Abstract
Idiopathic normal pressure hydrocephalus (iNPH) is a neuropsychiatric syndrome characterized by gait disturbance, cognitive impairment and urinary incontinence that affect elderly individuals. These symptoms can potentially be reversed by cerebrospinal fluid (CSF) drainage or shunt operation. Prior to shunt operation, drainage of a small amount of CSF or “CSF tapping” is usually performed to ascertain the effect of the operation. Unfortunately, conventional neuroimaging methods such as single photon emission computed tomography (SPECT) and functional magnetic resonance imaging (fMRI), as well as electroencephalogram (EEG) power analysis seem to have failed to detect the effect of CSF tapping on brain function. In this work, we propose the use of Neuronal Activity Topography (NAT) analysis, which calculates normalized power variance (NPV) of EEG waves, to detect cortical functional changes induced by CSF tapping in iNPH. Based on clinical improvement by CSF tapping and shunt operation, we classified 24 iNPH patients into responders (N = 11) and nonresponders (N = 13), and performed both EEG power analysis and NAT analysis. We also assessed correlations between changes in NPV and changes in functional scores on gait and cognition scales before and after CSF tapping. NAT analysis showed that after CSF tapping there was a significant decrease in alpha NPV at the medial frontal cortex (FC) (Fz) in responders, while nonresponders exhibited an increase in alpha NPV at the right dorsolateral prefrontal cortex (DLPFC) (F8). Furthermore, we found correlations between cortical functional changes and clinical symptoms. In particular, delta and alpha NPV changes in the left-dorsal FC (F3) correlated with changes in gait status, while alpha and beta NPV changes in the right anterior prefrontal cortex (PFC) (Fp2) and left DLPFC (F7) as well as alpha NPV changes in the medial FC (Fz) correlated with changes in gait velocity. In addition, alpha NPV changes in the right DLPFC (F8) correlated with changes in WMS-R Mental Control scores in iNPH patients. An additional analysis combining the changes in values of alpha NPV over the left-dorsal FC (∆alpha-F3-NPV) and the medial FC (∆alpha-Fz-NPV) induced by CSF tapping (cut-off value of ∆alpha-F3-NPV + ∆alpha-Fz-NPV = 0), could correctly identified “shunt responders” and “shunt nonresponders” with a positive predictive value of 100% (10/10) and a negative predictive value of 66% (2/3). In contrast, EEG power spectral analysis showed no function related changes in cortical activity at the frontal cortex before and after CSF tapping. These results indicate that the clinical changes in gait and response suppression induced by CSF tapping in iNPH patients manifest as NPV changes, particularly in the alpha band, rather than as EEG power changes. Our findings suggest that NAT analysis can detect CSF tapping-induced functional changes in cortical activity, in a way that no other neuroimaging methods have been able to do so far, and can predict clinical response to shunt operation in patients with iNPH.
Keywords: Neuronal Activity Topography, Normalized power variance, Idiopathic normal pressure hydrocephalus, Cerebrospinal fluid tapping, Electroencephalography, Tap test
Highlights
-
•
We aimed to detect brain functional changes by CSF tapping in iNPH patients.
-
•
Alpha NPV changes in the frontal cortex correlated with brain functional changes.
-
•
These NPV changes could predict the response of shunt operation in iNPH patients.
1. Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is a neuropsychiatric disease characterized by ventriculomegaly and a classic triad including gait disturbance, cognitive impairment (i.e., dementia) and urinary incontinence. Despite the typical ventricle enlargement, there is a normal cerebrospinal pressure, and no evidence of organic lesions, such as tumor, subarachnoid hemorrhage or meningitis. Meta analyses showed that the prevalence of iNPH was 1.1% of the elderly population (older than 65 years), with about 310,000 people suffering from this disease in Japan today (Hiraoka et al., 2008; Iseki et al., 2009; Mori et al., 2012; Tanaka et al., 2009). Epidemiologic study in Norway reported that the prevalence of iNPH was 0.1% of the elderly population (Brean and Eide, 2008). Also, a clinic-based study in east Denmark showed that the prevalence of iNPH was 3.5% among a series of 400 patients referred to a memory clinic (Bech-Azeddine et al., 2001).
Idiopathic NPH is attracting much attention in clinical practice of neurology and psychiatry as it is associated with a treatable form of dementia. Overall symptoms of iNPH can improve after shunt operation. However, all iNPH patients do not necessarily show clinical improvement after surgery. Therefore, it is a relevant issue finding predictive factors of a good surgical outcome. Clinical guidelines for iNPH in Japan recommended performing “cerebrospinal fluid (CSF) tapping,” drainage of a small amount of CSF (30–50 ml) by lumbar puncture, before shunt operation to predict shunt effectiveness (Mori et al., 2012). The clinical evaluation is performed before and after CSF tapping to measure changes in symptom severity. The whole procedure is called CSF tap test. If clinical changes are observed in the three main symptoms of the disease, and judged as positive, then shunt operation should be considered. However, there is no general consensus with regard to the type of clinical evaluation to be carried out before and after CSF tapping and the cut-off scores on commonly used scales to indicate clinical improvement. In addition, CSF tap test has a high positive predictive value of shunt operation outcome but has a low negative predictive value due to a high rate of false negative results. For instance, a multicenter prospective study (Ishikawa et al., 2012) revealed that CSF tap test had a high positive predictive value of 89% but a low negative predictive value of only 36% evaluating the CSF tapping effects on the classic triad with the total score of iNPH grading scale (iNPHGS). In case of evaluating CSF tapping effects on exclusively the gait symptom with the Timed Up and Go Test (TUG), positive predictive value was 83% but negative predictive value was 21%, and in case of evaluating CSF tapping effects on exclusively the cognitive symptom with the Mini-Mental State Examination (MMSE), positive predictive value was 78% but negative predictive value was 17%. In this study, CSF tapping was considered positive if the total score of iNPHGS improved more than one point, that of the TUG test more than 10%, and that of the MMSE more than three points, respectively (Ishikawa et al., 2012). They proposed the use of pre-shunt CSF pressure with the iNPHGS total sore to assess CSF tapping response, as it increased the negative predictive value of shunt outcome to 48%. There is also a growing criticism that the clinical evaluation of the iNPH triad relies on the subjective interpretation of the observer. Thus, efforts are being made to find reliable objective measures that can identify changes in brain functions induced by CSF tapping.
Conventional neuroimaging methods seem to have failed to detect brain functional changes related to CSF tapping, drainage of a small amount of CSF (30–50 ml). In fact, no single photon emission computed tomography (SPECT) and functional magnetic resonance imaging (fMRI) studies have identified significant regional cerebral blood flow (rCBF) changes after CSF tapping or correlation between rCBF changes and symptom improvement after CSF tapping (Kristensen et al., 1996). To our knowledge, only one diffusion tensor MRI study reported changes in regional fractional anisotropy (FA) or apparent diffusion coefficient (ADC) in the frontal periventricular region and the body of corpus callosum. However, there was no correlation with symptom changes (Demura et al., 2012). In only one study where a large volume (400 ml) drainage of CSF was performed by an indwelling lumbar catheter over three days (external lumbar drainage), fMRI succeeded in detecting rCBF increase in the left dorsal premotor and bilateral supplementary motor area (SMA) in right-handed iNPH patients during motor tasks (Lenfeldt et al., 2008).
EEG appears to be a useful tool to aid in the objective clinical assessment of brain functional changes induced by CSF drainage. Unlike fMRI and SPECT that measure hemodynamic changes that occur in response to neuronal activity, neurophysiological techniques like EEG and MEG measure directly the brain electrical activity (Ishii et al., 1999; Kurimoto et al., 2012). In particular, EEG time-series data relate to dynamic postsynaptic activity in the cerebral cortex with a high temporal resolution (Canuet et al., 2011, 2012). Because of these properties, its simplicity, and noninvasiveness, EEG has been widely used in neuroscience and clinical practice. Traditionally, the visual inspection of EEG recordings and power spectral analyses has been of value in the diagnosis of epilepsy, and consciousness and cognitive disturbances including dementia. However, these types of EEG analyses have failed to identify iNPH patients (Brown and Goldensohn, 1973) and to detect significant EEG power changes or correlation with clinical improvement after CSF drainage (Sand et al., 1994).
In the present study, we aimed to find an objective neurophysiological marker of brain response to CSF tapping in iNPH patients. For that purpose, we looked at normalized power variance (NPV) of EEG waves, calculated by NAT analysis, as NPV is thought to sensitively reflect the phase-instability of cortical electrical activity (Aoki et al., 2013; Chen et al., 2012). In a previous study, by using EEG and Neuronal Activity Topography (NAT) analysis in epilepsy, we could demonstrate the high sensitivity of this method to visualize the instability of cortical electrical activity at the seizure onset zone in the pre-ictal phase and its stabilization during transition to the ictal phase (Aoki et al., 2013). We hypothesized that the EEG phase-instability is related to functional impairment and that NPV decreases and increases after CSF drainage in iNPH patients reflects functional recovery and worsening, respectively. This would indicate that NAT analysis of CSF tapping data may be useful to predict clinical response to CSF removal.
2. Methods
2.1. Subjects
Patients with right handed possible iNPH were consecutively recruited from the neuropsychological clinic at the Department of Neuropsychiatry of Osaka University Hospital from November 2004 to June 2013.
The inclusion criteria were (1) age > 60 years; (2) at least one symptom of the triad: gait disturbance, cognitive impairment and urinary disturbance; (3) dilated ventricles and narrowed CSF space at the high convexity without severe cortical atrophy on MRI; (4) absence of diseases or conditions that could cause the clinical symptoms or radiological findings; (5) no apparent preceding disorders causing secondary NPH such as tumor, subarachnoid hemorrhage or meningitis; (6) normal CSF contents and pressure at lumbar puncture (< 200 mm H2O), and (7) right handedness. Exclusion criteria were (1) comorbidities of psychiatric or motor disorders such as Alzheimer's disease, schizophrenia and Parkinson's disease, where the comorbidity of Alzheimer's disease was defined in this study by the fact that severe preceding memory impairment existed (Ogino et al., 2006), and by a higher Alzheimer index (> 3438) evaluated by measuring amyloid beta and tau protein in CSF (Kanai et al., 1998) and (2) the shortage of 500-s artifact free epochs in the EEG recordings. Finally, twenty four iNPH patients were included in the study for analysis.
This study followed the clinical study guidelines of the Ethics Committee of Osaka University Hospital and was approved by the Internal Review Board. Written informed consent was obtained from the patients or their families.
2.2. Assessment of CSF tap outcome
2.2.1. CSF tap test
In the CSF tap test, 30 ml of CSF was removed by lumbar puncture with a 19 gauge or larger needle (CSF tapping). Because the speed of clinical recovery is different for each iNPH symptom (clinical recovery lasts about 10 days after CSF tapping) and also varies across subjects, the symptom triad was evaluated before, 1 day after, and 1 week after CSF tapping. Gait disturbance and cognitive impairment were assessed by following tests (see below). Urinary incontinence was excluded from clinical evaluation due to low reliability as the frequency of urination was sometimes self-reported. CSF tapping was judged as positive if at least one symptom had clinical improvement at either 1 day or 1 week after CSF tapping; otherwise it was judged as negative. Accordingly, the patients were classified as CSF tap responders or nonresponders.
2.3. Gait assessment
Gait disturbance was measured by the 3 m Timed Up and Go (TUG) test (Podsiadlo and Richardson, 1991), 10-meter reciprocating walking test (WT), and Gait Status Scale (GSS). In the TUG, the time a subject sitting in a chair takes to stand up, walk 3 m, walk back to the chair and sit down was measured. In the WT, the time a subject takes to walk forward 10 m and return to the starting position was measured. These tasks were performed as fast as the subject felt safe. In the GSS, eight features of the gait disturbance were assessed: postural stability (the score ranges from 0 to 4), independence of walking (0–2), wide based gait (0–1), lateral sway (0–2), petit-pas gait (0–2), festinating gait (0–2), freezing of gait (0–2), and disturbed tandem walking (0–2); higher scores indicate greater severity. The thresholds of walking tests were set at 10% improvement in the TUG and WT, and 1-point improvement in the GSS based on results of sensitivity and specificity for predicting shunt outcome derived from previous studies (Agren-Wilsson et al., 2007; Kubo et al., 2008). Improvement above those thresholds in all walking tests was identified as clinical improvement in gait.
2.4. Cognitive assessment
Cognitive impairment was assessed with the Mini-Mental State Examination (MMSE), Frontal Assessment Battery (FAB), Trail Making Test Part A (TMT-A), Wechsler Memory Scale-Revised (WMS-R)-Attention/Concentration Index, and Wechsler Adult Intelligence Scale-III (WAIS-III)-Block Design, -Digit Symbol Coding. The scores of WAIS-III subtests were age-corrected (scaled score). The thresholds of the cognitive tests were set at 4, 2 points, 30%, 15, 3, and 3 points based on previous articles (Abe et al., 2004; Ogino et al., 2006; Takagi et al., 2002; Thomas et al., 2005). Improvement above these thresholds in more than half of the cognitive tests was identified as clinical improvement in cognition.
2.5. Assessment of shunt outcome
Shunt operation was performed in the Department of Neurosurgery of Osaka University Hospital. Gait disturbance and cognitive impairment were evaluated before 1, 3, 6 months after, and 1 year after shunt operation in the same way. Shunt operation was judged as positive if at least one symptom had a clinical improvement at either 1, 3, 6 months after or 1 year after shunt operation; otherwise it was judged as negative. Accordingly, the patients were classified as shunt responders or nonresponders.
2.6. EEG recording and data acquisition
Subjects underwent EEG recording in a resting state, eyes closed condition for about 20 min before and two to seven days after CSF tapping. Prior to each recording, subjects were instructed to relax but stay awake. During the EEG sessions, the vigilance status was monitored by visual inspection of EEG traces: drowsiness was avoided by giving instructions once again. Spontaneous brain electrical activity was recorded with a 19-channel EEG system (EEG-1000/EEG-1200, Nihon Kohden, Inc., Tokyo, Japan) filtered with a frequency band of 0.53 to 120 Hz, sampled at 500 Hz.
EEG electrodes were positioned according to the International 10–20 system (i.e., Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, Pz) using a linked ears reference. Impedance was kept below 5 kΩ. Neuroworkbench software (Nihon Kohden, Inc., Tokyo, Japan) was used for visual inspection of the EEG recordings and manual selection of samples. For each subject, 500-s artifact-free, awake, resting-state segments were randomly selected. We carefully avoided, using the ear reference montage, particular epochs containing ocular movements, baseline shifts, drowsiness signs (i.e., emergence of slow wave activity with suppression of alpha rhythm), and muscle or cardiac contamination, so that reliable estimates of brain function in the steady state under awake, resting condition could be obtained. Finally, power spectral analysis was performed using the QP-220A Neuromap software (Nihon Kohden, Inc., Tokyo, Japan), and NAT analysis using the NAT analysis system provided at Brain Functions Laboratory, Inc., Yokohama, Japan.
2.7. Power spectral and NAT analyses
All electrodes were re-referenced to an average reference (i.e., a mean value of the 19 recorded potentials) for power spectral and NAT analyses. Power values of the EEG time-series data were calculated as the square of the amplitude of the EEG signal for each frequency band. Then, NPV was calculated in every segment of 2.56-s for each of the 19 recorded potential sequences, where NPV was defined as the variance of the power divided by the squared mean power to obtain relative values comparable among the different subjects. The output of the NAT analysis program was a z-score spatial distribution map, which shows how much the observed NPV values deviate from the mean NPV values of healthy controls in unit of its standard deviation (Musha and Matsuzaki, 2009). The healthy controls included in the NAT analysis program (N = 52, 71.5 ± 8.4 years old, 27 men, 25 women) had normal results in the Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), and magnetic resonance imaging (MRI), and no history of neurological or psychiatric disorders. Musha et al. (2013) reported that NAT analysis could discriminate MCI patients which later developed Alzheimer's disease from healthy controls with a small false positive rate of 15%.
In this study, the NPV time fluctuations were smoothed using a moving average filter method. The NPVs of 2.56-s EEG segments were calculated at 0.64-s steps for 500 s EEG segment as illustrated in Fig. 1. Then, all NPVs were averaged and the stationary mean NPV values were obtained for each subject. All analyses in our study were performed for five frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–40 Hz).
Fig. 1.
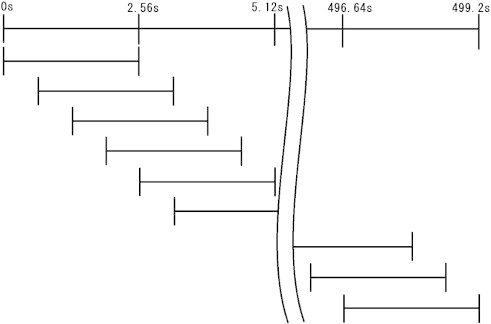
Moving average filter method was used to calculate the NPV. The NPVs of 2.56-s EEG segments were calculated advancing at 0.64-s steps for 500 s EEG segment. Then, all NPVs were averaged. Top line represents EEG time course, while lower lines represent 2.56-s EEG segments.
2.8. Statistical group analysis
The changes in NPV or EEG power at each electrode site for each frequency band between before and after CSF tapping were assessed by Paired Student t-test. The level of significance was set at P < 0.05 (uncorrected). In order to find EEG indexes that reflect brain functional changes, the correlations between the NPV or EEG power changes at each electrode site for each frequency band and clinical changes in gait and cognition scores were assessed by Pearson's correlation analysis with the significance level set at P < 0.01 (uncorrected) to reduce false negatives. These results were uncorrected for multiple comparisons, thus validity of each significant change in NPV and EEG power or significant correlation was explained in the Discussion section.
3. Results
3.1. Demographic and clinical results
We classified shunt responders as “responders” and shunt nonresponders or CSF tap nonresponders as “nonresponders”. The mean age of the 11 responders (5 men and 6 women) was 76 ± 4.6 (SD), and that of the 13 nonresponders (8 men and 5 women) was 77 ± 6.0 (SD). There were no differences in age and sex between groups. The clinical data are shown in Table 1.
Table 1.
Cognitive and gait function test scores before and after CSF tapping.
| Test |
Responders |
Nonresponders |
||
|---|---|---|---|---|
| Before tapping | After tapping | Before tapping | After tapping | |
| TUG | 16.9 ± 9.1 | 13.6 ± 8.7⁎⁎ | 14.7 ± 3.7 | 13.8 ± 3.6⁎ |
| WT | 26.2 ± 12.2 | 23.9 ± 12.2 | 24.2 ± 7.9 | 23.4 ± 8.7 |
| GSS | 5.8 ± 3.8 | 5.3 ± 3.8 | 4.5 ± 3.6 | 4.2 ± 3.5 |
| MMSE | 20.5 ± 4.5 | 22.0 ± 3.3⁎ | 22.9 ± 3.6 | 24.5 ± 3.9⁎⁎ |
| FAB | 10.7 ± 3.9 | 11.4 ± 3.3 | 11.6 ± 3.2 | 12.0 ± 3.0 |
| TMT-A | 151 ± 130 | 112 ± 90 | 152 ± 94 | 136 ± 96 |
| WMS-R_ Mental Control | 2.4 ± 1.6 | 3.2 ± 1.1 | 3.6 ± 1.0 | 3.6 ± 1.1 |
| WMS-R_ Attention/Concentration index | 77.5 ± 12.7 | 79.5 ± 7.8 | 87.6 ± 10.6 | 88.1 ± 11.7 |
| WAISIII_Digit Symbol-Coding | 5.4 ± 2.1 | 6.0 ± 2.1 | 6.6 ± 3.5 | 6.7 ± 3.0 |
| WAISIII_ Block Design (Scaled Score) | 5.4 ± 1.1 | 5.9 ± 1.5 | 6.9 ± 3.8 | 7.1 ± 4.6 |
Data are mean ± SD. TUG; 3 m Timed Up and Go, WT; 10-meter reciprocating walking test, GSS; Gait Status Scale, MMSE; Mini-Mental State Examination, FAB; Frontal Assessment Battery, TMT-A; Trail Making Test Part A, WMS-R; Wechsler Memory Scale-Revised, WAISIII; Wechsler Adult Intelligence Scale-III.
Significant improvement in cognitive and gait function test scores (p < 0.05).
Significant improvement in cognitive and gait function test scores (p < 0.01).
In 11 responders, two patients had a positive response in gait function to both CSF tapping and shunt operation. The other nine patients had a negative response to CSF tapping, although they showed improvements in some item scores. Subsequently, they underwent shunt operation, showing a positive surgical response in gait function. In 13 nonresponders, 11 patients showed a negative response to CSF tapping and did not undergo shunt operation. Two patients, however, despite having a negative response to CSF tapping showed improvements in some item scores, and subsequently underwent shunt operation, showing a negative surgical response.
3.2. Power analysis results
There were significant differences in theta and gamma EEG power after CSF tapping in nonresponders. These differences were observed in two frontal lobe regions. However, there was no correlation with functional changes (Tables 2 and 3). Pearson's correlation analysis for EEG power in all frequency bands and electrode sites in all iNPH patients showed several significant correlations between power changes and brain functional changes but not at frontal electrode sites (Table 3).
Table 2.
EEG power changes before and after CSF tapping.
| Nonresponders |
Mean NPV |
Mean difference |
p-Value |
|
|---|---|---|---|---|
| NPV at band-electrode | Before tapping | After tapping | ||
| Theta-Fp1 | 12.6 | 9.35 | − 3.3 | 0.015 |
| Gamma-F8 | 0.65 | 0.89 | 0.23 | 0.036 |
Table 3.
Pearson's correlation coefficients between EEG power changes and functional score changes of gait and cognition induced by CSF tapping in all patients.
| All iNPH patients | Test | Correlation coefficient | p-Value |
|---|---|---|---|
| Theta-Cz | GSS | 0.60 | 0.002 |
| Theta-Pz | TUG | 0.60 | 0.002 |
| Alpha-C3 | TUG | 0.52 | 0.008 |
| Beta-C4 | MMSE | 0.65 | 0.001 |
| Beta-Pz | FAB | − 0.54 | 0.006 |
GSS; Gait Status Scale, TUG; 3 m Timed Up and Go, MMSE; Mini-Mental State Examination, FAB; Frontal Assessment Battery.
3.3. NAT analysis results
The significant NPV differences before and after CSF Tapping in responders and nonresponders in each frequency band are listed in Table 4. Responders had a decrease in alpha NPV over the medial frontal cortex (FC) (Fz) after CSF tapping, while nonresponders had an increase in alpha NPV over right dorsolateral prefrontal cortex (DLPFC) (F8) after CSF tapping. Maps of alpha NPV results before and after CSF tapping and the statistical difference between conditions in responders and nonresponders are shown in Fig. 2. Pearson's correlation analysis for NPVs in all frequency bands and electrode sites in all iNPH patients found association between CSF tapping-related changes in NPVs and clinical changes. In particular, delta and alpha NPV changes in the left-dorsal FC (delta and alpha-F3-NPV) after CSF tapping correlated with GSS (r = 0.66, p = 0.0004; r = 0.55, p = 0.005). In addition, alpha and beta NPV changes in the right-anterior prefrontal cortex (PFC) (alpha and beta-Fp2-NPV) (r = 0.62, p = 0.001; r = 0.58, p = 0.003) and the left DLPFC (alpha and beta-F7-NPV) (r = 0.54, p = 0.006; r = 0.55, p = 0.005), as well as alpha NPV changes in the medial FC (alpha-Fz-NPV) (r = 0.55, p = 0.004) correlated with WT. Moreover, alpha NPV changes in the right DLPFC (alpha-F8-NPV) after CSF tapping correlated with WMS-R_Mental Control scores (r = − 0.52, p = 0.008) (Table 5, Figs. 3–5).
Table 4.
NPV changes between before and after CSF tapping.
| NPV at band-electrode | Mean NPV |
Mean difference | p-Value | |
|---|---|---|---|---|
| Before tapping | After tapping | |||
| Responders | ||||
| Theta-Fp1 | − 0.0026 | − 0.45 | − 0.45 | 0.013 |
| Alpha-Fz | 0.037 | − 0.28 | − 0.32 | 0.030 |
| Beta-P4 | − 0.24 | − 0.40 | − 0.15 | 0.047 |
| Beta-T4 | 0.33 | − 0.047 | − 0.38 | 0.023 |
| Gamma-T3 | 1.0 | 0.64 | − 0.44 | 0.022 |
| Nonresponders | ||||
| Delta-Fz | 0.20 | 0.51 | + 0.31 | 0.024 |
| Alpha-F8 | − 0.56 | − 0.25 | + 0.30 | 0.027 |
| Alpha-T5 | − 0.69 | − 0.062 | + 0.63 | 0.0073 |
| Gamma-C3 | 0.30 | 0.010 | − 0.29 | 0.031 |
Fig. 2.
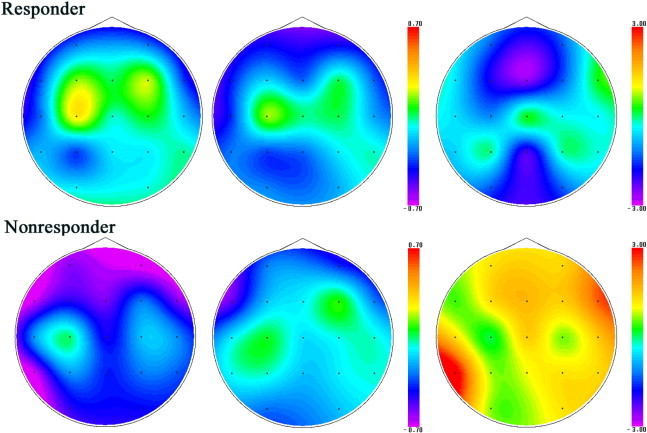
Alpha band NPV in responders (upper row) and nonresponders (lower row) before CSF tapping (left), after CSF tapping (center) and the statistical difference that is t-value between them (right). Responders have medial frontal (Fz)-alpha NPV decrease after CSF tapping, while nonresponders have right-dorsolateral prefrontal (F8)-alpha NPV increase.
Table 5.
Pearson's correlation coefficients between NPV changes and functional score changes of gait and cognition induced by CSF tapping in all patients.
| All iNPH patients | Test | Correlation coefficient | p-Value |
|---|---|---|---|
| Delta-F3 | GSS | 0.66 | 0.0004 |
| Delta-C4 | WT | 0.55 | 0.005 |
| Alpha-Fp2 | WT | 0.62 | 0.001 |
| Alpha-F3 | GSS | 0.55 | 0.005 |
| Alpha-F7 | WT | 0.54 | 0.006 |
| Alpha-F8 | WMS-R_ Mental Control | − 0.52 | 0.008 |
| Alpha-Fz | WT | 0.55 | 0.004 |
| Beta-Fp2 | WT | 0.58 | 0.003 |
| Beta-P3 | FAB | 0.52 | 0.009 |
| Beta-F7 | WT | 0.55 | 0.005 |
| Gamma-C4 | GSS | 0.57 | 0.004 |
| Gamma-Cz | WMS-R_ Mental Control | 0.65 | 0.0006 |
GSS; Gait Status Scale, WT; 10-meter reciprocating walking test, FAB; Frontal Assessment Battery, WMS-R; Wechsler Memory Scale-Revised.
Fig. 3.
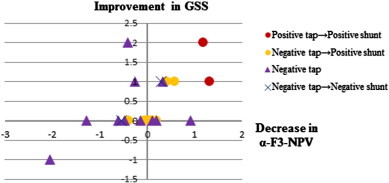
Scatterplots of significant correlations between difference of alpha-F3-NPV and difference of Gait Status Scale (GSS) before and after CSF Tapping (r = 0.55, p = 0.005). Decrease/increase of the alpha NPV at left-dorsal prefrontal cortex (F3) indicates improvement/worsening in gait status by CSF tapping.
Fig. 4.
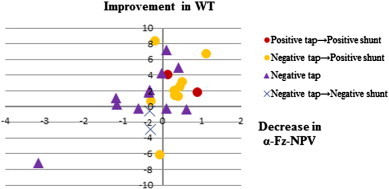
Scatterplots of significant correlations between difference of alpha-Fz-NPV and difference of 10-meter reciprocating walking test (WT) before and after CSF Tapping (r = 0.55, p = 0.004). Decrease/increase of the alpha NPV at medial frontal cortex (Fz) indicates improvement/worsening in gait velocity by CSF tapping.
Fig. 5.
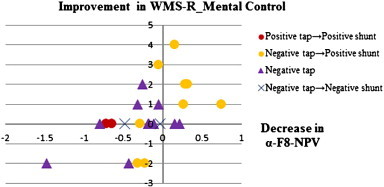
Scatterplots of significant correlations between difference of alpha-F8-NPV and difference of WMS-R_Mental Control before and after CSF Tapping (r = − 0.52, p = 0.008). Decrease/increase of the alpha NPV at right-dorsolateral-prefrontal cortex (F8) indicates improvement/worsening in response suppression by CSF tapping.
As functional improvement following CSF tapping and shunt operation mainly occurred in gait domain, we combined gait-related NPV changes induced by CSF tapping (∆alpha-F3-NPV, ∆alpha-Fz-NPV), where NPV decreases and increases after CSF removal indicated functional recovery and worsening, respectively. Thus, we set the threshold ∆alpha-F3-NPV + ∆alpha-Fz-NPV = 0. Using this additional analysis, we could correctly identify “shunt responders” and “shunt nonresponders” with a positive predictive value of 100% (10/10) and a negative predictive value of 66% (2/3) (Fig. 6).
Fig. 6.
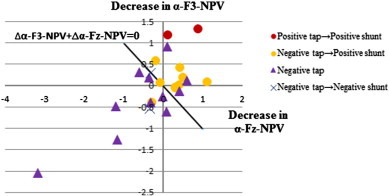
Scatterplots of differences in alpha-Fz-NPV (∆alpha-Fz-NPV) and differences in alpha-F3-NPV (∆alpha-F3-NPV) before and after CSF tapping. We could correctly identify “shunt responders (red circle and yellow circle)” and “shunt nonresponders (dark blue cross)” with a positive predictive value of 100% (10/10) and a negative predictive value of 66% (2/3) with the threshold of ∆alpha-F3-NPV + ∆alpha-Fz-NPV = 0.
4. Discussion
In the present study, we used NAT analysis, which is highly sensitive to instability of cortical electrical activity, to identify functional correlates of CSF tapping response in patients with iNPH. We found that NPV changes associated with clinical outcome, in particular gait and cognitive changes after CSF tapping. The main findings were that: (1) responders had a decrease in alpha NPV at the medial FC (Fz) after CSF tapping, (2) nonresponders had an increase in alpha NPV at the right DLPFC (F8) after CSF tapping, (3) delta and alpha NPV changes in the left-dorsal FC (F3) correlated with clinical outcome of gait status, and (4) alpha and beta NPV changes in the right anterior PFC (Fp2) and left DLPFC (F7) as well as alpha NPV changes in the medial FC (Fz) correlated with clinical outcome of gait velocity. Furthermore, (5) alpha NPV changes in the right DLPFC (F8) correlated with clinical outcome of WMS-R Mental Control scores in these patients.
Our NAT analysis findings [the main finding (1) and (2)] revealed that CSF tapping affected the cortical electrical activity in completely different ways in responders and nonresponders. In particular, medial frontal cortical electrical activity in alpha frequency band became stabilized (NPV decreased) in responders. In contrast, right dorsolateral prefrontal electrical activity in this frequency band became destabilized (NPV increased) in nonresponders (Fig. 2).
A striking finding of our analyses [the main finding (3) and (4)] is that the improvement and worsening in gait scores (i.e., gait velocity and gait status) induced by CSF tapping manifested as decreases and increases in alpha NPV, respectively. The findings of left-dorsal and medial frontal cortex engagement in gait improvement by CSF tapping are consistent with those of an fMRI study demonstrating that the left dorsal premotor cortex and bilateral SMA showed enhanced activation after a three-day continuous CSF drainage during hand motor task performance in right handed iNPH patients (Lenfeldt et al., 2008). Cortical regions subserving gait in humans have often been examined using fMRI and SPECT. These techniques, however, are associated with movement limitation, and subjects cannot walk inside the scanner. Wang et al. (2008) used fMRI with a walking video clip task in an egocentric perspective, which evoked mirror neuron system and walking imagery. They found that the observation of walking video clips activated the left dorsal premotor cortex, SMA and bilateral primary motor cortex in right-handed healthy subjects. Interestingly, bilateral SMA lesions have been reported in association with freezing and festination of gait (Della Sala et al., 2002), and lesions involving the premotor cortex have also been linked to freezing of gait when turning or negotiating narrow passages (Nutt et al., 1993). Others have found that intentional control of actions including gait engaged the left DLPFC (Frith et al., 1991; Hyder et al., 1997; Malouin et al., 2003). Together, these findings suggest that the SMA, the left dorsal premotor cortex, bilateral primary motor cortex and the left DLPFC are involved in human gait control of right handed healthy subjects. And that in addition to these cortical regions activated in healthy subjects during gait, there is recruitment of the anterior PFC in gait impaired patients (Caliandro et al., 2012). Further support to this view comes from fMRI evidence that patients with freezing of gait in Parkinson's disease employ right anterior PFC together with motor-related areas during continuous bimanual movements (Vercruysse et al., in press). Overall, our findings support and extend the notion that the cortical areas implicated in gait function in iNPH patients include: the left dorsal premotor cortex for gait status, and SMA, left DLPFC and right anterior PFC for gait velocity. NPV changes reflecting these gait functions were also seen in responders and nonresponders [the main finding (1)], although the some differences before and after CSF tapping did not reach statistical significance [alpha NPV decrease tendency at left-dorsal FC (F3) in responders (p = 0.085) and alpha NPV increase tendency at the medial FC (Fz) (p = 0.097) in nonresponders].
Like the clinical outcome of gait function, the improvement and worsening in WMS-R_Mental Control induced by CSF tapping also manifested as decrease and increase in alpha NPV, respectively, specifically in the right DLPFC [the main finding (5)]. The WMS-R_Mental Control includes three subtests: counting backward from 20 to 1, reciting the alphabet, and adding serial 3's. We found that in these subtests, counting backward test score mainly correlated with changes in alpha NPV at the right DLPFC (r = − 0.50, p = 0.011). The counting backward test from 20 to 1 is supposed to represent a suppression of overlearned response (i.e., counting forward). Kanno et al. (2012) reported that the first error score of counting backward test discriminated iNPH from AD patients with a sensitivity of 80% and a specificity of 85%, highlighting that deficits in suppression of overlearned response are one of the main cognitive characteristics of iNPH. Although this differs from previous findings that the left DLPFC is involved in the suppression of overlearned response in healthy subjects (Knoch et al., 2005), recruitment of the right DLPFC was frequently seen during a left DLPFC activation task (i.e., encoding) in high performance aged subjects. A possible interpretation of this result was that high performance aged subjects counteract age-related neuronal decline by reorganizing brain functional networks (Manenti et al., 2011). We can speculate that similar reorganization of brain functional networks may occur in iNPH patients.
Taken together, these findings suggest that gait and cognition-related NPV changes affect the frontal lobe. In support to this view, accumulating results from previous studies have led to the assumption that frontal lobe dysfunction may cause iNPH symptoms, as frontal lobe tests (FAB and TMT-A) were markedly impaired in iNPH patients (Saito et al., 2011). Although there was rCBF reduction in broad cortical areas, this dysfunction was more prominent in fronto-temporal regions (Kristensen et al., 1996) and FAB score, which assess frontal lobe dysfunction, significantly correlated with WT before CSF tapping. This support the notion that gait impairment is caused by frontal lobe dysfunction (Miyoshi et al., 2005).
Although power analysis before and after CSF tapping revealed some significant EEG power changes after CSF tapping, this activity showed no correlation with clinical changes. Also, Pearson's correlation analysis for EEG power in all frequency bands and electrode sites showed some significant correlations with functional changes but, their locations (central and parietal electrode site) were inconsistent with the frontal lobe dysfunction assumption. It is likely that these significant correlations of EEG power (p < 0.01, uncorrected) might be associated with false positives, as analyses were repeated 950 times for all frequency bands, electrode sites and for gait and cognitive tests. Overall, our findings suggest that EEG power did not reflect functional changes induced by CSF tapping. Likewise, additional NPV changes shown in Table 4, in particular theta-Fp1, beta-P4, beta-T4 and gamma-T3 activity in responders, and delta-Fz, alpha-T5 and gamma-C3 in nonresponders did not correlate with functional changes. Furthermore, other NPV correlations involving specifically delta-C4, beta-P3, gamma-C4 and gamma-Cz in Table 5 were not in line with a frontal lobe dysfunction assumption. Thus, we can also assume that these additional NPV changes, not previously described among our main study findings, are not associated with functional changes induced by CSF tapping.
In this study, functional improvement induced by CSF tapping or shunt operation occurred mainly in the gait domain but not in the cognitive domain. This tendency was also seen in previous reports of a predominant gait function recovery, sometimes accompanied by cognitive deterioration after shunt operation (Koivisto et al., 2013). In gait domain, TUG was more improved than WT as shown in Table 1. This may be because TUG, which requires standing and seating, depends on various gait factors and their synergistic effect led to a reduction of walking time. On the other hand, WT depends mainly on gait velocity. Therefore, correlation of the NPV changes with WT instead of TUG may indicate that NPV reflects a functional element rather than functional structure of elements.
It is noteworthy that the significant functional changes in our study affected the delta, alpha and beta frequency bands. Consistent with our results, many functional resting state networks were detected in the alpha and beta frequency band in EEG or MEG studies (Brookes et al., 2011; de Pasquale et al., 2010; Mantini et al., 2007). Our results suggest that resting state cortical electrical activity in alpha frequency band is strongly associated with overlearned response suppression, subserved by the right DLPFC, and with gait function in the left dorsal premotor, left DLPFC, right anterior PFC and SMA. This is consistent with the role of alpha frequency band in the neuronal mechanisms of attention and top-down modulation (Palva and Palva, 2007). Our results also suggests that resting state cortical electrical activity in beta frequency band in prefrontal cognitive control areas (Abe and Hanakawa, 2009; Kovach et al., 2012), specifically with the left DLPFC and right anterior PFC is strongly associated with gait function. This supports the role of beta frequency band in the neuronal mechanisms of top-down modulation (Arnal et al., 2011). Furthermore, our results suggest that resting state cortical electrical activity in delta frequency band in the left dorsal FC is strongly associated with gait function. This is consistent with EEG reports on connectivity using finger moving videos and a mirror neuron system paradigm, showing that motor areas including the premotor cortex had synchronization in delta and alpha frequency bands induced by related to finger movement (Holz et al., 2008).
As we have discussed, NAT analysis revealed the cortical areas responsible for clinical and functional impairments in iNPH, which may contribute to further understanding the mechanisms underlying iNPH pathophysiology. Overall results highlight the importance of the normalized variance of EEG power activity, namely NPV, in the identification of brain responses to CSF tapping and shunt operation. A decrease in NPV, which is thought to reflect a stabilization of cortical electrical activity, appears to represent a neurophysiological marker of cortical functional recovery after CSF drainage. An increase in NPV, however, which is likely associated with the destabilization of cortical electrical activity, may potentially indicate cortical functional impairment. Thus, these results confirm our working hypothesis.
This is the first study demonstrating that, unlike other methods such as SPECT, fMRI and EEG power spectral analysis that have been used to explore changes in brain activity induced by CSF tapping, NAT analysis can detect CSF tapping-related changes in brain activity associated with clinical outcome in iNPH patients. This is related to the fact that EEG directly relates to cortical electrical activity with a high temporal resolution and that NPV is particularly sensitive to the transition of cortical electrical activity. Although there are some other recently developed EEG methods which are connectivity (Fonseca et al., 2013), complexity (Mizuno et al., 2010) microstate (Nishida et al., 2013) and dimension analysis (Kouzuki et al., 2013), NAT analysis can more sensitively detect instability of cortical electrical activity prior to phase transition onset because theoretical model showed that NPV diverges to infinity as state approaches a phase transition point (Chen et al., 2012). Therefore, we selected NAT analysis to detect cortical activity changes in iNPH patients. However, the relationship of NAT with other EEG methods in neuropsychiatric diseases has not been clarified and further studies are needed.
Our results should be interpreted with caution based on the following limitations. First, our results were uncorrected for multiple comparisons. However, our main findings of NPVs correlating with clinical changes are consistent with neuroimaging findings of cortical functions, the role of each frequency band in cortical electrical oscillations and the notion of frontal lobe dysfunction proposed by previous iNPH studies. Therefore, we may assume that the EEG index (NPV) is a reliable measure of brain functional changes. Second, the sample size of shunt nonresponders was small. Although the small sample size of shunt nonresponders is due to the high positive predictive value of CSF tap test, which causes uncertainty of the negative predictive value of the NAT analysis (66%). So, further study is needed to determine the accurate predictive value of the NAT analysis by accumulating subjects. Third, NAT analysis program, which is EEG electrode-based without source localization method, has a limited anatomical specificity. Therefore, we estimated the corresponding cortical area of each electrode site based on rCBF findings in iNPH patients and neuroimaging findings of cortical functions. However, in support to our estimation of cortical correspondence, SMA epilepsy tends to show spike foci at Fz electrode (Aoki et al., 2013; Blume and Oliver, 1996; Canuet et al., 2008) and N30 cortical component of the somatosensory evoked potentials of median nerve, whose main power sources were estimated in the right dorsal premotor cortex by LORETA source localization method, had a maximum amplitude at F4 electrode (Cebolla et al., 2011).
5. Conclusion
NAT analysis is a sensitive and objective method to reliably detect changes in cortical functions induced by CSF tapping in iNPH patients. Based on these findings, it is promising that NAT analysis can be used as a powerful tool in assessing cortical functional changes in neuropsychiatric diseases such as dementia and head injury. The combination of CSF tapping and NAT analysis can correctly discriminate shunt responders and shunt nonresponders in iNPH patients. Thus, NAT results may potentially predict the effectiveness of shunt operation in patients suffering from this condition, which has important therapeutic implications.
Acknowledgments
This study was supported by the Research Committee of Normal Pressure Hydrocephalus and Related Disorders, Studies on the Etiology, Pathogenesis and Therapy from the Japanese Ministry of Health, Labour and Welfare (Tokyo, Japan).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abe M., Hanakawa T. Functional coupling underlying motor and cognitive functions of the dorsal premotor cortex. Behav. Brain Res. 2009;198(1):13–23. doi: 10.1016/j.bbr.2008.10.046. [DOI] [PubMed] [Google Scholar]
- Abe M., Suzuki K., Okada K., Miura R., Fujii T., Etsurou M., Yamadori A. Normative data on tests for frontal lobe functions: trail making test, verbal fluency, Wisconsin card sorting test (Keio version) No To Shinkei. 2004;56(7):567–574. [PubMed] [Google Scholar]
- Agren-Wilsson A., Lekman A., Sjöberg W., Rosengren L., Blennow K., Bergenheim A.T., Malm J. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2007;116:333–339. doi: 10.1111/j.1600-0404.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Aoki Y., Ishii R., Iwase M., Ikeda S., Hata M., Canuet L., Imajo K., Tanaka M., Matsuzaki H., Musha T., Takeda M. Normalized power variance change between pre-ictal and ictal phase of an epilepsy patient using NAT analysis: a case study. Conference Proceedings of the IEEE Engineering in Medicine and Biology Society. 2013;2013:437–440. doi: 10.1109/EMBC.2013.6609530. [DOI] [PubMed] [Google Scholar]
- Arnal L.H., Wyart V., Giraud A.L. Transitions in neural oscillations reflect prediction errors generated in audiovisual speech. Nat. Neurosci. 2011;14(6):797–801. doi: 10.1038/nn.2810. [DOI] [PubMed] [Google Scholar]
- Bech-Azeddine R., Waldemar G., Knudsen G.M., Høgh P., Bruhn P., Wildschiødtz G., Gjerris F., Paulson O.B., Juhler M. Idiopathic normal-pressure hydrocephalus: evaluation and findings in a multidisciplinary memory clinic. Eur. J. Neurol. 2001;8(6):601–611. doi: 10.1046/j.1468-1331.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- Blume W.T., Oliver L.M. Noninvasive electroencephalography in supplementary sensorimotor area epilepsy. Adv. Neurol. 1996;70:309–317. [PubMed] [Google Scholar]
- Brean A., Eide P.K. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol. Scand. 2008;118(1):48–53. doi: 10.1111/j.1600-0404.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- Brookes M.J., Woolrich M., Luckhoo H., Price D., Hale J.R., Stephenson M.C., Barnes G.R., Smith S.M., Morris P.G. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc. Natl. Acad. Sci. U. S. A. 2011;108(40):16783–16788. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.G., Goldensohn E.S. The electroencephalogram in normal pressure hydrocephalus. Arch. Neurol. 1973;29:70–71. doi: 10.1001/archneur.1973.00490250088014. [DOI] [PubMed] [Google Scholar]
- Caliandro P., Masciullo M., Padua L., Simbolotti C., Di Sante G., Russo G., Garattini C., Silvestri G., Rossini P.M. Prefrontal cortex controls human balance during overground ataxic gait. Restor. Neurol. Neurosci. 2012;30(5):397–405. doi: 10.3233/RNN-2012-120239. [DOI] [PubMed] [Google Scholar]
- Canuet L., Ishii R., Iwase M., Kurimoto R., Ikezawa K., Azechi M., Takahashi H., Nakahachi T., Takeda M. Cephalic auras of supplementary motor area origin: an ictal MEG and SAM(g2) study. Epilepsy Behav. 2008;13(3):570–574. doi: 10.1016/j.yebeh.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Canuet L., Ishii R., Pascual-Marqui R.D., Iwase M., Kurimoto R., Aoki Y., Ikeda S., Takahashi H., Nakahachi T., Takeda M. Resting-state EEG source localization and functional connectivity in schizophrenia-like psychosis of epilepsy. PLoS ONE. 2011;6(11):e27863. doi: 10.1371/journal.pone.0027863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuet L., Tellado I., Couceiro V., Fraile C., Fernandez-Novoa L., Ishii R., Takeda M., Cacabelos R. Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study. PLoS ONE. 2012;7(9):e46289. doi: 10.1371/journal.pone.0046289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla A.M., Palmero-Soler E., Dan B., Cheron G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. Neuroimage. 2011;54(2):1297–1306. doi: 10.1016/j.neuroimage.2010.08.060. [DOI] [PubMed] [Google Scholar]
- Chen L., Liu R., Liu Z.P., Li M., Aihara K. Detecting early-warning signals for sudden deterioration of complex diseases by dynamic network biomarkers. Sci. Rep. 2012;2(342) doi: 10.1038/srep00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F., Della Penna S., Snyder A.Z., Lewis C., Mantini D., Marzetti L., Belardinelli P., Ciancetta L., Pizzella V., Romani G.L., Corbetta M. Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci. U. S. A. 2010;107(13):6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala S., Francescani A., Spinnler H. Gait apraxia after bilateral supplementary motor area lesion. J. Neurol. Neurosurg. Psychiatry. 2002;72(1):77–85. doi: 10.1136/jnnp.72.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura K., Mase M., Miyati T., Osawa T., Hattori M., Kasai H., Hara M., Shibamoto Y., Yamada K. Changes of fractional anisotropy and apparent diffusion coefficient in patients with idiopathic normal pressure hydrocephalus. Acta Neurochir. Suppl. 2012;113:29–32. doi: 10.1007/978-3-7091-0923-6_6. [DOI] [PubMed] [Google Scholar]
- Fonseca L.C., Tedrus G.M., Carvas P.N., Machado E.C. Comparison of quantitative EEG between patients with Alzheimer's disease and those with Parkinson's disease dementia. Clin. Neurophysiol. 2013;124(10):1970–1974. doi: 10.1016/j.clinph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Friston K., Liddle P.F., Frackowiak R.S. Willed action and the prefrontal cortex in man: a study with PET. Proc. R. Soc. Biol. Sci. 1991;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Hiraoka K., Meguro K., Mori E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol. Med. Chir. 2008;48(5):197–199. doi: 10.2176/nmc.48.197. [DOI] [PubMed] [Google Scholar]
- Holz E.M., Doppelmayr M., Klimesch W., Sauseng P. EEG correlates of action observation in humans. Brain Topogr. 2008;21(2):93–99. doi: 10.1007/s10548-008-0066-1. [DOI] [PubMed] [Google Scholar]
- Hyder F., Phelps E.A., Wiggins C.J., Labar K.S., Blamire A.M., Shulman R.G. “Willed action”: a functional MRI study of the human prefrontal cortex during a sensorimotor task. Proc. Natl. Acad. Sci. U. S. A. 1997;94(13):6989–6994. doi: 10.1073/pnas.94.13.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki C., Kawanami T., Nagasawa H., Wada M., Koyama S., Kikuchi K., Arawaka S., Kurita K., Daimon M., Mori E., Kato T. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J. Neurol. Sci. 2009;277(1–2):54–57. doi: 10.1016/j.jns.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Ishii R., Shinosaki K., Ukai S., Inouye T., Ishihara T., Yoshimine T., Hirabuki N., Asada H., Kihara T., Robinson S.E., Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10(4):675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Hashimoto M., Mori E., Kuwana N., Kazui H. The value of the cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids and Barriers of the CNS. 2012;9(1):1. doi: 10.1186/2045-8118-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Matsubara E., Isoe K., Urakami K., Nakashima K., Arai H., Sasaki H., Abe K., Iwatsubo T., Kosaka T., Watanabe M., Tomidokoro Y., Shizuka M., Mizushima K., Nakamura T., Igeta Y., Ikeda Y., Amari M., Kawarabayashi T., Ishiguro K., Harigaya Y., Wakabayashi K., Okamoto K., Hirai S., Shoji M. Longitudinal study of cerebrospinal fluid levels of tau, A beta1-40, and A beta1-42(43) in Alzheimer's disease: a study in Japan. Ann. Neurol. 1998;44(1):17–26. doi: 10.1002/ana.410440108. [DOI] [PubMed] [Google Scholar]
- Kanno S., Saito M., Hayashi A., Uchiyama M., Hiraoka K., Nishio Y., Hisanaga K., Mori E. Counting-backward test for executive function in idiopathic normal pressure hydrocephalus. Acta Neurol. Scand. 2012;126(4):279–286. doi: 10.1111/j.1600-0404.2012.01644.x. [DOI] [PubMed] [Google Scholar]
- Knoch D., Brugger P., Regard M. Suppressing versus releasing a habit: frequency-dependent effects of prefrontal transcranial magnetic stimulation. Cereb. Cortex. 2005;15(7):885–887. doi: 10.1093/cercor/bhh196. [DOI] [PubMed] [Google Scholar]
- Koivisto A.M., Alafuzoff I., Savolainen S., Sutela A., Rummukainen J., Kurki M., Jääskeläinen J.E., Soininen H., Rinne J., Leinonen V. Poor cognitive outcome in shunt-responsive idiopathic normal pressure hydrocephalus. Neurosurgery. 2013;72(1):1–8. doi: 10.1227/NEU.0b013e31827414b3. [DOI] [PubMed] [Google Scholar]
- Kouzuki M., Asaina F., Taniguchi M., Musha T., Urakami K. The relationship between the diagnosis method of neuronal dysfunction (DIMENSION) and brain pathology in the early stages of Alzheimer's disease. Psychogeriatrics. 2013;13(2):63–70. doi: 10.1111/j.1479-8301.2012.00431.x. [DOI] [PubMed] [Google Scholar]
- Kovach C.K., Daw N.D., Rudrauf D., Tranel D., O'Doherty J.P., Adolphs R. Anterior prefrontal cortex contributes to action selection through tracking of recent reward trends. J. Neurosci. 2012;32(25):8434–8442. doi: 10.1523/JNEUROSCI.5468-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen B., Malm J., Fagerland M., Hietala S.O., Johansson B., Ekstedt J., Karlsson T. Regional cerebral blood flow, white matter abnormalities, and cerebrospinal fluid hydrodynamics in patients with idiopathic adult hydrocephalus syndrome. J. Neurol. Neurosurg. Psychiatry. 1996;60(3):282–288. doi: 10.1136/jnnp.60.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Kazui H., Yoshida T., Kito Y., Kimura N., Tokunaga H., Ogino A., Miyake H., Ishikawa M., Takeda M. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2008;25(1):37–45. doi: 10.1159/000111149. [DOI] [PubMed] [Google Scholar]
- Kurimoto R., Ishii R., Canuet L., Ikezawa K., Iwase M., Azechi M., Aoki Y., Ikeda S., Yoshida T., Takahashi H., Nakahachi T., Kazui H., Takeda M. Induced oscillatory responses during the Sternberg's visual memory task in patients with Alzheimer's disease and mild cognitive impairment. Neuroimage. 2012;59(4):4132–4140. doi: 10.1016/j.neuroimage.2011.10.061. [DOI] [PubMed] [Google Scholar]
- Lenfeldt N., Larsson A., Nyberg L., Andersson M., Birgander R., Eklund A., Malm J. Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain. 2008;131:2904–2912. doi: 10.1093/brain/awn232. [DOI] [PubMed] [Google Scholar]
- Malouin F., Richards C.L., Jackson P.L., Dumas F., Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum. Brain Mapp. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R., Cotelli M., Miniussi C. Successful physiological aging and episodic memory: a brain stimulation study. Brain Behav. Res. 2011;216(1):153–158. doi: 10.1016/j.bbr.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N., Kazui H., Ogino A., Ishikawa M., Miyake H., Tokunaga H., Ikejiri Y., Takeda M. Association between cognitive impairment and gait disturbance in patients with idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2005;20(2–3):71–76. doi: 10.1159/000085858. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Takahashi T., Cho R.Y., Kikuchi M., Murata T., Takahashi K., Wada Y. Assessment of EEG dynamical complexity in Alzheimer's disease using multiscale entropy. Clin. Neurophysiol. 2010;121(9):1438–1446. doi: 10.1016/j.clinph.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori E., Ishikawa M., Kato T., Kazui H., Miyake H., Miyajima M., Nakajima M., Hashimoto M., Kuriyama N., Tokuda T., Ishii K., Kaijima M., Hirata Y., Saito M., Arai H. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol. Med. Chir. (Tokyo) 2012;52(11):775–809. doi: 10.2176/nmc.52.775. [DOI] [PubMed] [Google Scholar]
- Musha T., Matsuzaki H. Conference Proceedings of the 9th International Conference on Alzheimer's and Parkinson's Disease. 2009. Neuronal abnormality topography and discrimination of MCI developing AD; pp. 199–203. [Google Scholar]
- Musha T., Matsuzaki H., Kobayashi Y., Okamoto Y., Tanaka M., Asada T. EEG markers for characterizing anomalous activities of cerebral neurons in NAT (neuronal activity topography) method. IEEE Trans. Biomed. Eng. 2013;60(8):2332–2338. doi: 10.1109/TBME.2013.2255101. [DOI] [PubMed] [Google Scholar]
- Nishida K., Morishima Y., Yoshimura M., Isotani T., Irisawa S., Jann K., Dierks T., Strik W., Kinoshita T., Koenig T. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer's disease. Clin. Neurophysiol. 2013;124(6):1106–1114. doi: 10.1016/j.clinph.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Nutt J.G., Marsden C.D., Thompson P.D. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43(2):268–279. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- Ogino A., Kazui H., Miyoshi N., Hashimoto M., Ohkawa S., Tokunaga H., Ikejiri Y., Takeda M. Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2006;21(2):113–119. doi: 10.1159/000090510. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Saito M., Nishio Y., Kanno S., Uchiyama M., Hayashi A., Takagi M., Kikuchi H., Yamasaki H., Shimomura T., Iizuka O., Mori E. Cognitive profile of idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. Extra. 2011;1(1):202–211. doi: 10.1159/000328924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand T., Bovim G., Gimse R. Quantitative electroencephalography in idiopathic normal pressure hydrocephalus: relationship to CSF outflow resistance and the CSF tap-test. Acta Neurol. Scand. 1994;89:317–322. doi: 10.1111/j.1600-0404.1994.tb02641.x. [DOI] [PubMed] [Google Scholar]
- Takagi R., Kajimoto Y., Kamiyoshi S., Miwa H., Kondo T. The frontal assessment battery at bed side (FAB) in patients with Parkinson's disease. No To Shinkei. 2002;54(10):897–902. [PubMed] [Google Scholar]
- Tanaka N., Yamaguchi S., Ishikawa H., Ishii H., Meguro K. Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: the Osaki–Tajiri project. Neuroepidemiology. 2009;32(3):171–175. doi: 10.1159/000186501. [DOI] [PubMed] [Google Scholar]
- Thomas G., McGirt M.J., Woodworth G., Heidler J., Rigamonti D., Hillis A.E., Williams M.A. Baseline neuropsychological profile and cognitive response to cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 2005;20(2–3):163–168. doi: 10.1159/000087092. [DOI] [PubMed] [Google Scholar]
- Vercruysse S., Spildooren J., Heremans E., Wenderoth N., Swinnen S.P., Vandenberghe W., Nieuwboer A. The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht170. (in press) [DOI] [PubMed] [Google Scholar]
- Wang C., Wai Y., Kuo B., Yeh Y.Y., Wang J. Cortical control of gait in healthy humans: an fMRI study. J. Neural Transm. 2008;115(8):1149–1158. doi: 10.1007/s00702-008-0058-z. [DOI] [PubMed] [Google Scholar]


