterone treated KS males.
| U-KS | T-KS | KS | Controls | p-Value |
t-Value |
|||
|---|---|---|---|---|---|---|---|---|
| U-KS vs. T-KS | KS vs. controls | U-KS vs. T-KS | KS vs. controls | |||||
| n | 21 | 44 | 65 | 65 | Df = 63 | Df = 128 | ||
| Total gray matter volume (mL) | 733.2 ± 47.1 | 723.9 ± 53.6 | 726.9 ± 51.4 | 756.9 ± 59.5 | 0.50 | < 0.01 | 0.68 | 3.08 |
| Total white matter volume (mL) | 532.5 ± 39.1 | 520.3 ± 40.5 | 524.3 ± 40.2 | 548.2 ± 52.3 | 0.26 | < 0.01 | 1.15 | 2.93 |
| Cerebrospinal fluid (mL) | 331.5 ± 29.5 | 338.8 ± 36.7 | 336.4 ± 34.5 | 340.7 ± 34,3 | 0.43 | 0.48 | − 0.78 | 0.71 |
Table 4.
Peak voxels in regions of gray matter with significant volumetric differences between KS patients and Controls (p < 0.05, FWE). L, left; R, right; MNI, Montreal Neurological Institute. Z-value, Inf, infinite.
| Putative region | Hemisphere | Cluster size (voxel) | MNI peak coordinates (X,Y,Z) | Z-value (peak voxel) |
|---|---|---|---|---|
| KS < controls | ||||
| Insula | L | 3133 | − 38,17,− 5 | Inf |
| Caudate | − 15,18,− 8 | Inf | ||
| Putamen | − 26,8,− 9 | Inf | ||
| Frontal Inf Orb | − 21,12,− 27 | 5.66 | ||
| Insula | R | 3503 | 38,14,− 5 | Inf |
| Putamen | 21.18,− 6 | 7.38 | ||
| Hippocampus | 24,− 9,− 18 | 6.85 | ||
| Parahippocampal extending into amygdala | 22,− 4,− 27 | 6.81 | ||
| Temporal Pole Sup | 27,8,− 27 | 6.67 | ||
| Frontal Inf Orb | 34,23,− 11 | 6.11 | ||
| Hippocampus extending into amygdala | L | 969 | − 20,− 6,− 20 | Inf |
| Temporal Pole Sup | L | 67 | − 40,6,− 24 | 6.66 |
| Caudate | R | 64 | 21,− 10,24 | 6.36 |
| Cerebellum | R | 29 | 28,− 57,− 32 | 5.64 |
| KS > controls | ||||
| Postcentral | R | 185 | 21,− 40,73 | 6.95 |
| Precuneus | 8,− 46,58 | 6.12 | ||
| Parietal superior | R | 79 | 26,− 49,60 | 5.97 |
| Precuneus | L | 70 | − 16,− 45,4 | 5.65 |
| Postcentral | L | 53 | − 21,− 43,69 | 6.98 |
| Parietal superior | − 18,− 51,69 | 6.62 | ||
| Parietal inferior | L | 51 | − 28,− 45,48 | 6.92 |
Fig. 1.
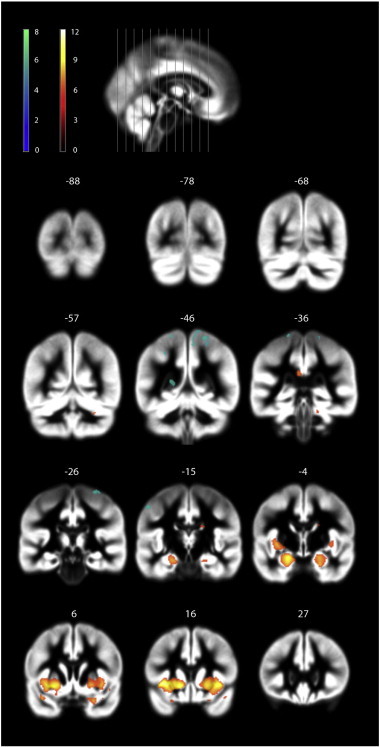
Coronal slice representation of gray matter differences between KS patients and controls. T-map displaying significant gray matter cluster differences between the KS patients and controls (p = 0.05, FWE corrected) is overlaid brain template. Significant gray matter clusters were KS < controls is displayed in orange-red colors. Significant gray matter clusters were KS > controls is shown in green-blue colors (t (df), df = 128).
Fig. 2.
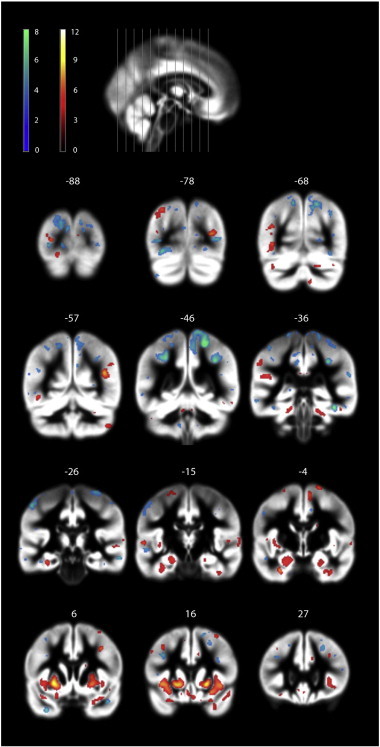
Whole-brain representation of classification analysis (reproducible) brain map (p < 0.05FDR). Increasing the signal locally in cold areas will make a particular brain scan more likely to be classified as a KS patient. Increasing the signal locally in warm areas will make a brain scan more likely to be classified as a control.
3.4. Correlation between global and regional brain volume and neuropsychological performance
In the initial across group analyses, we found a significant positive correlation between intelligence and GMV (rho = 0.18, p = 0.04), between intelligence and WMV (rho = 0.20, p = 0.02) and between visual performance and WMV (rho = 0.23, p = 0.009) in the 130 subject group. Within controls, we found a positive significant correlation between visual performance and GMV (rho = 0.26, p = 0.04) and between visual performance and WMV (rho = 0.30, p = 0.02), and a significant negative correlation between processing speed and GMV (rho = − 0.30, p = 0.02). No correlations were found within the group of KS patients. In the subsequent analyses which included Bonferroni adjustment, we found no significant correlation between psychological and cognitive domains and global GMV, WMV and CSF in the 130 subject group or within controls separately. Furthermore, we did not find any significant correlations between voxel-wise regional brain volumes and the 3 psychological and 10 cognitive domains (Table 5).
Table 5.
Principal component analysis: Domains, tests included and 1.component. WAIS-III, Wechsler Adult Intelligence Scale — third edition; RCFT, Rey Complex Figure Test and Recognition Trial; RAVLT, Rey Auditory Verbal Learning Test; SCWT, Stroop Color and Word Test; WCST, Winconsin Card Sorting Test; TOL, Tower of London Test.
| Domain | Test | Variability accounted for by 1.component (%) |
|---|---|---|
| Psychological domain | ||
| Psychological distress (3 subtests) | SCL-DEP4, SCL-ANX 6, SCL-8 | 87.5 |
| Personality (5 subscales from NEO PI-R) | Neuroticism, extraversion, openness, agreeableness, conscientiousness | 42.2 |
| Autism traits (5 subscales from AQ) | Communication, social skills, imagination, attention to detail, attention switching | 42.4 |
| Cognitive domain | ||
| Intelligence (3 subscales) | Full scale IQ, verbal IQ, performance IQ | 80.9 |
| Processing speed (3 subtests) | WAIS-III C, TMA, TMB | 73.9 |
| Working memory (2 subtests) | WAIS-III DS, WAIS-III LN | 78.7 |
| Visual performance (3 subtests) | WAIS-III MR, WAIS-III BD, RCFT copy | 60.4 |
| Visual learning and memory (2 subtests) | RCFT immediate recall, RCFT delayed recall | 95.8 |
| Verbal learning and memory (3 subscales) | RAVL 1.trail, RAVL total, RAVL delayed recall | 78.2 |
| Verbal performance (4 subtests) | Fluency, sentence span, WAIS-III S, WAIS-III V | 71.4 |
| Response inhibition (1 subtest) | SCWT diff | – |
| Executive function: planning (2 subscales) | TOL total move score, TOL total correct score | 93.0 |
| Executive function: flexibility (5 subscales) | WCST cards administered, WCST error(%), WCST persev. responses %, WCST categories completed, WCST failure to maintain set | 74.2 |
4. Discussion
The present study documents significantly reduced global and regional brain volumes in KS patients. Based on the brain scans we could predict a KS diagnosis with 96.9% accuracy. We did not find any significant correlation between the reduced brain volumes and neuropsychological outcome measures, nor did we demonstrate any significant effect of testosterone treatment on brain volumes.
Globally, KS patients had significantly smaller TBV, in agreement with previous studies (Bryant et al., 2011; DeLisi et al., 2005; Giedd et al., 2007; Shen et al., 2004; Warwick et al., 2003), and significantly smaller GMV and WMV. Other VBM studies of KS patients demonstrate similar results, i.e., either a significantly decreased WMV and GMV in boys and adolescents (Shen et al., 2004), or a trend towards decreased WMV (p = 0.059) and GMV (p = 0.07) in boys (Bryant et al., 2011). However, studies using ROI and LowD techniques found only an isolated GMV decrease in boys and adolescents (Giedd et al., 2007) or an isolated WMV decrease in adults (Rezaie et al., 2009). No volumetric difference in CSF was found in our study. No study has earlier reported CSF measurements in KS patients. Studies using ROI have found either enlarged lateral ventricles (Giedd et al., 2007; Shen et al., 2004; Warwick et al., 2003) or no volumetric differences (DeLisi et al., 2005; Patwardhan et al., 2000). As we found no volumetric difference in CSF, a putatively larger lateral ventricle would require smaller CFS volumes in other brain regions which seems unlikely. In accordance with Rezaie et al. (2009) we found no difference in left and right hemisphere global GMV and WMV between KS patients and controls.
The concordance between our findings of changes in TBV, GMV and WMV in our adult group of KS patients and other VBM studies of boys and adolescents with KS, indicates that these differences in brain volumes probably develop during fetal life or early childhood, either due to the gene dosage of having an extra X-chromosome or to differences in endogenous testosterone level. Testosterone influences brain development in the human fetus (Knickmeyer and Baron-Cohen, 2006). If – and to what degree – prenatal and postnatal testosterone levels are affected in KS is uncertain. KS is associated with an increased prevalence of microphallus and cryptorchidism (Ross et al., 2005) indicating low intrauterine testosterone levels. However, studies in KS neonates report either decreased or normal/high testosterone levels (Aksglaede et al., 2007; Lahlou et al., 2004). A recent study actually does suggest that intrauterine testosterone is decreased in KS fetuses (Manning et al., 2013). The brain size in girls with congenital adrenal hyperplasia exposed to excessive testosterone, prenatally, is not larger than brains in female controls, indicating that testosterone prenatally does not determine global brain size in girls (Merke et al., 2003). In addition, we found no significant difference in global brain sizes between testosterone treated and untreated KS patients in accordance with previous studies (Itti et al., 2006; Patwardhan et al., 2000), indicating that exogenous testosterone does not affect global brain volumes in adult KS patients either. In conclusion, the observed difference in global brain size seems to be an inherited trait specific to KS, but it remains to be determined whether genetic or endocrine factors or a combination of these factors are to blame. This is supported by a study of brain volumes in females with 47,XXX in which reduced brain volume has been demonstrated (Warwick et al., 1999).
We observed pervasive differences in regional brain volumes in brain regions involved in cognitive functions. We found a significantly smaller volume of the caudate nucleus and putamen among KS patients, also observed by Giedd et al. (2007) but not in other studies (Shen et al., 2004; Warwick et al., 2003). The caudate nucleus is part of the circuits involved in learning (Poldrack et al., 2001) and executive functions (Alexander et al., 1986). Smaller caudate volume has been found to be associated with ADHD (Castellanos et al., 1994), which is seen with an increased prevalence in KS (Bruining et al., 2009). In accordance with others (DeLisi et al., 2005; Giedd et al., 2007; Patwardhan et al., 2000; Shen et al., 2004), we demonstrated regional volumetric differences in the temporal and frontal lobes, regions involved in adaptive learning and social functions (Kringelbach and Rolls, 2004). We also observed decreased hippocampal and parahippocampal volumes in KS patients, which was also reported by Bryant et al. (2011). Hippocampal/parahippocampal regions are involved in memory processing (Young et al., 1997) known to be affected in KS (Fales et al., 2003). Decreased amygdala volume in KS patients as we found, has been reported previously (Bryant et al., 2011; Patwardhan et al., 2000; Rose et al., 2004; Shen et al., 2004). Amygdala is part of the limbic system which is involved in social and emotional processing (Wallentin et al., 2011), which is known to be affected in KS patients (van Rijn et al., 2008). Furthermore, we found a significantly smaller insula in KS patients. Reduced volume of the insula has been associated with impaired social communication (Baron-Cohen and Belmonte, 2005), impairments which have also been described in KS patients (van Rijn et al., 2008).
The regional volume abnormalities seen in KS have been proposed to be due to either hypogonadism or gene dosage effect. A recently published study found fetal testosterone to influence gray matter volume in specific temporal, parietal and orbitofrontal areas (Lombardo et al., 2012). We found no volumetric GM abnormalities in these specific brain regions in our KS patients, indicating that the regional volumetric abnormalities seen in KS most likely are due to the gene dosage effect of the extra X-chromosome and not directly to the effects of low testosterone. The hypothesis that an altered X-chromosome dosage can influence regional brain volume is supported by a study who found evidence of dosage-sensitive X-linked locus influencing the volume of amygdala (Good et al., 2003). We found an effect in the same region, albeit extended to the entire medial and frontal temporal lobe. Furthermore, women with Turner syndrome who have only one X-chromosome, have reduced the volume of the parietal lobe and increased the volume of the temporal lobe (Cutter et al., 2006; Kesler et al., 2004), indicating that X-chromosome gene dosage may influence the size of the parietal and temporal lobes. Contrary to this there is evidence that testosterone has an organizational effect on brain volumes. A recent voxel-based morphometry study reported that gray matter volume in parahippocampal gyri and putamen were increased in boys with early excess of androgen secretion compared to healthy age-matched boys (Mueller et al., 2011). Our finding of decreased gray matter volume in the same area in KS patients, a syndrome associated with androgen insufficiency, is in line with these findings, suggesting an influence of androgen on brain regions such as the parahippocampal area and putamen and perhaps a more general organizational effect on the brain.
We aimed to associate the neuroanatomical phenotype with the neuropsychological phenotype seen in KS. It has long been the perception that regional volumetric abnormalities associated with KS underlie the neuropsychological phenotype seen in KS, although few studies have investigated this. We did not find any correlation between neuropsychological test scores and regional brain volumes. This lack of correlation has also been observed in four out of the five existing publications, although these four publications suffer from a limited number of participants (n = 10–31) and a limited neuropsychological test battery (Bryant et al., 2011; DeLisi et al., 2005; Patwardhan et al., 2000; Warwick et al., 2003). The only published study in KS using a thorough neuropsychological test battery found an inverse correlation between ventricular volume and processing speed and executive function, whereas left temporal lobe volume showed a positive correlation with language scores and processing speed in right-handed subjects (Itti et al., 2006). These analyses, however, may reflect known between-group effects, as the analysis was done on the KS patients and controls combined (Steinman et al., 2009). The lack of correlation between brain volume and neuropsychological test scores found within our large sample of KS patients indicates that the explanation of the neuropsychological deficits in KS may be related to the micro-architecture, rather than the mesoscopic anatomical brain level investigated using VBM. Our data indicate that the neuroanatomical etiology of the neuropsychological phenotype of KS is much more complicated than just simply differences in volumetric measurements. This complexity is mirrored in well-known gender difference in GMV. Although females on average have 10% smaller GMV, it is very difficult to point to any significant neuropsychological effects of this (Wallentin, 2009). To understand the neuroanatomical etiologies of the neuropsychological phenotype, we need to investigate the brain by functional scans and investigate the interaction between behavior, brain function and the possible relation to X-linked genes and changes in gene expression.
4.1. Strength
This study is the largest study conducted investigating brain volumes in KS participants. Our KS patients and controls were carefully matched on age and educational background. We used a comprehensive neuropsychological test battery. Compared to the ROI, the VBM-method is an objective method not biased by subjectivity. Furthermore, the VBM-method allows investigation of the entire brain, not just of easily quantified brain structures.
4.2. Limitations
Our data concerning the effect of testosterone on global brain volumes are weakened by a lack of standardization of duration and pharmaceutical form of testosterone therapy and the age when initiating testosterone therapy. The lack of correlation between brain volumes and neuropsychological test score could be due to the fact that significant changes in neuropsychological test scores are too weak a measurement to correlate to brain volume in general, or that the neuropsychological test score are influenced by other parameters (such as depression) blurring the test score.
5. Conclusion
In summary, we present global and regional brain volumetric measurements and their possible associations to the neuropsychological phenotype and testosterone therapy in the largest cohort of KS patients to date. Our data suggest that KS is associated with global and regional brain volumetric changes, and that these changes probably are due to a gene dosage effect rather than hypogonadism. We did not find evidence that testosterone therapy affects brain volume in KS or that the neuropsychological phenotype can be explained by differences in brain volume.
The following is the supplementary data related to this article.
Principal component analysis: Domains, tests included and 1.component. WAIS-III, Wechsler Adult Intelligence Scale — third edition; RCFT, Rey Complex Figure Test and Recognition Trial; RAVLT, Rey Auditory Verbal Learning Test; SCWT, Stroop Color and Word Test; WCST, Winconsin Card Sorting Test; TOL, Tower of London Test.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.013.
Acknowledgments
This study was supported by grants from the Lundbeck Foundation, the Augustinus Foundation and Aase and Einar Danielsen Foundation. A.S. received a research fellowship from the University of Aarhus. C.H.G. was supported by a personal clinical research grant from the Novo Nordisk Foundation. We would like to thank Eva Schriver, Merete Møller, Dorte Wulff, Susanne Sørensen, Lone Kvist Dora Zeidler and Michael Geneser for their technical assistance. The authors declare no competing financial interests.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Aksglaede L., Petersen J.H., Main K.M., Skakkebaek N.E., Juul A. High normal testosterone levels in infants with non-mosaic Klinefelter's syndrome. Eur. J. Endocrinol. 2007;157:345–350. doi: 10.1530/EJE-07-0310. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barker T.E., Black F.W. Klinefelter syndrome in a military population. Electroencephalographic, endocrine, and psychiatric status. Arch. Gen. Psychiatry. 1976;33:607–610. doi: 10.1001/archpsyc.1976.01770050059009. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Belmonte M.K. Autism: a window onto the development of the social and the analytic brain. Annu. Rev. Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bech P. Frederiksborg amt; Hillerød, Danmark: 2005. Rating scales for affektive lidelser: kompendium, Psykiatrisk Forskningsenhed, Psykiatrik sygehus. [Google Scholar]
- Bender B.G., Harmon R.J., Linden M.G., Bucher-Bartelson B., Robinson A. Psychosocial competence of unselected young adults with sex chromosome abnormalities. Am. J. Med. Genet. 1999;88:200–206. doi: 10.1002/(sici)1096-8628(19990416)88:2<200::aid-ajmg18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Bojesen A., Juul S., Gravholt C.H. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J. Clin. Endocrinol. Metab. 2003;88:622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- Bruining H., Swaab H., Kas M., van E.H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123:e865–e870. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Bryant D.M., Hoeft F., Lai S., Lackey J., Roeltgen D., Ross J., Reiss A.L. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. J. Neurosci. 2011;31:6654–6660. doi: 10.1523/JNEUROSCI.5899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Giedd J.N., Eckburg P., Marsh W.L., Vaituzis A.C., Kaysen D., Hamburger S.D., Rapoport J.L. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. Am. J. Psychiatry. 1994;151:1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- Christensen K.S., Fink P., Toft T., Frostholm L., Ornbol E., Olesen F. A brief case-finding questionnaire for common mental disorders: the CMDQ. Fam. Pract. 2005;22:448–457. doi: 10.1093/fampra/cmi025. [DOI] [PubMed] [Google Scholar]
- Christensen K.S., Bech P., Fink P. Measuring mental health by questionnaires in primary care — unidimentionality, responsiveness and compliance. Eur. Psychiatr. Rev. 2010;3:8–12. [Google Scholar]
- Cooke B., Hegstrom C.D., Villeneuve L.S., Breedlove S.M. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. Psychological Assessment Resources, Inc.; Odessa, FL: 1992. Revised NEO Personality Inventory and NEO Five-factor Inventory. [Google Scholar]
- Culbertson W.C., Zillmer E.A. Technical Manual. Multi-Health Systems Inc.; NY: 2005. Tower of London — Dextrel University: 2nd Edition. [Google Scholar]
- Cutter W.J., Daly E.M., Robertson D.M., Chitnis X.A., van Amelsvoort T.A., Simmons A., Ng V.W., Williams B.S., Shaw P., Conway G.S., Skuse D.H., Collier D.A., Craig M., Murphy D.G. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol. Psychiatry. 2006;59:273–283. doi: 10.1016/j.biopsych.2005.06.026. [DOI] [PubMed] [Google Scholar]
- DeLisi L.E., Maurizio A.M., Svetina C., Ardekani B., Szulc K., Nierenberg J., Leonard J., Harvey P.D. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. Am. J. Med. Genet.B Neuropsychiatr. Genet. 2005;135B:15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- Derogatis L.R., Lipman R.S., Covi L. SCL-90: an outpatient psychiatric rating scale–preliminary report. Psychopharmacol. Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- Fales C.L., Knowlton B.J., Holyoak K.J., Geschwind D.H., Swerdloff R.S., Gonzalo I.G. Working memory and relational reasoning in Klinefelter syndrome. J. Int. Neuropsychol. Soc. 2003;9:839–846. doi: 10.1017/S1355617703960036. [DOI] [PubMed] [Google Scholar]
- Fink P., Ewald H., Jensen J., Sorensen L., Engberg M., Holm M., Munk-Jorgensen P. Screening for somatization and hypochondriasis in primary care and neurological in-patients: a seven-item scale for hypochondriasis and somatization. J. Psychosom. Res. 1999;46:261–273. doi: 10.1016/s0022-3999(98)00092-0. [DOI] [PubMed] [Google Scholar]
- Fink P., Orbol E., Hansen M.S., Sondergaard L., De J.P. Detecting mental disorders in general hospitals by the SCL-8 scale. J. Psychosom. Res. 2004;56:371–375. doi: 10.1016/S0022-3999(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Fink P., Ornbol E., Huyse F.J., De J.P., Lobo A., Herzog T., Slaets J., Arolt V., Cardoso G., Rigatelli M., Hansen M.S. A brief diagnostic screening instrument for mental disturbances in general medical wards. J. Psychosom. Res. 2004;57:17–24. doi: 10.1016/S0022-3999(03)00374-X. [DOI] [PubMed] [Google Scholar]
- Geschwind D.H., Gregg J., Boone K., Karrim J., Pawlikowska-Haddal A., Rao E., Ellison J., Ciccodicola A., D'Urso M., Woods R., Rappold G.A., Swerdloff R., Nelson S.F. Klinefelter's syndrome as a model of anomalous cerebral laterality: testing gene dosage in the X chromosome pseudoautosomal region using a DNA microarray. Dev. Genet. 1998;23:215–229. doi: 10.1002/(SICI)1520-6408(1998)23:3<215::AID-DVG7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Clasen L.S., Wallace G.L., Lenroot R.K., Lerch J.P., Wells E.M., Blumenthal J.D., Nelson J.E., Tossell J.W., Stayer C., Evans A.C., Samango-Sprouse C.A. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case–control study. Pediatrics. 2007;119:e232–e240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Golden C.J., Freshwater S.M. Stoelting Co.; Wood Dale, IL: 2002. Stroop Color and Word Test. A Manual for Clinical and Experimental Uses. [Google Scholar]
- Good C.D., Lawrence K., Thomas N.S., Price C.J., Ashburner J., Friston K.J., Frackowiak R.S., Oreland L., Skuse D.H. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Graham J.M., Jr., Bashir A.S., Stark R.E., Silbert A., Walzer S. Oral and written language abilities of XXY boys: implications for anticipatory guidance. Pediatrics. 1988;81:795–806. [PubMed] [Google Scholar]
- Hastie T., Buja A., Tibshirani R. Penalized discriminant analysis. Ann. Stat. 1995;23:73–102. [Google Scholar]
- Hastie T., Tibshirani R., Friedman J. Springer; 2009. The Elements of Statistical Learning: Data Mining, Inference and Prediction. [Google Scholar]
- Heaton R.K., Chelune G.J., Talley J.L., Kay G.G., Curtiss G. Psychological Assessment Resources, Inc.; Odessa, FL: 1993. Wisconsin Card Sorting Test Manual, Revised and Expanded. [Google Scholar]
- Itti E., Gaw Gonzalo I.T., Pawlikowska-Haddal A., Boone K.B., Mlikotic A., Itti L., Mishkin F.S., Swerdloff R.S. The structural brain correlates of cognitive deficits in adults with Klinefelter's syndrome. J. Clin. Endocrinol. Metab. 2006;91:1423–1427. doi: 10.1210/jc.2005-1596. [DOI] [PubMed] [Google Scholar]
- Kesler S.R., Garrett A., Bender B., Yankowitz J., Zeng S.M., Reiss A.L. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 2004;42:1971–1978. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems U., Hansen L.K., Anderson J., Frutiger S., Muley S., Sidtis J., Rottenberg D., Strother S.C. The quantitative evaluation of functional neuroimaging experiments: mutual information learning curves. Neuroimage. 2002;15:772–786. doi: 10.1006/nimg.2001.1033. [DOI] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B., Chiang M.C., Christensen G.E., Collins D.L., Gee J., Hellier P., Song J.H., Jenkinson M., Lepage C., Rueckert D., Thompson P., Vercauteren T., Woods R.P., Mann J.J., Parsey R.V. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R.C., Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J. Child Neurol. 2006;21:825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- Kompus K., Westerhausen R., Nilsson L.G., Hugdahl K., Jongstra S., Berglund A., Arver S., Savic I. Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res. 2011;189:135–140. doi: 10.1016/j.psychres.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lahlou N., Fennoy I., Carel J.C., Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004;89:1864–1868. doi: 10.1210/jc.2003-031624. [DOI] [PubMed] [Google Scholar]
- Lee N.R., Wallace G.L., Clasen L.S., Lenroot R.K., Blumenthal J.D., White S.L., Celano M.J., Giedd J.N. Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J. Int. Neuropsychol. Soc. 2011;1–9 doi: 10.1017/S1355617711000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W. 4 ed. Oxford University Press; Oxford: 2004. Neuropsychological Assessment. [Google Scholar]
- Lombardo M.V., Ashwin E., Auyeung B., Chakrabarti B., Taylor K., Hackett G., Bullmore E.T., Baron-Cohen S. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 2012;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J.T., Kilduff L.P., Trivers R. Digit ratio (2D:4D) in Klinefelter's syndrome. Andrology. 2013;1:94–99. doi: 10.1111/j.2047-2927.2012.00013.x. [DOI] [PubMed] [Google Scholar]
- Mardia K.V., Kent L.P., Bibby J.M. Multivariate Analysis. Academic Press; London: 1979. [Google Scholar]
- Merke D.P., Fields J.D., Keil M.F., Vaituzis A.C., Chrousos G.P., Giedd J.N. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J. Clin. Endocrinol. Metab. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Meyers J.E., Meyers K.R. Psychological Assessment Resources; Odessa, FL.: 1995. Rey Complex Figure Test and Recognition Trail. [Google Scholar]
- Mueller S.C., Merke D.P., Leschek E.W., Fromm S., VanRyzin C., Ernst M. Increased medial temporal lobe and striatal grey-matter volume in a rare disorder of androgen excess: a voxel-based morphometry (VBM) study. Int.J Neuropsychopharmacol. 2011;14:445–457. doi: 10.1017/S1461145710001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Pelsen B. Follow-up 20 years later of 34 Klinefelter males with karyotype 47, XXY and 16 hypogonadal males with karyotype 46, XY. Hum. Genet. 1987;77:188–192. doi: 10.1007/BF00272390. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Knudsen L., Daugbjerg O. Normative data for eight neuropsychological tests based on a Danish sample. Scand. J. Psychol. 1989;30:37–45. doi: 10.1111/j.1467-9450.1989.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Patwardhan A.J., Eliez S., Bender B., Linden M.G., Reiss A.L. Brain morphology in Klinefelter syndrome: extra X chromosome and testosterone supplementation. Neurology. 2000;54:2218–2223. doi: 10.1212/wnl.54.12.2218. [DOI] [PubMed] [Google Scholar]
- Phoenix C.H., Goy R.W., Gerall A.A., Young W.C. Organizing action of prenatally administred testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 2013;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Clark J., Pare-Blagoev E.J., Shohamy D., Creso M.J., Myers C., Gluck M.A. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Rasmussen P.M., Hansen L.K., Madsen K.H., Churchill N.W., Strother S.C. Model sparisity and brain pattern interpretation of classification models in neuroimaging. Pattern Recogn. 2012;45:2085–2100. [Google Scholar]
- Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch. Dis. Child. 1999;80:192–195. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8:271–286. [Google Scholar]
- Rezaie R., Daly E.M., Cutter W.J., Murphy D.G., Robertson D.M., DeLisi L.E., Mackay C.E., Barrick T.R., Crow T.J., Roberts N. The influence of sex chromosome aneuploidy on brain asymmetry. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:74–85. doi: 10.1002/ajmg.b.30772. [DOI] [PubMed] [Google Scholar]
- Rose A.B., Merke D.P., Clasen L.S., Rosenthal M.A., Wallace G.L., Vaituzis A.C., Fields J.D., Giedd J.N. Effects of hormones and sex chromosomes on stress-influenced regions of the developing pediatric brain. Ann. N.Y. Acad. Sci. 2004;1032:231–233. doi: 10.1196/annals.1314.027. [DOI] [PubMed] [Google Scholar]
- Ross J.L., Samango-Sprouse C., Lahlou N., Kowal K., Elder F.F., Zinn A. Early androgen deficiency in infants and young boys with 47, XXY Klinefelter syndrome. Horm. Res. 2005;64:39–45. doi: 10.1159/000087313. [DOI] [PubMed] [Google Scholar]
- Shen D., Liu D., Liu H., Clasen L., Giedd J., Davatzikos C. Automated morphometric study of brain variation in XXY males. Neuroimage. 2004;23:648–653. doi: 10.1016/j.neuroimage.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Spreen O., Benton L. 1969. Neurosensory Center Comprehensive Examination for Aphasia. [Google Scholar]
- Steinman K., Ross J., Lai S., Reiss A., Hoeft F. Structural and functional neuroimaging in Klinefelter (47, XXY) syndrome: a review of the literature and preliminary results from a functional magnetic resonance imaging study of language. Dev. Disabil. Res. Rev. 2009;15:295–308. doi: 10.1002/ddrr.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemkens D., Roza T., Verrij L., Swaab H., van Werkhoven M.K., Alizadeh B.Z., Sinke R.J., Giltay J.C. Is there an influence of X-chromosomal imprinting on the phenotype in Klinefelter syndrome? A clinical and molecular genetic study of 61 cases. Clin. Genet. 2006;70:43–48. doi: 10.1111/j.1399-0004.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- Strother S.C., Anderson J., Hansen L.K., Kjems U., Kustra R., Sidtis J., Frutiger S., Muley S., LaConte S., Rottenberg D. The quantitative evaluation of functional neuroimaging experiments: the NPAIRS data analysis framework. Neuroimage. 2002;15:747–771. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- Tartaglia N.R., Ayari N., Hutaff-Lee C., Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J. Dev. Behav. Pediatr. 2012;33(4):309–318. doi: 10.1097/DBP.0b013e31824501c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Aleman A., Swaab H., Kahn R. Klinefelter's syndrome (karyotype 47, XXY) and schizophrenia-spectrum pathology. Br. J. Psychiatry. 2006;189:459–460. doi: 10.1192/bjp.bp.105.008961. [DOI] [PubMed] [Google Scholar]
- van Rijn S., Swaab H., Aleman A., Kahn R.S. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J. Autism Dev. Disord. 2008;38:1634–1641. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2009;108:175–183. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Wallentin M., Nielsen A.H., Vuust P., Dohn A., Roepstorff A., Lund T.E. Amygdala and heart rate variability responses from listening to emotionally intense parts of a story. Neuroimage. 2011;58:963–973. doi: 10.1016/j.neuroimage.2011.06.077. [DOI] [PubMed] [Google Scholar]
- Warwick M.M., Doody G.A., Lawrie S.M., Kestelman J.N., Best J.J., Johnstone E.C. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J. Neurol. Neurosurg. Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick M.M., Lawrie S.M., Beveridge A., Johnstone E.C. Abnormal cerebral asymmetry and schizophrenia in a subject with Klinefelter's syndrome (XXY) Biol. Psychiatry. 2003;53:627–629. doi: 10.1016/s0006-3223(02)01484-1. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Third edition. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Young B.J., Otto T., Fox G.D., Eichenbaum H. Memory representation within the parahippocampal region. J. Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Principal component analysis: Domains, tests included and 1.component. WAIS-III, Wechsler Adult Intelligence Scale — third edition; RCFT, Rey Complex Figure Test and Recognition Trial; RAVLT, Rey Auditory Verbal Learning Test; SCWT, Stroop Color and Word Test; WCST, Winconsin Card Sorting Test; TOL, Tower of London Test.


