Abstract
Children born preterm, and more specifically those with intrauterine growth restriction (IUGR), are prone to exhibit scholastic difficulties and behavioral problems later in development. Neuropsychological studies showed that their deficits in response inhibition and attention could be at the heart of these difficulties. Functional magnetic resonance imaging (fMRI) studies using a Go/No-go task in preterm adolescents and adults suggest their use of an alternative neuronal pathway to compensate for a possible delayed development. However, little is known about the impact of IUGR at a functional neural network level. This study used fMRI to explore brain regions activated during a Go/No-go task in 20 preterm children aged 6–7 years, 10 of which were born with IUGR. Results showed that preterm children without IUGR preferentially activated fronto-temporal regions including the inferior frontal cortex, region known to be involved in successful response inhibition. In contrast, IUGR preterm children exhibited greater activation in the putamen, in the medial frontal cortex and parietal regions, specifically involved in attention demanding tasks, some being part of the default-mode network. These findings suggest that IUGR preterm children use different brain regions and a more diffuse network to perform the task, which interfere with goal-directed activity and may reflect inefficient attentional control. The differences observed in IUGR preterm children might relate to their higher risk for neurodevelopmental and behavioral disorders.
Keywords: IUGR, Prematurity, Response inhibition, Attention, fMRI, Children
Highlights
-
•
We investigate inhibition abilities with fMRI in 6- to 7-year-old preterm children.
-
•
IUGR children showed different patterns of brain activation compared with controls.
-
•
IUGR preterm children seem to rely on more posterior, parietal regions and SMA.
-
•
Control preterm children seem to preferentially activate fronto-temporal regions.
-
•
Results suggest more attentional control difficulties in IUGR children.
1. Introduction
During these past years, an important increase of infants born very preterm and with very low birth weight was observed. This population has been reported to be at particular risk of long term cognitive difficulties (Soria-Pastor et al., 2009; Taylor and Jakobson, 2010) and psychiatric disorders such as anxiety, autism spectrum, and especially attention deficit hyperactivity disorders (e.g. Arpino et al., 2010; Hack et al., 2009; Johnson and Marlow, 2011; Schreuder et al., 2002). Additionally, several studies showed that such risk was enhanced when premature birth was associated with intrauterine growth restriction (IUGR — Baschat, 2011; Geva et al., 2006; Lagercrantz, 1997; Morsing et al., 2011).
A fetus with IUGR is a fetus born small for gestational age (SGA) with abnormal umbilical artery blood flow prior to birth. The most common and important cause of IUGR is placental insufficiency (Morsing et al., 2011). Follow-up studies of children have shown that IUGR/SGA is associated with significant difficulties in school achievements (Kok et al., 1998; Morsing et al., 2011; Shah and Kingdom, 2011). Such difficulties are thought to be consequences of specific deficits in executive function at school age, particularly in inhibition abilities and attentional control (Anderson and Doyle, 2004; Böhm et al., 2002, 2004; Mulder et al., 2009; Taylor et al., 2004; Tideman et al., 2007).
In the current study, we will particularly focus on response inhibition which is critical to executive control (Aron et al., 2004; Miyake et al., 2000; Nigg, 2000). Response inhibition is the ability to withhold a prepotent or dominant response. It develops in early childhood and continues to mature into adolescence (Abdullaev et al., 2012; Luna and Sweeney, 2004). One of the most used tests to measure response inhibition is the Go/No-go task (Donders, 1969), in which participants are required to respond to one type of stimuli frequently appearing (go trials) and to inhibit responding to another infrequent type of stimuli (no-go trials). The simple pattern of a Go/No-go task makes it an appropriate task to use with young children in functional magnetic resonance imaging (fMRI). In healthy school-age children, Go/No-go tasks activate a large cortical network, principally left motor and precentral areas during execution (go) conditions and frontal and temporal regions during inhibition (no-go) conditions (Abdullaev et al., 2012; Braet et al., 2009; Bunge et al., 2002; Durston et al., 2002, 2006; Suskauer et al., 2008). fMRI studies pointed at the importance of frontal systems, particularly the prefrontal cortex (PFC), in response inhibition. However, whereas adults primarily activate prefrontal regions specifically linked to inhibition ability such as the inferior frontal cortex (IFC), children and adolescents seem to preferentially activate more discrete regions of the PFC (e.g. Aarnoudse-Moens et al., 2012; Casey et al., 1997b; Luna et al., 2001; Tamm et al., 2002). The frontal lobe, linked to the development of higher order cognitive skills, has a prolonged maturational course, which makes it more sensitive to developmental perturbations (Taylor et al., 2011).
In two studies, fMRI was used to look into brain activation associated with successful response inhibition in prematurely born male adolescents (Nosarti et al., 2006) and adults (Lawrence et al., 2009). A Go/No-go task controlling for attention allocation to low frequency stimuli was used, to prevent the attribution of differences in cerebral activity to the “oddball” character of the no-go stimuli. In the first study, adolescents born preterm demonstrated reduced bold signal response in prefrontal areas including IFC (BA44/45) and subcortical areas including globus pallidus, but also increased bold signal response in right prefrontal areas, temporal cortex and more posterior regions like the cingulate and cuneus (Nosarti et al., 2006). The authors suggested that these regions where increased activation was observed in preterm adolescents could compensate for a potential dysfunction of fronto-striatal-cerebellar circuit, by engaging alternative but effective response pathways. In the other study, adults born preterm exhibited decreased activation in the cerebellum and increased activation in the right posterior cingulate, precuneus, and postcentral gyrus (Lawrence et al., 2009). Both studies showed that despite equal behavioral performances, participants born preterm demonstrated different brain activation related to inhibition of prepotent response compared to healthy controls, which was suggestive of a reorganization/alteration of the functional network in order to compensate for a possible maturational delay. These findings confirmed brain particularities related to response inhibition processes in prematurity but no fMRI study has yet considered the impact of IUGR on these processes in preterm population, which would be important considering the additional risk for cognitive deficits associated with this pathology. So far, the literature has reported that prenatal growth restriction can affect the frontal lobe and subsequently neuropsychological capacities and school achievements throughout the first decade of life (Baschat, 2011; Geva et al., 2006) but little is known considering IUGR at a neuronal level. Borradori Tolsa et al. (2004) studied a population of infants born preterm with and without IUGR. Infants were first studied with MRI at birth and then a second MRI and a neurobehavioral assessment at term (40 weeks GA) were performed. The results showed less mature behavioral scores in IUGR infants compared with control preterm infants at term. Specifically, they had more difficulties in maintaining attention to, and interacting with, various social stimuli. Moreover, premature infants born with IUGR demonstrated a significant reduction in total brain tissue volume and cortical gray matter volume both at birth and term, confirming the brain vulnerability associated with IUGR.
In the present work, we used event-related fMRI to explore the neural correlates associated with response execution and response inhibition during a simple colored Go/No-go task in a population of 6- to 7-year-old children born preterm with IUGR (IUGR group) and without IUGR (control group). The task, constructed with attractive colored stimuli, was based on a previous study measuring response selection in children (Suskauer et al., 2008). It was then adapted to have both a context of inhibition and a context of execution (see Section 2.2 (fMRI Go/No-go task) in Section 2 (Methods)). The simplicity of the task was also chosen with the purpose of minimizing the cognitive demands other than response inhibition or execution (Mostofsky et al., 2003; Suskauer et al., 2008). Based on previous MRI and behavioral results with the same population of children at birth (Borradori Tolsa et al., 2004) and considering that the frontal lobe function is especially vulnerable to fetal nutrient deficiency in the third trimester (Baschat, 2011), we hypothesized to observe differences in brain activation between IUGR and control children during response inhibition. Given the literature about specific cognitive and scholar deficits in IUGR population (e.g. Morsing et al., 2011; Tideman et al., 2007) and knowing that this population has less compensatory resources (Geva et al., 2006), we also expected the IUGR children to obtain poorer inhibition performances on the task.
2. Methods
2.1. Participants
The 6- to 7-year-old preterm children included, are part of a longitudinal study on neurodevelopmental outcome of premature infants already studied in the newborn period (Borradori Tolsa et al., 2004) from the Division of Child Development and Growth at the University Hospitals of Geneva. The study group consisted of children born prematurely with IUGR defined as birth weight below the 10th percentile for gestational age and gender (Arbuckle et al., 1993) and the presence of placental insufficiency defined as a resistance to arterial umbilical blood flow higher than the 95th percentile measured by the two indexes (RI and S/D) within one or several measurements (Sonesson et al., 1993) (for more details, see Borradori Tolsa et al., 2004). The control group was composed of children born prematurely with birth weight appropriate for gestational age and normal flow in the umbilical artery. Out of the 60 families selected, 38 agreed to full assessment. Of these, three refused to enter the scanner and five fell asleep during the experiment. Ten children were excluded due to head motion on the MRI1 or a very high rate of non-responses on the Go/No-go task. Finally, twenty children born preterm were included in the present study. Ten children (GA at birth 30.73 weeks ± 2.85) born with placental insufficiency and birth weights (1038 g ± 292) < 10th percentile (IUGR group) were compared with 10 children (mean birth weights 1273 g ± 478) of equivalent GA at birth (see Table 1). There were no age or gender differences between the IUGR group (5 boys; mean age 6.7) and the control group (3 boys; mean age 6.7). The participants had all normal standard neurologic examination and were free of any neurological disability (cerebral pathology, blindness, hearing loss).
Table 1.
Neonatal and socio-demographic characteristics for IUGR and control children.
| Mean (SD), range |
Statistics | p-Value | ||
|---|---|---|---|---|
| IUGR | Controls | |||
| GA, weeks | 30.73 (± 2.85), 26.29–34 | 29.37 (± 3.50), 24.29–34 | t = − 0.95 | .355 |
| Birth weight, grams | 1038 (± 292), 630–1450 | 1273 (± 478), 730–1970 | ||
| Age, years | 6.7 (± 0.6) | 6.6 (± 0.6) | t = − 0.25 | .806 |
| Gender, % | χ2 = 0.83 | .361 | ||
| Female | 50% | 70% | ||
| Male | 50% | 30% | ||
Threshold of significance: p < .05.
As part of our preterm-born follow-up, the Kaufman Assessment Battery for Children (K-ABC; Kaufman and Kaufman, 1993) was used to assess the participants' general cognitive abilities. Mean Mental Processing Composite scores were 96.1 (± 15.2) for IUGR children and 98.4 (± 12.2) for control children, and did not differ between groups (t(18) = 0.37, p > .10). Mean scores on the Sequential Processing Scale were 94.9 (± 15.2) for IUGR children and 96.1 (± 11.6) for controls (t(18) = 0.2, p > .10) and mean scores on the Simultaneous Processing Scale were 96.8 (± 10.3) and 93.9 (± 8.9) respectively for IUGR and control children (t(18) = − 0.67, p > .10).
This study was approved by the medical ethical review board of the University Hospital of Geneva and written informed consent was obtained from parents. Participants gave their oral consent to take part in the study and were free to withdraw from the procedure at any time.
2.2. fMRI Go/No-go task
The participants were individually assessed and completed a simple Go/No-go task associated with an event-related fMRI paradigm. In order to make the task more attractive for children, green and red spaceships (Mostofsky et al., 2003; Suskauer et al., 2008) were used respectively as go and no-go stimuli. In most studies using Go/No-go tasks, go trials outnumbered no-go trials, in order to make responding prepotent and the inhibition of response rare, so fitting the response inhibition definition. Nevertheless, in these studies, differences in cerebral activity between trial types could possibly be attributed to the “oddball” character of the no-go stimuli (Aron and Poldrack, 2005; Liddle et al., 2001). Thus, in the present study, we designed the task in creating two types of context, a context of inhibition (where no-go trials outnumbered go trials) and a context of execution (where go trials outnumbered no-go trials) making the number of go and no-go events overall equal and allowing us to measure response execution as well as response inhibition. All children viewed the stimuli displayed on a dark blue screen at the head of the scanner via a 45° angled mirror fixed to the MRI head coil. They responded by pressing a button, using their right index finger, on a button box held in their right hand. Paradigm programming, stimuli display and responses logging were done using E-prime (Psychology Software Tools, Pittsburgh, PA, USA).
The children were instructed to respond as quickly and accurately as possible when a green spaceship appeared on the screen and to withhold their response when a red spaceship appeared on the screen. The task consisted of 4 runs, each run lasting approximately 4 min, and contained a total of 40 trials, presented in a pseudorandomized order. Each trial started with a white crosshair fixation point remaining for 2.9 s on average (jittered with SD of 928 ms, min duration 674 ms and max duration 5309 ms). Then, the stimulus was presented until the child pressed the response button within a limit of 500 ms. Finally, the trial ended with a blank screen remaining between 2.5 and 3 s, to complete exactly 3 s with the presentation of the stimulus (Fig. 1). Two runs were performed in the GO context, with 75% of go trials and 25% of no-go trials, and the two others in the NOGO context, with the opposite proportions (75% of no-go trials and 25% of go trials). Three rest periods in which a friendly shooting star appeared on screen for 15 s separated the four runs. The order of the runs was counterbalanced across participants.
Fig. 1.
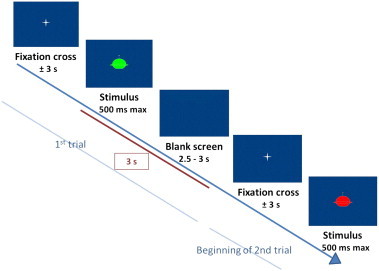
Time course of a trial.
2.3. Scanning procedure
Scanning was performed using a 3 Tesla system with a 12-channel receiver head coil (Magnetom Tim-Trio, Siemens Medical Solutions, Erlangen, Germany). High resolution 3D anatomical images were collected for each participant using a 3D MPRAGE sequence (TR = 2500 ms, TE = 2.98 ms, TI = 1100 ms, flip angle = 9°, 0.9 × 0.9 mm2 resolution, FOV = 230 mm, 0.9 mm slice thickness) acquired in the sagittal orientation. For the functional scans, echo-planar imaging (EPI) blood oxygenated level-dependent (BOLD) images were acquired (repetition time [TR] = 2000 ms, echo time [TE] = 30 ms, 30 axial slices, slice thickness = 4 mm, no spacing between slices, flip angle = 85°, matrix size 128 × 110, field of view [FOV] = 220 mm). Subject's head was stabilized with cushions to minimize motion. The anatomical images were first collected in 6 min during which the participants were shown a Tom & Jerry cartoon. The functional images were then collected in 20 min.
2.4. fMRI data analysis
Matlab (Mathworks, Natick, MA, USA) and SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK: http://www.fil.ion.ucl.ac.uk/spm/) were used to process and analyze the functional data. Images were motion corrected using rigid-body realignment. Estimated motion parameters were examined on a subject-by-subject basis and subjects demonstrating a rate of corrupted scans greater than 20% were excluded (N = 7). Slice time correction was conducted with the use of the middle slice as reference. Image slices were acquired in descending order. T1-weighted anatomical image of each individual was then coregistered to the mean realigned functional images. Realigned and slice-timed images were segmented and spatially normalized to a pediatric template corresponding to 6 years old, created with the Template-o-Matic toolbox (Wilke et al., 2008). The normalized images were then spatially smoothed using a Gaussian kernel of 8 × 8 × 8 mm3.
The fit of the data to a general linear model (Friston et al., 1994) was constructed and examined using SPM5. A statistical random effects two-stage analysis was performed on successful trials. First, voxelwise t-maps were computed for each individual. Activation in each voxel was contrasted with an implicit baseline corresponding to time between trials and rest periods. Both conditions (go and no-go) in both contexts (GO and NOGO) were computed resulting in four conditions per subject: go vs. baseline in context GO (go_GO), condition go vs. baseline in context NOGO (go_NOGO), condition no-go vs. baseline in context GO (nogo_GO) and condition no-go vs. baseline in context NOGO (nogo_NOGO).
At a second-level, random-effects of these maps were analyzed for hypothesis testing. The group contrasts employed a statistical threshold of p < .001, uncorrected for multiple comparisons, with an extent threshold of six contiguous voxels. Within group contrasts for IUGR and controls in conjunction (Nichols et al., 2005) and then separately were performed. In order to perform between groups analyses with regions of interest (ROIs), we extracted mean first eigenvariates from the regions that showed activation in the IUGR or the control group at a statistical threshold p < .001, uncorrected, with an extent threshold of six contiguous voxels for the corresponding contrast. ROIs were centered at the peak statistical value and defined on a sphere of 5 mm radius. Finally, in the purpose of confirming the finding that several regions were more activated in one group than the other, whole-brain between groups analysis, still separated by context, were performed with two-sample t-tests.
2.5. Behavioral data analysis
Mean reaction times and error rate were examined for each participant. Errors were computed according to its type: omission (failure to respond to “go” stimuli) or false alarm (failure to inhibit responding to “no-go” stimuli). Errors were then divided according to context (GO vs. NOGO). Behavioral data were analyzed using Student's tests or non-parametrical Mann–Whitney U-tests.
3. Results
3.1. Behavioral results
Despite a tendency for IUGR children to commit an overall greater number of errors, especially in omissions, the IUGR and control groups did not significantly differ in task performance in any of the dependent variables, separated or not by context. Additionally, there was no difference between groups in reaction time to “go” stimuli (all p > .05). Performances are reported in Table 2.
Table 2.
Behavioral data for IUGR and control children.
| Mean (SE) |
Statistics | p-Value | ||
|---|---|---|---|---|
| IUGR | Controls | |||
| % Omission errors | 11.9 (± 3.9) | 4.6 (± 1.2) | z = − 1.21 | .223 |
| % False alarm errors (FA) | 10.9 (± 2.6) | 9.1 (± 1.8) | z = − 0.35 | .730 |
| % Omissions context GO | 11.8 (± 4.4) | 4.3 (± 1.3) | z = − 1.30 | .195 |
| % Omissions context NOGO | 12.0 (± 4.8) | 5.5 (± 2.3) | z = − 0.91 | .362 |
| % FA context GO | 17.5 (± 2.7) | 23.0 (± 4.5) | t = − 1.04 | .313 |
| % FA context NOGO | 8.7 (± 3.3) | 4.5 (± 1.1) | z = − 0.73 | .467 |
| Reaction time (ms) | 652.9 (± 52.1) | 664.5 (± 29.4) | t = 0.20 | .849 |
Threshold of significance: p < .05 (Student's t-tests — t, or Mann–Whitney U-tests — z). SE = standard error.
3.2. Brain activation
As IUGR children committed a greater number of errors compared to controls, the differences in the number of event-related trials analyzed might have increased the variability of the results. However, there were no significant differences in the number of event-related trials analyzed per group.
3.2.1. Within group analyses
Firstly, group conjunction analyses were used to identify regions commonly activated in both groups of children. All the conditions were compared to baseline. The execution condition in both contexts (go_GO and go_NOGO contrasts) was associated with left activation in the primary sensorimotor cortex (BA2/3/4) and bilateral activation in the area including supplementary motor area (SMA, BA6) and cingulate gyrus (BA24/32) (Table 3). None of these regions were significantly more activated in one group than the other. In the inhibition conditions (nogo_GO and nogo_NOGO contrasts), groups did not share activation.
Table 3.
Activation foci for control & IUGR children for go contrasts.
| Region | Hem | BA | Peak location | t(peak voxel) | Cluster level | ||
|---|---|---|---|---|---|---|---|
| IUGR & Controls (Conjunction) | |||||||
| Go_GO | |||||||
| Primary motor & sensorimotor cortex | L | 2,3,4 | − 45 | − 15 | 54 | 5.20 | 270 |
| Cingulate gyrus/SMA | B | 6,24,32 | 3 | 9 | 48 | 4.12 | 17 |
| Medial frontal gyrus-SMA/cingulate gyrus | L | 6,24,31 | − 6 | − 6 | 51 | 3.91 | 7 |
| Go_NOGO | |||||||
| Primary motor & sensorimotor cortex | L | 2,3,4 | − 39 | − 27 | 57 | 5.28 | 44 |
| Cingulate gyrus/SMA | B | 6,24,32 | 3 | 6 | 51 | 4.94 | 63 |
Activation at statistic threshold p < .001 (≥ 6 voxels). Coordinates are in MNI space. Hem = hemisphere. BA = Broadmann Area. B = bilateral (both L and R).
Second, within group analyses were performed to identify regions significantly activated in each group. The go_GO contrast was associated with activation of the right inferior parietal lobule (BA40) in IUGR participants whereas in controls it was associated with activation in the right lateral globus pallidus. In the go_NOGO contrast (Fig. 2), IUGR children displayed activation in the right posterior cerebellum, bilateral putamen, paracentral lobule (BA5), premotor cortex (BA5/6) and left precuneus (BA7). In control children this contrast was associated with activations in the inferior frontal (BA44) and precentral gyri (BA6) bilaterally, insula (BA13), left superior temporal gyrus (BA41), and right lateral globus pallidus (Table 4). During the nogo_GO contrast, activation survived neither the statistical threshold of p < .001 uncorrected nor the more lenient threshold of p < .005 with the extent threshold of 6 contiguous voxels. In IUGR children, the nogo_NOGO contrast (Fig. 3) was associated with activation in the right paracentral lobule (BA4/5) and SMA (BA6), bilaterally in the area including cingulate gyrus (BA24/32) and SMA (BA6) and in the putamen. At the more lenient threshold of p < .005 uncorrected with 6 contiguous voxels, controls showed activation in the left pre-SMA (BA6) and a left cerebral region including the inferior frontal cortex (BA44), superior temporal gyrus (BA22) and insula (BA 13). Results are reported in Table 5.
Fig. 2.
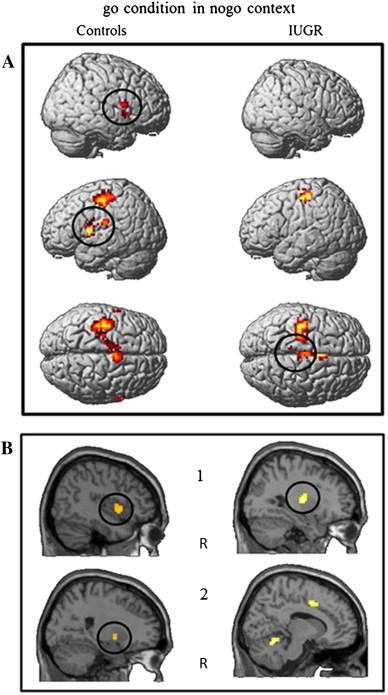
Within-group contrasts for activation in go condition in a context of inhibition. Activation at statistic threshold p < .001 (≥ 6 voxels) for controls (left) and IUGR (right). (A) 3-Dimensional reconstructed brain images, (B) sagittal views. R = right hemisphere. Black circles = regions where group differences are significant. For both groups, go_NOGO related activation is seen in the left primary motor and sensorimotor cortex and the SMA. In control children, additional go-NOGO activation is visible in the frontal lobe and superior temporal gyrus (A), with significantly higher effects in the inferior frontal and precentral gyri (A) as well as in the right insula (A, B1) and right lateral globus pallidus (B2). In IUGR children, additional activation is visible in the right posterior cerebellum (B2) and significantly higher effects are seen in premotor and parietal cortex (A) as well as in the putamen (B1).
Table 4.
Within-group activation foci (ROI) for controls & IUGR for go contrasts and between-group differences.
| t-Tests within group |
t-Tests between groups |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Hem | BA | Peak location: ROI | t(peak voxel) | Cluster level | IUGR (Mean ± SD) |
Controls (Mean ± SD) |
t | p-Value | Cohen's d | ||
| IUGR | ||||||||||||
| Go_GO | ||||||||||||
| Inferior parietal lobule/supramarginal gyrus | R | 40 | 60 | − 39 | 42 | 4.52 | 18 | 1.29 ± 2.01 | 2.48 ± 1.75 | − 1.74 | .098 | − 0.67 |
| Go_NOGO | ||||||||||||
| Putamen | R | 30 | − 12 | 9 | 5.07 | 92 | 3.17 ± 2.87 | 0.78 ± 1.18 | − 2.44 | .031 | 1.15 | |
| Putamen | L | − 30 | − 15 | 3 | 4.64 | 61 | 2.17 ± 1.96 | 0.79 ± 1.13 | − 1.92 | .071 | 0.91 | |
| Posterior cerebellum | R | 12 | − 57 | − 18 | 5.43 | 53 | 2.00 ± 1.14 | 1.14 ± 1.18 | − 1.64 | .118 | 0.78 | |
| Paracentral lobule, premotor cortex | B | 5,6 | 0 | − 21 | 57 | 4.96 | 50 | 2.08 ± 1.37 | 0.27 ± 1.33 | − 2.93 | .008 | 1.41 |
| Paracentral lobule, precuneus | L | 5,7 | − 9 | − 36 | 60 | 4.20 | 6 | 1.52 ± 0.95 | 0.04 ± 1.34 | − 2.95 | .011 | 1.34 |
| Controls | ||||||||||||
| Go_GO | ||||||||||||
| Lateral globus pallidus | R | 21 | − 3 | 0 | 3.87 | 7 | 0.15 ± 0.79 | 2.2 ± 2.33 | 2.63 | .023 | − 1.24 | |
| Go_NOGO | ||||||||||||
| Inferior frontal gyrus, precentral gyrus | L | 44,6 | − 60 | 6 | 12 | 6.67 | 154 | 2.54 ± 1.02 | 2.45 ± 1.39 | 1.68 | .110 | 0.08 |
| Inferior frontal gyrus, precentral gyrus | R | 44/45,6 | 63 | 9 | 12 | 7.22 | 24 | 1.14 ± 0.94 | 2.78 ± 1.64 | 2.78 | .012 | − 1.29 |
| Superior temporal gyrus, insula | L | 41,13 | − 51 | − 21 | 21 | 5.54 | 36 | 1.22 ± 1.57 | 2.47 ± 1.50 | 1.82 | .085 | − 0.86 |
| Insula | R | 13 | 42 | 6 | − 3 | 4.21 | 36 | 0.88 ± 2.57 | 3.80 ± 3.19 | 2.25 | .037 | − 1.06 |
| Lateral globus pallidus | R | 24 | 0 | − 6 | 4.02 | 10 | 1.29 ± 2.03 | 3.05 ± 1.12 | 2.24 | .038 | − 1.13 | |
Activation at statistic threshold p < .001 (≥ 6 voxels). Group differences significant at p < .05 (in bold) in SPSS version 19. Coordinates are in MNI space. Hem = hemisphere. BA = Broadmann Area. B = bilateral (both L and R).
Fig. 3.
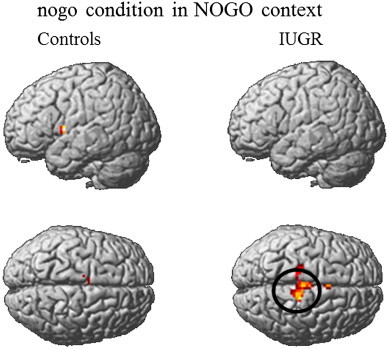
Within-group contrasts for activation in nogo condition in a context of inhibition. Activation at statistic threshold p < .005 (≥ 6 voxels) for controls (left) and activation at statistic threshold p < .001 (≥ 6 voxels) for IUGR (right). Black circle = region where group differences are significant. In control children, left activation is seen in the region including the inferior frontal cortex, superior temporal gyrus and insula and in the medial frontal and cingulate gyri. In IUGR children, activation is visible in the medial frontal and cingulate gyri and significantly higher effects are visible in the right medial frontal region including paracentral lobule and SMA.
Table 5.
Within-group activation foci (ROI) for controls & IUGR for nogo_NOGO contrast and between-group differences.
| t-Tests within group |
t-Tests between groups |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Hem | BA | Peak location: ROI | t(peak voxel) | Cluster level | IUGR (Mean ± SD) |
Controls (Mean ± SD) |
t | p-Value | Cohen's d | ||
| IUGR | ||||||||||||
| Nogo _NOGOa | ||||||||||||
| Medial frontal gyrus/SMA, paracentral lobule | R | 4,5,6 | 9 | − 30 | 63 | 5.71 | 117 | 1.60 ± 0.87 | 0.06 ± 1.02 | − 3.62 | .002 | 1.72 |
| Cingulate gyrus, SMA | B | 6,24,32 | 3 | 9 | 45 | 4.35 | 26 | 2.70 ± 2.31 | 1.28 ± 1.73 | − 1.56 | .136 | 0.73 |
| Putamen | B | 30 | − 9 | 6 | 4.24 | 13 | 2.81 ± 2.57 | 0.31 ± 1.39 | − 2.71 | .014 | 1.28 | |
| Controls | ||||||||||||
| Nogo _NOGOb | ||||||||||||
| Medial frontal gyrus/SMA, cingulate gyrus | L | 6,31 | − 12 | − 12 | 54 | 4.35 | 19 | |||||
| Superior temporal gyrus, IFC, insula | L | 22,44,13 | − 48 | 3 | 6 | 3.40 | 17 | |||||
Group differences significant at p < .05 (in bold) in SPSS version 19. Coordinates are in MNI space. Hem = hemisphere. BA = Broadmann Area. B = bilateral (both L and R).
Activation at statistic threshold p < .001 (≥ 6 voxels).
Activation at statistic threshold p < .005 (≥ 6 voxels).
3.2.2. Between groups analysis
Between groups analyses with ROI enabled the identification of the regions significantly more activated in one group than the other. All peak values of the regions activated within the IUGR or control group at the voxel threshold of p < .001 uncorrected and a cluster threshold of 6 were taken as ROIs (15 in total).
In the go conditions, 12 ROIs were identified; 2 in the execution context and 10 in the inhibition context (see Table 4). In the go_GO contrast, control children demonstrated stronger activation in lateral globus pallidus compared to the IUGR. In the go_NOGO contrast, compared to controls, IUGR participants showed increased activation in the right putamen and in regions extending from the paracentral lobule to the premotor cortex in the right hemisphere, and to the precuneus in the left hemisphere. The control group demonstrated greater right activation in the region from the inferior frontal gyrus to the precentral gyrus, the insula and the lateral globus pallidus.
In the nogo conditions, 3 ROIs were identified at current statistical threshold; all in the inhibition context and within the IUGR group (see Table 5). In the nogo_NOGO contrast, the IUGR group displayed greater activation relative to controls in the ROIs in the region encompassing the right paracentral lobule and SMA and in the putamen but no significant group difference in activation was found in the region including bilateral cingulate gyrus and SMA.
Additionally, whole-brain between groups analysis was performed for each of the four contrasts (Table 6, Fig. 4) to confirm the presence of group differences in activation (Tables 4 & 5). The group differences were sometimes due to positive effect (above baseline) of one group and sometimes due to negative effect (below baseline) of the other group. Four main regions showing group differences could be identified: (1) a superior bilateral fronto-parietal region encompassing medial frontal gyrus, SMA (BA6) and paracentral lobule (BA5), (2) the right parieto-temporal junction (BA13), (3) a right fronto-temporal region encompassing the inferior frontal gyrus (BA44), superior temporal gyrus (BA22), and insula (BA13), and (4) a posterior region straddling the parietal lobule and precuneus (BA7/19). Confirming ROI analysis, the fronto-parietal region was more activated in IUGR compared to controls, in the go_NOGO and nogo_NOGO contrasts. The right parieto-temporal junction was activated in IUGR and deactivated in controls in the go_NOGO and nogo_NOGO contrasts. Also confirming ROI analyses, activation in the inferior frontal region was significantly greater in controls than IUGR during the go_NOGO contrast. The right posterior parietal region was activated in IUGR and deactivated in controls in the go_GO and nogo_GO contrasts. The left posterior parietal region was also deactivated in IUGR during the go_NOGO contrast at the lower statistical threshold of p < .005, uncorrected, with an extent threshold of six contiguous voxels.
Table 6.
Between-group activation foci for each contrast.
| Region | Hem | BA | Peak location | t(peak voxel) | Cluster level | IUGR | Controls | ||
|---|---|---|---|---|---|---|---|---|---|
| IUGR > Controls | |||||||||
| Go_GO | |||||||||
| Inferior/superior parietal lobule/precuneusa | R | 7 | 30 | − 54 | 45 | 4.78 | 39 | ↑ | ↓ |
| Go_NOGO | |||||||||
| Medial frontal gyrus-SMA/paracentral lobuleb | B | 5,6,7 | 0 | − 21 | 60 | 3.28 | 46 | ↑ | − |
| Parieto-temporal junctionb | R | 48 | − 42 | 21 | 3.56 | 19 | ↑ | ↓ | |
| Nogo_GO | |||||||||
| Superior parietal lobule/precuneusb | R | 19,7 | 27 | − 66 | 48 | 3.53 | 19 | ↑ | ↓ |
| Nogo _NOGO | |||||||||
| Medial frontal gyrus-SMA/paracentral lobulea | B | 4,5,6 | 3 | − 27 | 60 | 4.52 | 30 | ↑ | − |
| Parieto-temporal junctionb | R | 48 | − 42 | 21 | 3.40 | 9 | ↑ | ↓ | |
| Controls > IUGR | |||||||||
| Go_NOGO | |||||||||
| Superior temporal gyrus/IFC/insulaa | R | 22,44,13 | 52 | − 6 | − 2 | 4.95 | 56 | − | ↑ |
| Superior parietal lobule/precuneusb | L | 19,7 | − 27 | − 75 | 48 | 3.67 | 22 | ↓ | − |
Coordinates are in MNI space. Hem = hemisphere. BA = Broadmann Area. ↑ = activation, ↓ = deactivation, − = close to “0”. B = bilateral (both L and R).
Activation at statistic threshold p < .001 (≥ 6 voxels).
Activation at statistic threshold p < .005 (≥ 6 voxels).
Fig. 4.
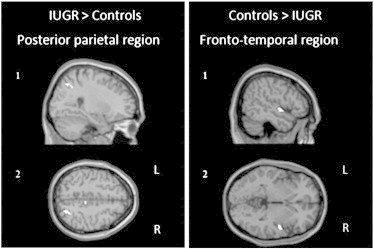
Between-group major positive contrasts. Main activation at statistic threshold p < .001 (≥ 6 voxels). (1) Sagittal views, (2) axial views. R = right hemisphere, L = left hemisphere. IUGR > Controls: activation of the superior parietal lobule/precuneus, region activated both in go and nogo conditions in Go context. Controls > IUGR: activation of the superior temporal gyrus/insula/inferior frontal cortex, region activated in go condition in NOGO context.
4. Discussion
In the current study, functional activations of young children born preterm with and without IUGR, while performing a Go/No-go task, were presented. As hypothesized, the results indicated that IUGR children displayed different patterns of activation in selective brain areas compared with controls. Globally, these results showed that IUGR children seem to rely on more posterior, parietal regions and on the putamen in a context of inhibition while control children seem to preferentially activate fronto-temporal regions including the IFC, superior temporal gyrus and insula as well as the globus pallidus. In addition, IUGR children showed more activation during inhibition conditions compared with controls and a tendency to a higher error rate, which may imply the task to be more demanding for these children (Casey et al., 1997a).
We will first discuss performances and regions activated in both groups together. Secondly, group differences of activation will be discussed, starting with results regarding task particularity. We will then focus on results of our control group, based on ROI and whole-brain between groups analyses, and discuss them in the light of previous research with different populations. Finally, results concerning the IUGR group (ROI, whole-brain between groups analyses and significant correlations with performance) will be discussed.
4.1. Task performances and joint activation underlying response execution
On a behavioral level, even though no significant between-group differences concerning task performances were observed, the IUGR group tended to commit a greater number of errors, as hypothesized. The IUGR group also presented with higher response variability compared to controls. This is consistent with previous studies in which greater response variability has been identified in clinical groups with frontal brain deficits and has been associated with more immature pattern of activation (Braet et al., 2009; Bunge et al., 2002).
Regarding activations related to go processes (response execution), the regions activated in both groups, independently of context, are in accordance with previous studies with children (Durston et al., 2002, 2006; Suskauer et al., 2008). In these studies, activity in left primary motor and sensorimotor cortex has been related to the control of execution and planning of movement. Furthermore, the left pattern of activation was expected as our participants responded with their right hand. Also, the activation we observed in the bilateral field from the ACC to the SMA has been related to the preparation for visually cued movements of the finger of the right hand, which had to press the button (Watanabe et al., 2002). According to Mostofsky and colleagues, the caudal (SMA proper) and rostral (pre-SMA) portions of the SMA have both a role in the execution of response; in the motor act of executing the response and in the selection of response, respectively (Mostofsky et al., 2003), while the ACC may play an error monitoring function, providing performance feed-back to the pre-SMA (Mostofsky and Simmonds, 2008). In conclusion, these go-related regions activated in both groups seem to play a role in preparation and execution of motor response. In contrast, regarding no-go processes (response inhibition), the two groups did not share any activation, as revealed by conjunction analysis.
4.2. Direct activation comparison between IUGR and control preterm children
The overall results showed less brain activation related to no-go events than to go events in both groups and this was more pronounced in control children. Similarly, a lack of brain activation related to inhibition performances (nogo > neutral) has been found in a group of 16 children ages 8 to 12 by Bunge et al. (2002) where no activation had survived the statistical threshold of p < .001 uncorrected. However, this is inconsistent with most previous Go/No-go studies (Abdullaev et al., 2012; Braet et al., 2009; Casey et al., 1997b; Durston et al., 2002, 2006; Suskauer et al., 2008), which have found major activation related to response inhibition (in a classical context of execution). This inconsistency may be based on the specificity of our task, designed in the purpose of controlling for the oddball character of the no-go stimuli. As a matter of fact, our results revealed a particular pattern of activation: most activation in each group was found in a context of inhibition. In this context, the child had to inhibit his response in 75% of the trials and actually execute his response in 25% of the trials. It is thus possible that this specific design created a “prepotent nonresponse” as well as a prepotent response, so making the inhibition process as automatic as the execution process (Verbruggen and Logan, 2008). Finally, the lack of differences we found between go- and no-go-related activation in inhibition context in each group is consistent with previous theories which have suggested that the neural correlates of preparing to inhibit a response were quite similar to the neural correlates of actually inhibiting a response (Goghari and MacDonald, 2009; Miller and Cohen, 2001).
4.2.1. Predominant activation in control preterm children
When the two groups were compared, control children exhibited increased activation in IFC (pars opercularis), precentral gyrus as well as insula and superior temporal gyrus in a context of inhibition and particularly in the right hemisphere. Control children also demonstrated increased go-related activation in the right globus pallidus in both contexts. Similar activations have been reported in previous Go/No-go studies with typically developing children (Abdullaev et al., 2012; Durston et al., 2002, 2006; Suskauer et al., 2008). Interestingly, prefrontal and temporal areas have been related to successful inhibition, (e.g. Rubia et al., 2003) while in our study it was related to successful execution in inhibition context. Even though previous research in healthy population have highlighted the important role of the IFC, when inhibition is required (Casey et al., 1997b; Durston et al., 2002; Luna et al., 2001; Tamm et al., 2002), recent research have postulated that the IFC region plays an important role in the executive control of updating and selection of actions; so it could be activated by cues to update the plan of behavior, rather than cues specific to response inhibition (Smith et al., 2013; Verbruggen et al., 2010). Following this theory, in the present study, the main plan was to inhibit a response and children had to activate an alternative plan, so they activated the IFC, when a go stimulus was presented.
The prefrontal cortex is thought to be involved in response inhibition early in childhood, but its functional development continues into adulthood (e.g. Adleman et al., 2002; Aron and Poldrack, 2005; Bunge et al., 2002; Rubia et al., 2000; Tamm et al., 2002). This prolonged maturational course makes it more sensitive to developmental perturbations (Taylor et al., 2011). We therefore suggest that the lack of prefrontal activation found in IUGR children compared to controls could translate to a less mature pattern of response selection. In a study by Nosarti et al. (2006), adolescents born preterm have shown decreased activation in the IFC (BA44,45) and in the globus pallidus. The authors have concluded to a rerouting of function to compensate for a possibly altered neurodevelopment. In the present study, these regions were greatly activated in control preterm compared to IUGR preterm children so we could argue that a lack of activation in those regions is even more important when prematurity is associated with IUGR. Nevertheless, a further comparison with Nosarti et al.'s results is difficult as they have not reported go-related activation and have not provided specific information about IUGR in their preterm population.
4.2.2. Predominant activation in IUGR preterm children
When the two groups were compared, IUGR children showed increased bilateral activation in regions comprising the medial frontal gyrus and SMA, paracentral lobule, precuneus and go-related activation in the right inferior parietal lobule in a context of inhibition. This is consistent with Lawrence et al. (2009) who have shown that the precuneus was more activated in adults born preterm compared to adults born at term in inhibition condition. Our results also revealed that in the context of execution where IUGR activated posterior parietal regions (comprising the superior parietal lobule and precuneus), controls seemed to deactivate it. In typically developing population, parietal regions and the medial frontal gyrus were found to be more activated in children than adults during successful response inhibition (Braet et al., 2009; Durston et al., 2002; Tamm et al., 2002). However, a previous study among healthy adolescents and adults has reported that decreased activity in the precuneus and inferior parietal lobule was associated with successful response inhibition (Stevens et al., 2007). The medial, lateral and inferior parietal cortex as well as the precuneus and medial frontal cortex have been identified as regions being part of the default mode network (Raichle et al., 2001; Sonuga-Barke and Castellanos, 2007) which engagement has been negatively related to response inhibition ability (Congdon et al., 2010). The default mode network being usually activated during resting cognition and deactivated during cognitive tasks (Binder, 2012), it has been suggested that brain regions deactivate when the required attentional resources that maintain them active are needed for processing other information (McKiernan et al., 2003). Thus, increased activation or attenuated deactivation of this network during task performance was thought to underlie impaired attentional control (Congdon et al., 2010; Sonuga-Barke and Castellanos, 2007). Following this idea, the fact that IUGR children recruited several regions known to be part of the default-mode network and therefore interfere with goal-directed activity may express an inability to reallocate their attention resources in order to focus on the task. This hypothesis is reinforced with the fact that inferior and superior parietal cortex have been reported to activate in more complex response inhibition (Simmonds et al., 2008) or attention demanding tasks (Garavan et al., 1999).
Results finally showed that the increase of activation in inhibition context found in IUGR in the putamen and in the region encompassing SMA and paracentral lobule during no-go trials was negatively correlated to accuracy. These findings corroborate those of Congdon et al. (2010) who have postulated that individuals with poorer response inhibition had increased activation in regions responsible for the execution of a motor response, including SMA and putamen. Overall, these results are in favor of attentional control difficulties that seem to influence poorer response selection (execution and inhibition) in IUGR preterm children compared to control preterm children.
4.3. Limitations
Differences in brain activation in the presence of normal cognitive development were found in former studies with premature population (Lawrence et al., 2009; Ment and Constable, 2007; Nosarti et al., 2006). However, the lack of significant group differences in performance in our study seems inconsistent with previous research with IUGR population (e.g. Geva et al., 2006). Nevertheless, given the large variability within each group, the lack of group differences in accuracy could be due to a lack of statistical power due to the small number of participants in each group. This small case numbers and the lack of paired children born at term are mainly caused by difficulty to recruit and test 6- and 7-year-old children for fMRI studies. In addition, in order to ensure adequate data quality, almost half of the subjects were excluded from analyses, due to important head motion or task interruption.
4.4. Conclusion
Our results showed that preterm children with IUGR exhibited higher variability in executing or inhibiting a response compared to preterm children without IUGR, and demonstrated decreased reliance on regions classically involved in successful response inhibition, like the inferior frontal cortex. IUGR children also showed greater activation in the medial frontal cortex and in posterior and parietal regions, like the precuneus and parietal lobules, regions known to be either part of the default-mode network or specifically involved in attention demanding tasks. fMRI studies using a Go/No-go task with preterm population have reported their dependence on an alternative neuronal pathway to compensate for a possible altered or delayed neurodevelopment. Together, our findings suggest the use of different brain regions or a more diffuse network in IUGR children to execute or inhibit a response in a context of inhibition, which reflects impaired attentional control mechanism. This study shows that preterm children do not form a homogenous population when it comes to brain functions, and specific factors related to prematurity, like IUGR, should be considered to identify the preterm children more at risk for later neurodevelopmental disorders.
Acknowledgments
This work was supported by the Swiss National Science Foundation (PI PS Hüppi No 32000B0-113632), the Swiss Confederation Grant (IP K. Barisnikov, No UN7528), the Leenaards Foundation, and the Center for Biomedical Imaging (CIBM). The authors thank Stéphane Simon, Martial Van der Linden and Andres Posada for their precious help in building this study and Arnaud Saj for his constructive suggestions.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Note: The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
See fMRI data analysis.
Contributor Information
Morgane Réveillon, Email: Morgane.Reveillon@unige.ch.
Sébastien Urben, Email: Sebastien.Urben@chuv.ch.
Koviljka Barisnikov, Email: Koviljka.Barisnikov@unige.ch.
Cristina Borradori Tolsa, Email: Cristina.BorradoriTolsa@hcuge.ch.
Petra S. Hüppi, Email: Petra.Huppi@hcuge.ch.
François Lazeyras, Email: Francois.Lazeyras@unige.ch.
References
- Aarnoudse-Moens C.S.H., Duivenvoorden H.J., Weisglas-Kuperus N., Van Goudoever J.B., Oosterlaan J. The profile of executive function in very preterm children at 4 to 12 years. Dev. Med. Child Neurol. 2012;54:247–253. doi: 10.1111/j.1469-8749.2011.04150.x. [DOI] [PubMed] [Google Scholar]
- Abdullaev Y., Bruce J., Fisher P.A. Functional MRI of brain activity during response inhibition in children. Int. J. Psychophysiol. 2012;85:366. [Google Scholar]
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the Stroop color–word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Anderson P.J., Doyle L.W. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- Arbuckle T.E., Wilkins R., Sherman G.J. Birth weight percentiles by gestational age in Canada. Obstet. Gynecol. 1993;81:39–48. [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Arpino C., Compagnone E., Montanaro M.L., Cacciatore D., De Luca A., Cerulli A., Di Girolamo S., Curatolo P. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv. Syst. 2010;26:1139–1149. doi: 10.1007/s00381-010-1125-y. [DOI] [PubMed] [Google Scholar]
- Baschat A.A. Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011;37:501–514. doi: 10.1002/uog.9008. [DOI] [PubMed] [Google Scholar]
- Binder J.R. Task-induced deactivation and the “resting” state. Neuroimage. 2012;62:1086–1091. doi: 10.1016/j.neuroimage.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm B., Katz-Salamon M., Institute K., Smedler A.C., Lagercrantz H., Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birth weight children. Dev. Med. Child Neurol. 2002;44:508–516. doi: 10.1017/s001216220100247x. [DOI] [PubMed] [Google Scholar]
- Böhm B., Smedler A.C., Forssberg H. Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatr. 2004;93:1363–1371. doi: 10.1080/08035250410021379. [DOI] [PubMed] [Google Scholar]
- Borradori Tolsa C., Zimine S., Warfield S.K., Freschi M., Sancho Rossignol A., Lazeyras F., Hanquinet S., Pfizenmaier M., Huppi P.S. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr. Res. 2004;56:132–138. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]
- Braet W., Johnson K.A., Tobin C.T., Acheson R., Bellgrove M.A., Robertson I.H., Garavan H. Functional developmental changes underlying response inhibition and error-detection processes. Neuropsychologia. 2009;47:3143–3151. doi: 10.1016/j.neuropsychologia.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Castellanos F.X., Giedd J.N., Marsh W.L., Hamburger S.D., Schubert A.B., Vauss Y.C., Vaituzis A.C., Dickstein D.P., Sarfatti S.E. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Am. Acad. Child Adolesc. Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R.J., Orendi J.L., Schubert A.B., Nystrom L.E., Giedd J.N., Castellanos F.X., Haxby J.V., Noll D.C., Cohen J.D. A developmental functional MRI study of prefrontal activation during performance of a Go–No-Go task. J. Cogn. Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Congdon E., Mumford J.A., Cohen J.R., Galvan A., Aron A.R., Xue G., Miller E., Poldrack R.A. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders F.C. On the speed of mental processes. In: Koster W.G., editor. Attention and Performance II. North Holland; Amsterdam: 1969. pp. 412–431. (Original work published in 1868) [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Uluğ A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002;5:F9–F16. [Google Scholar]
- Durston S., Mulder M., Casey B.J., Ziermans T., van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol. Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva R., Eshel R., Leitner Y., Valevski A.F., Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118:91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- Goghari V.M., MacDonald A.W., III The neural basis of cognitive control: response selection and inhibition. Brain Cogn. 2009;71:72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M., Taylor H.G., Schluchter M., Andreias L., Drotar D., Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J. Dev. Behav. Pediatr. 2009;30:122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011;69:11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- Kaufman A., Kaufman N. ECPA; Paris: 1993. Batterie pour l'examen psychologique de l'enfant. [Google Scholar]
- Kok J.H., Lya den Ouden A., Verloove-Vanhorick S.P., Brand R. Outcome of very preterm small for gestational age infants: the first nine years of life. BJOG. 1998;105:162–168. doi: 10.1111/j.1471-0528.1998.tb10046.x. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H. Better born too soon than too small. Lancet. 1997;350:1044–1045. doi: 10.1016/S0140-6736(05)70450-5. [DOI] [PubMed] [Google Scholar]
- Lawrence E.J., Rubia K., Murray R.M., McGuire P.K., Walshe M., Allin M., Giampietro V., Rifkin L., Williams S.C.R., Nosarti C. The neural basis of response inhibition and attention allocation as mediated by gestational age. Hum. Brain Mapp. 2009;30:1038–1050. doi: 10.1002/hbm.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle P.F., Kiehl K.A., Smith A.M. Event-related fMRI study of response inhibition. Hum. Brain Mapp. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann. N. Y. Acad. Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J., Keshavan M.S., Genovese C.R., Eddy W.F., Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Ment L.R., Constable R.T. Injury and recovery in the developing brain: evidence from functional MRI studies of prematurely born children. Nat. Clin. Pract. Neurol. 2007;3:558–571. doi: 10.1038/ncpneuro0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognit. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morsing E., Asard M., Ley D., Stjernqvist K., Marsal K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics. 2011;127:e874–e882. doi: 10.1542/peds.2010-1821. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Simmonds D.J. Response inhibition and response selection: two sides of the same coin. J. Cogn. Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Schafer J.G., Abrams M.T., Goldberg M.C., Flower A.A., Boyce A., Courtney S.M., Calhoun V.D., Kraut M.A., Denckla M.B. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res. Cogn. Brain Res. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Mulder H., Pitchford N.J., Hagger M.S., Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev. Neuropsychol. 2009;34:393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nigg J.T. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nosarti C., Rubia K., Smith A.B., Frearson S., Williams S.C., Rifkin L., Murray R.M. Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Dev. Med. Child Neurol. 2006;48:265–271. doi: 10.1017/S0012162206000582. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Andrew C., Bullmore E.T. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci. Biobehav. Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Schreuder A.M., McDonnell M., Gaffney G., Johnson A., Hope P.L. Outcome at school age following antenatal detection of absent or reversed end diastolic flow velocity in the umbilical artery. Arch. Dis. Child. Fetal Neonatal Ed. 2002;86:F108–F114. doi: 10.1136/fn.86.2.F108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Kingdom J. Long-term neurocognitive outcomes of SGA/IUGR infants. Obstet. Gynaecol. Reprod. Med. 2011;21:142–146. [Google Scholar]
- Simmonds D.J., Pekar J.J., Mostofsky S.H. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.L., Jamadar S., Provost A.L., Michie P.T. Motor and non-motor inhibition in the Go/NoGo task: an ERP and fMRI study. Int. J. Psychophysiol. 2013;87(3):244–253. doi: 10.1016/j.ijpsycho.2012.07.185. [DOI] [PubMed] [Google Scholar]
- Sonesson S.E., Fouron J.C., Drblik S.P., Tawile C., Lessard M., Skoll A., Guertin M.C., Ducharme G.R. Reference values for Doppler velocimetric indices from the fetal and placental ends of the umbilical artery during normal pregnancy. J. Clin. Ultrasound. 1993;21:317–324. doi: 10.1002/jcu.1870210505. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S., Padilla N., Zubiaurre-Elorza L., Ibarretxe-Bilbao N., Botet F., Costas-Moragas C., Falcon C., Bargallo N., Mercader J.M., Junque C. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:E1161–E1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Kiehl K.A., Pearlson G.D., Calhoun V.D. Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Fotedar S., Blankner J.G., Pekar J.J., Denckla M.B., Mostofsky S.H. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J. Cogn. Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor N.M., Jakobson L.S. Representational momentum in children born preterm and at term. Brain Cogn. 2010;72:464–471. doi: 10.1016/j.bandc.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Taylor H.G., Minich N.M., Klein N., Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J. Int. Neuropsychol. Soc. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Donner E.J., Pang E.W. fMRI and MEG in the study of typical and atypical cognitive development. Neurophysiol. Clin.Clin. Neurophysiol. 2011;42:19–25. doi: 10.1016/j.neucli.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Tideman E., Marsal K., Ley D. Cognitive function in young adults following intrauterine growth restriction with abnormal fetal aortic blood flow. Ultrasound Obstet. Gynecol. 2007;29:614–618. doi: 10.1002/uog.4042. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008;137:649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Aron A.R., Stevens M.A., Chambers C.D. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J., Sugiura M., Sato K., Sato Y., Maeda Y., Matsue Y., Fukuda H., Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wilke M., Holland S.K., Altaye M., Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–913. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]


