Abstract
Background
Flexor tendon grafting is often required to reconstruct a failed tendon repair. Previous reports have demonstrated flexor grafts coated with lubricants such as carbodiimide derivatized hyaluronic acid (cd-HA) decrease adhesion formation and improve digit function. However, whether this surface modification would affect graft adhesion and cellularity is unknown.
Questions/Purposes
Adhesion score and the cellularity of the graft of untreated and cd-HA surface-modified autograft and allograft tendons were studied using a canine forepaw in vivo model.
Methods
The peroneus longus tendons (n = 6) and flexor digitorum profundus tendons (n = 8) were used as extrasynovial autograft and intrasynovial allograft, respectively. The flexor digitorum profundus (FDP) tendons in the second and fifth digits in each dog were reconstructed with one digit treated with cd-HA and the other treated with saline as a control. Six weeks after surgery, the grafted tendons were harvested for histological evaluation with hematoxylin and eosin staining. During dissection, the adhesions were observed and scored.
Results
The adhesion score was greatest in the extrasynovial autograft without surface modification and the least in the intrasynovial allograft with surface modification. Autograft tendons had a higher cell density than the allografts regardless of surface treatment. Cd-HA graft treatment did not affect cellularity when compared with controls.
Conclusions
Our observations suggest surface modification of a tendon graft with cd-HA decreased the adhesion formation without altering the cellularity in either autologous or allograft tendon. We therefore presume this surface modification would not adversely affect graft healing.
Introduction
Flexor tendon injuries in the hand are common, and successful functional recovery after flexor tendon repair is very challenging [18, 21]. In complex cases, tendon grafts play an important role in the restoration of hand function [4, 7, 19]. Unfortunately, clinical data [9, 13, 17, 20, 23] have shown that restrictive adhesions and poor digital function occur in 20% to 50% of patients after flexor tendon grafting. Although flexor tendons in the hand are optimized for intrasynovial function, the tendons most often available for grafting are extrasynovial in origin. Clinical and laboratory studies have shown that for intrasynovial recipient sites, an extrasynovial donor tendon has more adhesion formation than an intrasynovial donor [1, 12, 17]. This may be the result of the higher friction and absence of a gliding surface structure in extrasynovial tendons [24]; laboratory studies suggest that the higher the friction, the more the adhesion formation [25].
Clinically, there are few sources of autologous intrasynovial tendons. As an alternative, intrasynovial tendon allografts have been suggested [3, 6, 16]. One recent study concluded that the biomechanical advantages for tendon reconstruction using autografts over devitalized allograft were minimal, but this study evaluated an extrasynovial tendon injury, which raises some questions about its clinical applicability [10]. More recently, Ikeda et al. [11] investigated methods to prepare intrasynovial tendon allografts and found that lyophilization, a common preservation technique, damaged the intrasynovial tendon surface and caused increased tendon gliding resistance. Therefore, surface improvement for allograft intrasynovial tendon graft may have benefits to decrease adhesion formation, which served as a rationale for the current study.
Surface modification with carbodiimide derivatized hyaluronic acid (cd-HA) improves both intrasynovial and extrasynovial tendon graft gliding ability in vitro [14, 22], whereas it also improved digit function in vivo [28, 29]. However, the impact of cd-HA surface modification on adhesion status and the cell viability and graft regeneration have not been studied.
Based on these considerations, the purpose of this study was to assess and compare adhesion and cell density after cd-HA surface modification in autograft and allograft tendons in an in vivo animal model.
Materials and Methods
We studied five conditions including autologous extrasynovial graft with and without surface treatment (n = 12), allograft intrasynovial tendon with and without surface treatment (n = 16), and normal surgical digits (n = 8) in 14 mixed breed dogs and compared adhesion scores and cell density between the conditions. Six dogs had autograft peroneus longus (PL) tendons as the donor grafting tendons and eight dogs had allograft flexor digitorum profundus (FDP) tendons as the graft donor tendons. Because this was a preliminary study, the sample size power analysis was not performed. In each dog, the second and fifth digits were randomly selected for a graft, treated either with saline solution as a control or cd-HA gelatin (ie, a total of 28 grafts). For the tendon cellularity study, the normal FDP tendon was used as the normal control group. Therefore, a total of five groups were designed: (1) autograft control (n = 6); (2) autograft treated (n = 6); (3) allograft control (n = 8); (4) allograft treated (n = 8); and (5) normal FDP tendon (n = 8). The cd-HA graft tendons were immersed in 1% sodium hyaluronate (Acros, 95%), 10% gelatin (from porcine skin; Sigma Chemical Co, St Louis, MO, USA), 1% 1-ethy1-3 [(3-dimethylaminopropyl) carbodiimide hydrochloride] [9] (Sigma Chemical Co), and 1% N-hydroxysuccinimide (Sigma Chemical Co) in 0.1 M NaCl pH 6.0 and 0.9% phosphate-buffered saline for 5 minutes [27]. We had prior approval of our Institutional Animal Care and Use Committee (IACUC).
The dogs were anesthetized using intravenous ketamine (10 mg/kg) and diazepam (0.6 mg/kg) and isoflurane inhalational during the surgical procedure. For the autograft dogs, both PL tendons from the hind paws of the same dog were harvested and immediately replaced the FDP tendons of the second and fifth digits of one paw in Zone II with one graft tendon treated with cd-HA and the other treated with saline as a control. For the allograft treated with cd-HA or saline control dog, however, a FDP tendon repair failure model was used to mimic the clinical scenario, ie, flexor tendon reconstruction in a scarred digit. In this clinically relevant model, the FDP tendon in Zone II was lacerated and repaired first. The dogs were immediately allowed free cage activity after surgery. All repaired FDP tendons ruptured within 1 week as a result of this unprotected postoperative ambulation, which we used as a mechanism to create a scarred tendon bed before reconstruction [29]. Six weeks after primary repair, the digits underwent FDP tendon reconstruction. The allograft FDP tendons were harvested from dogs that were euthanized for other IACUC-approved studies not related to musculoskeletal disorders. The allograft tendons were immediately immersed in liquid nitrogen for 1 minute and then thawed for 5 minutes in warmed saline solution at 37°C. This procedure was repeated five times to induce tenocyte necrosis [23]. The tendons were lyophilized with a custom-made lyophilizer and then gas-sterilized. The graft was rehydrated in a 0.9% NaCl bath in a closed, sterilized container for 24 hours in an incubator at 37°C before graft surgery.
The surgical procedures of the FDP reconstruction either in autograft or allograft followed a standard one-stage flexor tendon reconstruction as previously described for the canine model [28, 29]. Briefly, a space for the graft was created by removing the FDP tendon in Zone II. For both autograft and allograft procedures, one of the surgical digits was randomly selected for treatment with a graft coated with cd-HA gelatin, and the other digit was treated with a graft soaked in saline. A 3-mm drill was used to make a hole at the distal phalanx beneath the FDP tendon attachment. The graft distal end was sutured with 3/0 Ethibond suture (Ethicon, Inc, Somerville, NJ, USA) with modified Pennington locking loops [26]. The suture was passed through the tunnel and out onto the dorsal aspect of the digit, and the distal graft was pulled into the bone tunnel. We used a dorsal button to fix the suture [28]. The proximal end of the graft was sutured to the proximal recipient FDP tendon using a Pulvertaft weave suture. After the tendon graft, we performed a proximal radial neurectomy on the selected surgical forelimb to denervate the triceps muscle and prevent elbow extension and thus weightbearing [5, 27]. The operated paw was then immobilized. For the analgesics, a Buprenex intramuscular injection with a dose of 0.01 mg/kg every 8 hours for 2 days and then 4 mg/kg carprofen was given by subcutaneous injection for 1 week. On postoperative Day 5, rehabilitation was started with a synergistic wrist digit motion protocol (wrist flexion with the digit joints in extension and wrist extension with the digit joints in flexion) performed once daily for 6 weeks, after which the animal was euthanized with an overdose of pentobarbital.
During dissection, the adhesion status in Zone II was grossly assessed by two investigators (RLK, CZ) in a blinded fashion. The adhesion score system was modified based on previous flexor tendon repair grading criteria [26]. The rating scale at each site ranged from 0 (no adhesion) to 4 (very severe) (Table 1). The graft tendons were graded for adhesion formation at two sites: (1) between the tendon and sheath including the pulley and the synovial lining; and (2) between the tendon and tendon bed, including the flexor digitorum superficialis tendon and the surrounding soft tissues of the phalanx. Thus, the total of the scores at the two sites ranged from 0 to 8. Any disagreements in adhesion score were resolved by consensus.
Table 1.
Score for gross evaluation of the adhesion
| Score | Adhesion |
|---|---|
| 0 | None |
| 1 | Light (< 5 mm in length and easy to separate) |
| 2 | Moderate (5–20 mm in length and easy to separate) |
| 3 | Severe (> 20 mm in length and can be separated) |
| 4 | Very severe (> 20 mm in length and cannot be separated) |
After graft dissection, six autograft and eight allograft tendons were cut in 10 mm length at the proximal interphalangeal joint level. The graft tendons were then fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned longitudinally in 5-μm thickness. Hematoxylin and eosin staining was performed. Two to three slides from each sample were examined in a high-resolution microscope linked to a digital image analysis system (microscope: Olympus BX51, Tokyo, Japan; camera: Sony DXC-970MD, 3CCD color camera, Tokyo, Japan) for gross observation of cell distribution, morphology, and appearance of foreign body giant and inflammatory cells. For the cellularity quantification, the cells in one slide from each tendon sample were counted by one investigator (FEK) in a blinded fashion from longitudinally sectional samples with the following protocol. The tendon slides were first observed under low-power magnification (20×), which included the entire tendon section (Fig. 1). Using a stereological randomization technique, 10 areas of interest were randomly selected for cell counting. The scope was moved and focused on one circle (with the letter and number) centered in each grid (Fig. 1) with medium magnification (100×). The microscope was switched to high-power magnification (400×) without any movement on the slide, and cells were counted. Then, the scope was switched to low magnification and moved to the other circle for cell counting. The cell number in 10 viewed areas in each slide was averaged for data analysis.
Fig. 1A–B.
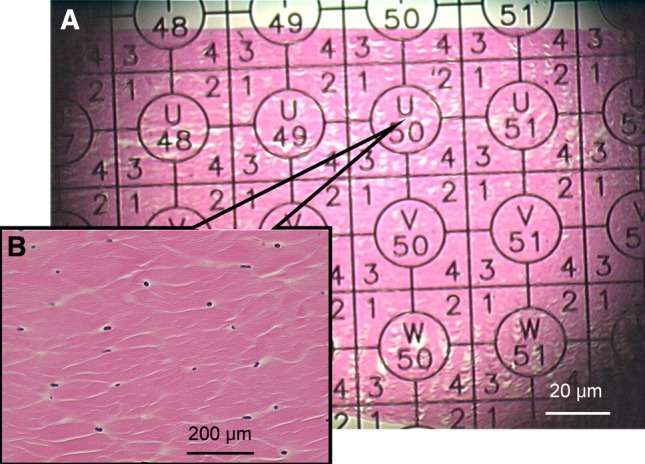
This is a typical slide showing a grid slide at 20× low magnification (A). For cell counting, the scope was moved and focused on the circle (with the letter and number) centered in each grid with a magnification of 100× (B).
Differences in cell count among five groups (autograft-cd-HA, autograft-saline, allograft-cd-HA, allograft-saline, and normal FDP tendon) and adhesion score in four groups (without normal FDP tendon group) were analyzed with analysis of variance and the post hoc Tukey’s honestly significantly different test was applied to assess differences among groups.
Results
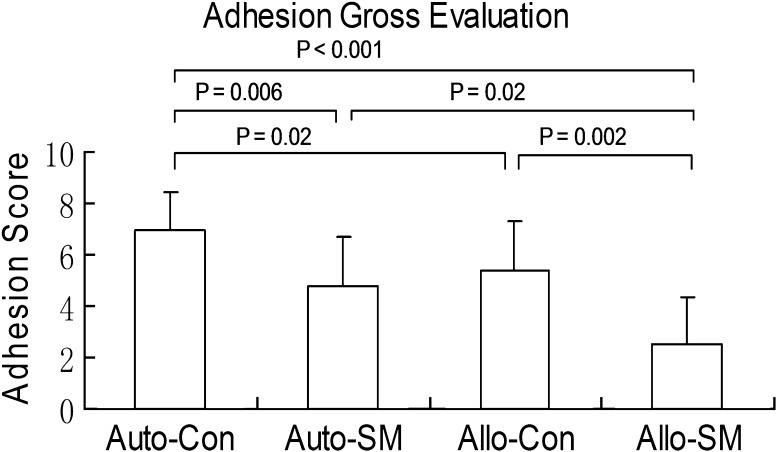
The mean adhesion score of the grafts treated with cd-HA was lower than the nontreated control graft regardless of graft source (p = 0.006 for autograft and p = 0.002 for allograft). The mean adhesion score for the intrasynovial allografts was lower than the extrasynovial autografts for both cd-HA-treated (p = 0.02) and nontreated tendons (p = 0.02) (Fig. 2). The graft tendons with cd-HA treatment displayed a smooth and shining surface. The nontreated graft presented severe adhesions around the graft in Zone II (Fig. 3). However, the adhesions could be easily peeled off the allograft tendons, but not the autografts.
Fig. 2.
Adhesion score was determined by gross observation. Auto-Con = extrasynovial control autograft; Auto-SM = extrasynovial autograft with surface modification; Allo-Con = intrasynovial allograft control group; Allo-SM = intrasynovial allograft with surface modification. This showed that the autograft control group had the most adhesion and allograft with surface treatment had the least adhesion.
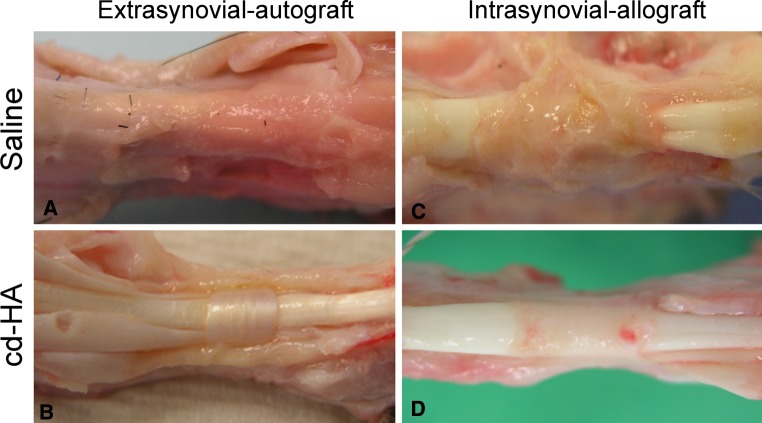
Fig. 3A–D.
Graft treated with cd-HA after 6-week reconstruction displayed a smooth tendon surface without adhesions in both autologous extrasynovial tendon (B) and allograft intrasynovial tendon (D). However, the graft tendons treated with saline presented severe adhesion around the whole flexor sheath in extrasynovial autograft (A) and adhesions around the pulley area in allograft intrasynovial tendons (C).
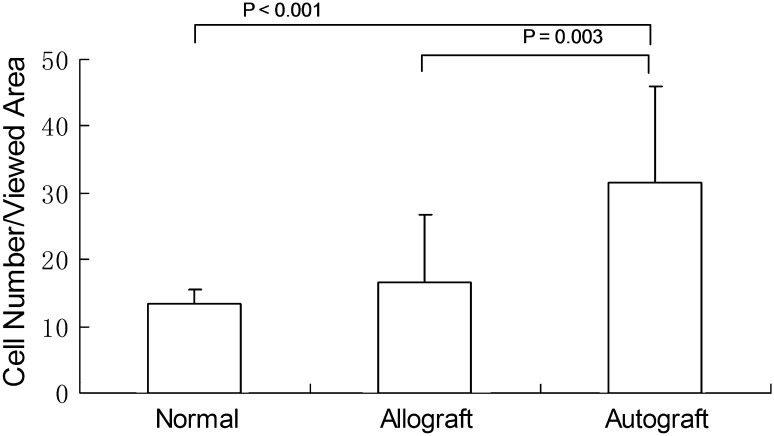
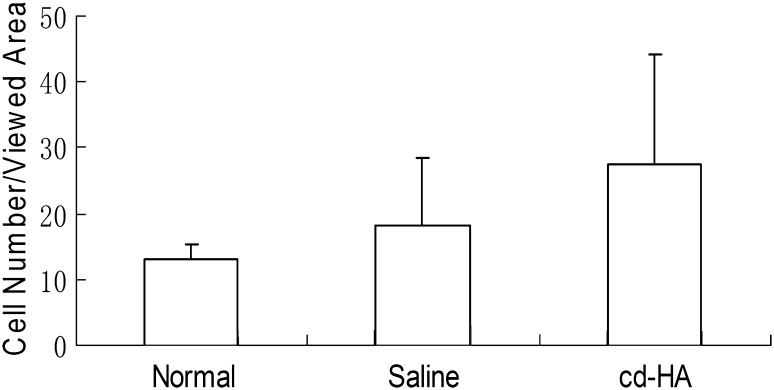
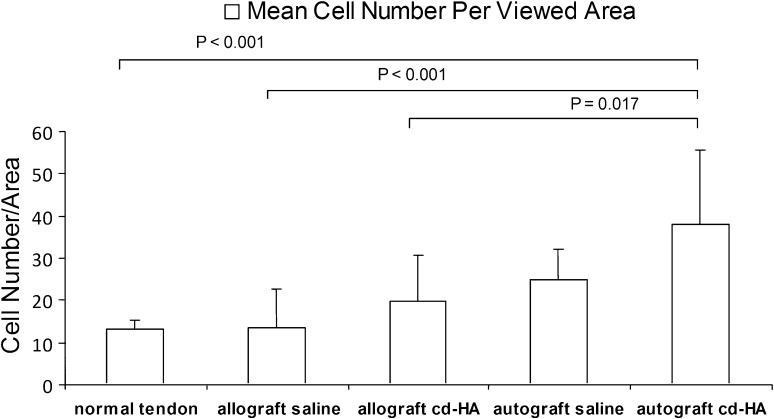
No inflammatory cells or lymphocytes were observed in either allograft or autograft tendons regardless of cd-HA treatment. The mean cell number per viewed area of the autograft tendons was higher than the allograft (p = 0.003) and normal FDP tendons (p = 0.001). There was no difference between normal FDP tendons and allograft (Fig. 4). The mean cell number per viewed area was similar (p = 0.088) among three groups (normal FDP tendon, cd-HA-treated graft, and saline-treated graft) (Fig. 5). The data from each individual construct (normal, auto-cd-HA, auto-saline, allo-cd-HA, and allo-saline) showed a difference among the five groups with an analysis of variance (Pr > F 0.001). The data were analyzed by post hoc test (Tukey’s honestly significant difference) showing the cell number for the autografts treated with cd-HA was higher than the normal FDP tendon (p < 0.001), allograft saline (p < 0.001), and allograft cd-HA (p = 0.017) groups (Fig. 6).
Fig. 4.
Cell density grouped by tendon type in the representative areas of the autograft, allograft, and normal FDS tendon showed that the autograft had higher cell density than the normal and allograft groups.
Fig. 5.
A comparison of the tenocyte counts in the grafts grouped by cd-HA, saline, and normal FDS tendon was not different among groups.
Fig. 6.
The Tukey honestly significant difference for pairwise comparisons was used to analyze the tenocyte counts in all groups with a confidence interval of 95%. Cell density of autograft cd-HA was higher than the normal tendon (p < 0.001), allograft saline (p < 0.001), and allograft cd-HA groups (p = 0.017).
Discussion
Flexor tendons are intrasynovial tendons, ie, a tendon system that includes a synovially lined sheath [2, 8]. This surface structure gives intrasynovial tendons less gliding resistance and more durability (ie, less surface damage with repetitive motion) compared with extrasynovial tendons [24]. Experimental and clinical studies have demonstrated that autologous intrasynovial tendon grafts have less adhesion formation and superior functional outcomes than autologous extrasynovial tendon grafts [1, 12, 15, 17]. Unfortunately, potential sources of intrasynovial tendons available for use as autografts are limited. Recent studies have shown that graft surface modification with cd-HA improved the tendon gliding ability and improved the digit function using either intrasynovial allografts or extrasynovial autograft. The current study focused on the adhesion formation and the cell penetration or population after tendon grafting.
There are several limitations in the current study. First, although there were four parameters, including graft source (allograft, autograft) and tendon type (intrasynovial, extrasynovial), we only compared extrasynovial autograft and intrasynovial allograft. The limited selection was based on the usual clinical situation, in which intrasynovial autograft is not usually available and clinically extrasynovial allografts are not used for finger flexor tendon reconstruction. Second, the autograft model was performed as an acute primary reconstruction in a normal tendon sheath, whereas the allograft model was based on a repair failure/secondary reconstruction model. However, the differences between these two models would probably not affect the final conclusion because the allograft reconstructed based on a scar digit likely had more adhesions compared with the autograft that was reconstructed on a normal digit. Third, we did not assess collagen types, cytokines, or growth factors because we focused on the adhesion formation and cellularity between these two types of graft model, both of which could be done by simple hematoxylin and eosin staining. Fourth, the inter- and intraobserver reliability for adhesion and cell counting was not tested. Adhesion score was graded by two investigators and the disagreements in adhesion score resolved by consensus. The cell counting was performed by one investigator in a blinded fashion. Fifth, the histology was only studied at one time point, 6 weeks after grafting. Long-term followup may be necessary in future studies. Finally, the study design and scope were limited on adhesions score and cell density. However, these important data have been not reported.
We found adhesion formation decreased with cd-HA surface modification in both autograft and allograft tendons. These results are consistent with previous reports [28, 29], which have shown that cd-HA surface modification in both extrasynovial autograft and intrasynovial allograft improved the digit function and decreased tendon friction. We also found cd-HA treatment of grafted tendons did not affect cellularity in either the extrasynovial autografts or intrasynovial allografts. Previous studies demonstrated that graft distal attachment strength was similar between surface modification and control for both extrasynovial autograft tendons and intrasynovial allograft tendons [28, 29]. However, comparison between autograft and allograft in the current study demonstrated that the cellularity of the autografts was higher than the allografts. This is expected because the autografts were not decellularized before surgery, whereas the allografts were. It is likely that at least some autograft cells survived during tissue regeneration, and we believe that this explains the difference in cellularity.
In conclusion, cd-HA surface treatment decreased adhesions for flexor tendon reconstruction using either autograft or allograft tendons compared with the graft treated with saline. These findings were in consensus with previous reports that showed improved digit function and graft gliding ability after graft tendons were treated with cd-HA. The current study also revealed that adhesion formation in allograft intrasynovial tendon was less than the autograft extrasynovial tendon. However, the cellularity in the allograft decreased compared with the autograft, which could interpret the delayed tendon/bone interface healing in allograft reported previously [29]. cd-HA treatment did not affect the cellularity regardless of auto- or allograft model.
Acknowledgments
We thank Drs Y. L. Sun, S. L. Moran, K. N. An, and P. C. Amadio and Ms Ramona L. Kirk for their great contributions and assistance on animal surgeries and care, data collection and interpretation, and manuscript editing.
Footnotes
The institution of one of the authors (CZ) received funding from the Orthopedic Research Education Foundation and the Musculoskeletal Transplant Foundation.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Mayo Clinic, Rochester, MN, USA.
References
- 1.Abrahamsson SO, Gelberman RH, Amiel D, Winterton P, Harwood F. Autogenous flexor tendon grafts: fibroblast activity and matrix remodeling in dogs. J Orthop Res. 1995;13:58–66. doi: 10.1002/jor.1100130110. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsson SO, Gelberman RH, Lohmander SL. Variations in cellular proliferation and matrix synthesis in intrasynovial and extrasynovial tendons: an in vitro study in dogs. J Hand Surg [Am]. 1994;19:259–265. doi: 10.1016/0363-5023(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 3.Asencio G, Abihaidar G, Leonardi C. Human composite flexor tendon allografts. A report of two cases. J. Hand Surg [Br]. 1996;21:84–88. doi: 10.1016/S0266-7681(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 4.Bertelli JA, Santos MA, Kechele PR, Rost JR, Tacca CP. Flexor tendon grafting using a plantaris tendon with a fragment of attached bone for fixation to the distal phalanx: a preliminary cohort study. J Hand Surg [Am]. 2007;32:1543–1548. doi: 10.1016/j.jhsa.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Bishop AT, Cooney WP, 3rd, Wood MB. Treatment of partial flexor tendon lacerations: the effect of tenorrhaphy and early protected mobilization. J Trauma. 1986;26:301–312. doi: 10.1097/00005373-198604000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cameron RR, Conrad RN, Sell KW, Latham WD. Freeze-dried composite tendon allografts: an experimental study. Plast Reconstr Surg. 1971;47:39–46. doi: 10.1097/00006534-197101000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Coyle MP, Jr, Leddy TP, Leddy JP. Staged flexor tendon reconstruction fingertip to palm. J Hand Surg [Am]. 2002;27:581–585. doi: 10.1053/jhsu.2002.34319. [DOI] [PubMed] [Google Scholar]
- 8.Duffy FJ, Seiler JG, Hergrueter CA, Kandel J, Gelberman RH. Intrinsic mitogenic potential of canine flexor tendons. J Hand Surg [Br]. 1992;17:275–277. doi: 10.1016/0266-7681(92)90114-H. [DOI] [PubMed] [Google Scholar]
- 9.Finsen V. Two-stage grafting of digital flexor tendons: a review of 43 patients after 3 to 15 years. Scand J Plast Reconstr Surg Hand Surg. 2003;37:159–162. doi: 10.1080/03844310310007773. [DOI] [PubMed] [Google Scholar]
- 10.Hasslund S, Jacobson JA, Dadali T, Basile P, Ulrich-Vinther M, Soballe K, Schwarz EM, O’Keefe RJ, Mitten DJ, Awad HA. Adhesions in a murine flexor tendon graft model: autograft versus allograft reconstruction. J Orthop Res. 2008;26:824–833. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda J, Zhao C, Sun Y-L, An K-N, Amadio PC. Carbodiimide-derivatized hyaluronic acid surface modification of lyophilized flexor tendon: a biomechanical study in a canine in vitro model. J Bone Joint Surg Am. 2010;92:388–395. doi: 10.2106/JBJS.H.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leversedge FJ, Zelouf D, Williams C, Gelberman RH, Seiler JG., 3rd Flexor tendon grafting to the hand: an assessment of the intrasynovial donor tendon—a preliminary single-cohort study. J Hand Surg [Am]. 2000;25:721–730. doi: 10.1053/jhsu.2000.9413. [DOI] [PubMed] [Google Scholar]
- 13.Liu TK, Yang RS. Flexor tendon graft for late management of isolated rupture of the profundus tendon. J Trauma. 1997;43:103–106. doi: 10.1097/00005373-199707000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Momose T, Amadio PC, Sun YL, Zhao C, Zobitz ME, Harrington JR, An KN. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219–224. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi M, Seiler JG, 3rd, Boardman ND, 3rd, Tramaglini DM, Gelberman RH, Woo SL. Tensile properties of canine intrasynovial and extrasynovial flexor tendon autografts. J Hand Surg [Am]. 1997;22:457–463. doi: 10.1016/S0363-5023(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 16.Peacock EE, Jr, Madden JW. Human composite flexor tendon allografts. Ann Surg. 1967;166:624–629. doi: 10.1097/00000658-196710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiler JG, 3rd, Chu CR, Amiel D, Woo SL, Gelberman RH. The Marshall R. Urist Young Investigator Award. Autogenous flexor tendon grafts. Biologic mechanisms for incorporation. Clin Orthop Relat Res. 1997;345:239–247. doi: 10.1097/00003086-199712000-00034. [DOI] [PubMed] [Google Scholar]
- 18.Singer M, Maloon S. Flexor tendon injuries: the results of primary repair. J Hand Surg [Br]. 1988;13:269–272. doi: 10.1016/0266-7681(88)90083-6. [DOI] [PubMed] [Google Scholar]
- 19.Smith P, Jones M, Grobbelaar A. Two-stage grafting of flexor tendons: results after mobilisation by controlled early active movement. Scand J Plast Reconstr Surg Hand Surg. 2004;38:220–227. doi: 10.1080/02844310410024566. [DOI] [PubMed] [Google Scholar]
- 20.Stark HH, Anderson DR, Zemel NP, Boyes JH, Ashworth CR, Rickard TA. Bridge flexor tendon grafts. Clin Orthop Relat Res. 1989;242:51–59. [PubMed] [Google Scholar]
- 21.Strickland JW. Management of acute flexor tendon injuries. Orthop Clin North Am. 1983;14:827–849. [PubMed] [Google Scholar]
- 22.Sun YL, Yang C, Amadio PC, Zhao C, Zobitz ME, An KN. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Tejwani SG, Shen W, Fu FH. Soft tissue allograft and double-bundle reconstruction. Clin Sports Med. 2007;26:639–660. doi: 10.1016/j.csm.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama S, Amadio PC, Coert JH, Berglund LJ, An KN. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg Am. 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma Inj Infect Crit Care. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Amadio PC, Momose T, Couvreur P, Zobitz ME, An KN. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor digitorum profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg Am. 2002;84:78–84. [PubMed] [Google Scholar]
- 27.Zhao C, Amadio PC, Zobitz ME, Momose T, Couvreur P, An KN. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop Relat Res. 2002;396:223–230. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Sun Y-L, Amadio PC, Tanaka T, Ettema AM, An K-N. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao C, Sun Y-L, Ikeda J, Kirk RL, Thoreson AR, Moran SL, An K-N, Amadio PC. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am. 2010;92:2817–2828. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]