Abstract
Background
The antifibrinolytic tranexamic acid reduces surgical blood loss, but studies have not identified an optimal regimen.
Questions/purposes
We studied different dosages, timings, and modes of administration to identify the most effective regimen of tranexamic acid in achieving maximum reduction of blood loss in TKA.
Methods
We prospectively studied five regimens (four intravenous, one local; 40 patients each) with a control group (no tranexamic acid). The four intravenous (10-mg/kg dose) regimens included (1) intraoperative dose (IO) given before tourniquet deflation, (2) additional preoperative dose (POIO), (3) additional postoperative dose (IOPO), and (4) all three doses (POIOPO). The fifth regimen was a single local application (LA). Two independent parameters of drain loss and total blood loss, calculated by the hemoglobin balance method, were evaluated statistically.
Results
Both parameters were reduced in all five regimens as against the control. A significant reduction in drain loss was seen in the POIO, IOPO, and POIOPO groups whereas total blood loss was significantly reduced in the POIO, POIOPO, and LA groups. The POIOPO group had the least drain loss (303 mL) and least total blood loss (688 mL). The IO group had the greatest drain loss and the IOPO group the greatest total blood loss.
Conclusions
Single-dose tranexamic acid did not give effective results. The two-dose regimen of POIO was the least amount necessary for effective results. When compared against the control, this regimen produced reduction of drain loss and total blood loss, whereas the IOPO regimen did not. The three-dose regimen of POIOPO produced maximum effective reduction of drain loss and total blood loss.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Patients undergoing TKA are at an increased risk of perioperative bleeding. Estimated blood loss reported for TKA varies between 800 mL to 1800 mL [2, 7, 13, 20, 27], mostly related to surgical trauma. Trauma also induces fibrinolysis [2, 3, 16, 26], which in TKA is enhanced by the use of a pneumatic tourniquet [11, 17, 24, 26]. This further contributes to bleeding during and after surgery [5]. Replacing blood loss by blood transfusions is considered undesirable owing to associated risks of immunologic reactions and disease transmission. Control of bleeding with antifibrinolytic agents may be a preferable alternative.
Tranexamic acid inhibits fibrinolysis by competitively blocking the lysine-binding sites of plasminogen [8, 10]. It has been used successfully to reduce the blood loss in cardiac surgery [22], liver surgery [4], and gynecology [30]. The efficacy of tranexamic acid administered intravenously in preventing blood loss in TKA has been established in numerous studies [2, 6, 12–15, 29]. Topical application of tranexamic acid intraoperatively has shown a decrease in blood loss after TKA without the disadvantage of systemic absorption and thromboembolic complications [31].
Despite several clinical studies proving the efficacy of tranexamic acid with single or multiple boluses of different sizes with or without subsequent infusions, no consensus has been reached regarding the optimal regimen for tranexamic acid administration. A previous study established the need for a therapeutic plasma concentration of 10 ng/mL and an 80% reduction in the activity of plasminogen activator for adequate suppression of fibrinolysis in tissues [25]. An intravenous dose of tranexamic acid of 10 mg/kg maintains such a plasma concentration for approximately 3 hours [25].
A literature review shows studies where tranexamic acid was administered either as a single dose just before the release of the tourniquet [1, 13] or along with an additional postoperative dose [2, 6, 12, 14, 32]. Inflation of the tourniquet stimulates the fibrinolytic system; administrating tranexamic acid before tourniquet inflation was studied by Tanaka et al. [29] and Jansen et al. [15]. There also are indications that local enhancement of fibrinolysis elicited by tourniquet application may affect hemostasis for a considerably longer time than the surgical procedure [14]. Thus, it is believed it also may be beneficial to maintain antifibrinolytic treatment in the postoperative period. Use of antifibrinolytic agents such as aprotinin has been associated with increased thrombotic tendency in the form of myocardial infarction, renal dysfunction, or cerebral thrombosis [21, 28]. However some studies [29, 32] suggest tranexamic acid per se is not the cause of increased thrombosis in TKA, but the concern persists.
We have used tranexamic acid in TKA since March 2009. The dramatic reduction in blood loss we experienced prompted this study, with the aim of identifying a regimen that is the most effective. We compared two independent parameters, drain loss and total blood loss, between five administration regimens of tranexamic acid (four intravenous, one local application) and a control group (no tranexamic acid). In addition, the incidence of thromboembolic events was studied during the postoperative period and compared among all groups.
Patients and Methods
The study was approved by our institutional review board. We conducted the study between August 2010 and April 2011. We prospectively enrolled 206 consecutive patients with a diagnosis of osteoarthritis scheduled to have primary, unilateral TKA. All patients had normal preoperative platelet count, normal prothrombin time, normal partial thromboplastin time, and normal international normalized ratio. The exclusion criteria were a known allergy to tranexamic acid; preoperative hepatic or renal dysfunction; serious cardiac or respiratory disease; congenital or acquired coagulopathy; and a history of thromboembolic disease. Patients taking antiplatelet agents were asked to stop them at least 7 days before surgery. For the control group, we selected a similar cohort of 40 patients who underwent TKA by the same surgeon in January and February 2009, when tranexamic acid was not in use.
The patients were randomized into five groups and received tranexamic acid as follows: (1) 10 mg/kg 15 minutes before deflation of the tourniquet as an intraoperative dose (IO group); (2) 10 mg/kg 15 minutes before deflation of the tourniquet as an intraoperative dose and 10 mg/kg 3 hours after the first dose as a postoperative dose (IOPO group); (3) 10 mg/kg at least 20 minutes before tourniquet inflation as a preoperative dose and 10 mg/kg 15 minutes before deflation of the tourniquet as an intraoperative dose (POIO group); (4) 10 mg/kg 20 minutes before tourniquet application as a preoperative dose, 10 mg/kg 15 minutes before deflation of the tourniquet as an intraoperative dose, and 10 mg/kg 3 hours after the second dose as a postoperative dose (POIOPO group); and (5) 3 g diluted in 100 mL normal saline applied locally after cementing the implant and before tourniquet release (LA group). At least 5 minutes of contact time was allowed before the tourniquet was deflated.
In our earlier patients not treated with tranexamic acid, the average postoperative hemoglobin loss over 5 days was approximately 150 g (SD, 90 g). We considered a reduction in this loss by 1/3 to approximately 100 g (SD, 60 g) as clinically important. If any of the treatment groups caused such a reduction, the study should have a power of 80% to detect it at a significance level of 5%. Using these inputs, the Stata® 8.2 software (Stata Corp, College Station, TX, USA) found a sample size of 37 per group would be needed, which we rounded to 40 per group.
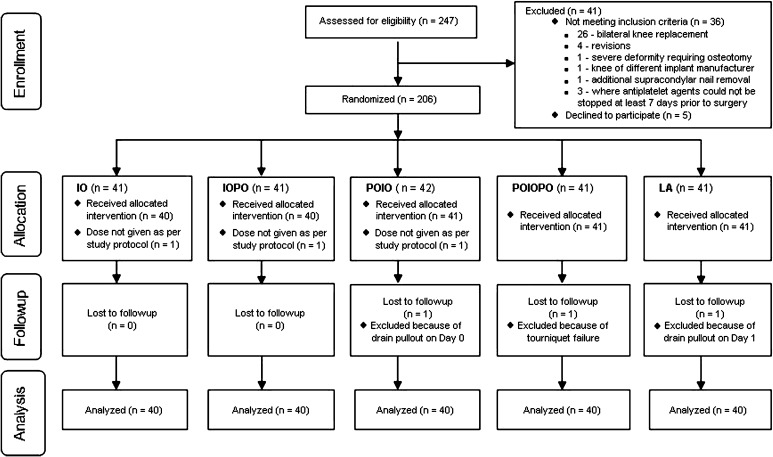
We randomized 206 patients to the study regimens to achieve our target sample size of 200 patients (40 in each study group). The additional six recruits were required because we had to exclude six patients after randomization for various reasons (Fig. 1). The trial ended when each group had 40 patients available for analysis. There were no differences in the general demographics among the groups (Table 1). Tourniquet time and pressure among the groups also were comparable (Table 2).
Fig. 1.
A flow diagram shows the number of patients assessed and included at each stage of the trial.
Table 1.
Preoperative comparison of groups
| Variable | Control | IO | IOPO | POIO | POIOPO | LA | p value* |
|---|---|---|---|---|---|---|---|
| Number of patients | 40 | 40 | 40 | 40 | 40 | 40 | |
| Sex | 0.37 | ||||||
| Male | 4 (10) | 10 (25) | 11 (28) | 8 (20) | 7 (18) | 6 (15) | |
| Female | 36 (90) | 30 (75) | 29 (72) | 32 (80) | 33 (82) | 34 (85) | |
| Age (years)† | 66.2 (7.2) | 67.3 (9.1) | 68.3 (8.0) | 67.4 (8.4) | 66.8 (7.0) | 67.4 (7.9) | 0.90 |
| BMI†,‡ | 30.8 (5.6) | 29.4 (5.5) | 28.6 (4.2) | 27.8 (4.8) | 31.2 (7.3) | 30.9 (5.2) | 0.025 |
| Peripheral blood volume (mL)† | 4085 (648) | 4015 (631) | 3989 (625) | 3933 (681) | 4066 (614) | 3956 (544) | 0.87 |
* Using chi-square test for sex and ANOVA for the others; †values are expressed as mean, with SD in parentheses; the remaining values are expressed as number of patients, with percentage in parentheses, ‡using Scheffe’s test, p > 0.05 for all pairwise comparisons; therefore, no significant differences; IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
Table 2.
Comparison of groups regarding tourniquet time and pressure
| Variable | Control | IO | IOPO | POIO | POIOPO | LA | p value* |
|---|---|---|---|---|---|---|---|
| Number of patients | 40 | 40 | 40 | 40 | 40 | 40 | |
| Tourniquet time (minutes)† | 78 (12.5) | 79 (10.7) | 79 (10.6) | 78 (8.5) | 80 (14.3) | 79 (8.7) | 0.94 |
| Tourniquet pressure (mm Hg)†,‡ | 294 (22.8) | 288 (18.7) | 281 (14.3) | 282 (13.8) | 288 (18.8) | 292 (18.6) | 0.013 |
* Using ANOVA for comparison among groups; †values are expressed as mean, with SD in parentheses; ‡using Scheffe’s test, p > 0.05 for all pairwise comparisons; therefore, no significant differences; IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
The randomization was performed on the day of surgery from a set of 200 envelopes (40 envelopes for each group). The envelope was picked randomly by a junior resident on the day of the surgery and it was conveyed to the anesthetist. If a patient was removed from the study after recruitment, a new envelope for the same regimen was added to the box and mixed. There were no changes in methods after commencement of the trial.
All surgeries were performed by the senior author (RNM) using computer navigation and the PFC® Sigma® series of total knee prostheses (DePuy Orthopaedics Inc, Warsaw, IN, USA). After elevation of the limb and exsanguination with an Esmarch bandage, the tourniquet was inflated to between 270 to 320 mm Hg. Skin incisions were midline, and a midvastus arthrotomy was performed for all patients. Cement with gentamicin was used for fixation of the prosthesis. After cementing the implant, the tourniquet was deflated and hemostasis was achieved by electrocoagulation. The polyethylene liner then was inserted followed by layered closure of the wound. There was an interval of approximately 40 to 50 minutes between tourniquet deflation and conclusion of surgery. In each knee, one intraarticular drain and one subcutaneous drain were placed and connected to vacuum drain bottles. The deep drain was blocked for 2 hours after the procedure. The day of operation was considered Day 0. For all patients who underwent surgery between 8 AM and 12 noon, Day 1 drains were recorded at 8 AM; and for all patients who underwent surgery between 12 noon to 4 PM, the Day 1 drains were recorded at 12 noon. Thus, there was a variable duration ranging from 20 to 24 hours for Day 1 drain collection, but this was distributed among the groups as the patients were randomized. The Day 2 drains were recorded at the same time, ie, 8 AM for all patients. All patients began continuous passive motion on Day 2 after surgery. Our protocol for postoperative rehabilitation was the same and fixed for all patients. Each patient was made to stand on Day 2 after surgery and discharged on Day 5 after surgery. The blood hemoglobin concentration was determined preoperatively and on Days 2 and 5 after surgery for all patients and on Day 1 where indicated.
Blood transfusions were given to patients as per the following protocol: (1) all patients whose hemoglobin was less than 8.5 g/dL; (2) all patients with cardiac disorders whose hemoglobin was less than 10 g/dL; and (3) patients whose hemoglobin was 8.5 to 10 g/dL but who had symptoms related to anemia develop, such as tachycardia, tachypnea, or decreased exercise tolerance.
We recorded blood transfusions for quantity and determined the hemoglobin concentration of each transfused unit. We had advised blood transfusions to patients based on the protocol mentioned above, but not all patients consented to transfusion. There was a variability factor in the number of patients who were advised regarding transfusions and those receiving transfusions. Although recorded, we did not include the transfusion requirements as a parameter for comparison.
We studied two independent parameters to determine the efficacy of tranexamic acid on reducing blood loss. One was the drain loss which was the sum of the drain collection on Day 1 (Day 1 drain loss) and on Day 2 (Day 2 drain loss). The other parameter was the total blood loss calculated by the hemoglobin balance method [12, 19], which involved measuring hemoglobin levels at several stages. This calculation gave a better estimation of the combined external loss, ie, intraoperative loss plus drain loss and the internal (hidden) blood loss. The patient, the floor nurse who recorded the drain collections, and the hospital laboratory technician who estimated hemoglobin were blinded to the regimen received by the patient. Local application of tranexamic acid had to occur during surgery, therefore the senior author who was the operating surgeon could not have been blinded to the regimen being given to the patient.
For calculation of the total blood loss by the hemoglobin balance method, the patient’s blood volume was first calculated using the formula of Nadler et al. [23] as follows: patient’s blood volume = (k1 * height3 [meters]) + (k2 * weight [kilograms]) + k3, where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for men and k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women.
The loss of hemoglobin then was estimated according to the following formula [11, 18]: Hbloss = patient’s blood volume * (Hbi − Hbe) * 0.001 + Hbt, where Hbloss (grams) was the amount of hemoglobin lost up to Day 5 after surgery, Hbi (grams/liter) was the hemoglobin concentration before surgery, Hbe (grams/liter) was the hemoglobin concentration on the fifth day after surgery, and Hbt (grams) was the amount of hemoglobin transfused. From these, the total blood loss in milliliters was calculated as follows [12, 19]: total blood loss = 1000 * Hbloss/Hbi.
Our deep vein thrombosis (DVT) prophylaxis protocol was as follows: (1) ankle and foot movement exercises were started as soon the anesthesia effect wore off; (2) low-molecular-weight heparin was given to all patients beginning on Day 1 and continued until the time of discharge; and (3) below-knee stockings were given to all patients.
The patients were monitored for occurrence of any complications, particularly DVT and thromboembolism during the hospital stay and for 3 months postoperatively.
We analyzed sex differences among the groups using the chi-square test. We analyzed group differences in the study parameters (drain loss, Day 1 drain loss, Day 2 drain loss, total blood loss) and demographic and operative parameters (age, BMI, patient’s blood volume, tourniquet time, and pressure) using one-way ANOVA; if a significant difference among the groups was found, pairwise differences between groups were examined by Scheffe’s test. The significance level used for all tests was p ≤ 0.05. Analyses were performed using Stata® 8.2 software.
Results
The overall drain loss was lower in each study group than in the control group (Table 3). However, the difference was significant only in the IOPO, POIO, and POIOPO groups versus the control group. In the study groups, the mean drain loss was least (303 mL) in the POIOPO group and greatest (436 mL) in the IO group (p = 0.03). The other three study groups showed comparable drain loss with no significance among all groups. When we studied drain loss on Days 1 and 2 separately (Table 4), we observed the Day 2 drain loss was comparable in all five study groups and the control group. However, the Day 1 drain loss differed and accounted for the difference in the total drain loss.
Table 3.
Drain loss compared among groups
| Variable | Control | IO | IOPO | POIO | POIOPO | LA | p value* |
|---|---|---|---|---|---|---|---|
| Drain loss (mL)† | 500 (184.1) | 436 (164.8) | 349 (179.4) | 361 (162.4) | 303 (133.0) | 385 (186.2) | 0.000 |
| p value‡ | |||||||
| IO | 0.722 | ||||||
| IOPO | 0.008 | 0.384 | |||||
| POIO | 0.023 | 0.569 | 1.000 | ||||
| POIOPO | 0.000 | 0.033 | 0.915 | 0.792 | |||
| LA | 0.109 | 0.880 | 0.967 | 0.995 | 0.446 | ||
* Using ANOVA for comparison among groups; †values are expressed as mean, with SD in parentheses; ‡using Scheffe’s test for pairwise comparisons; IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
Table 4.
Days 1 and 2 drain loss compared among groups
| Variable | Control | IO | IOPO | POIO | POIOPO | LA | p value* |
|---|---|---|---|---|---|---|---|
| Day 1 drain loss (mL)† | 366 (149.3) | 268 (108.0) | 201 (100.9) | 198 (103.5) | 159 (86.4) | 244 (142.2) | 0.000 |
| p value‡ | |||||||
| IO | 0.018 | ||||||
| IOPO | 0.000 | 0.268 | |||||
| POIO | 0.000 | 0.211 | 1.000 | ||||
| POIOPO | 0.000 | 0.005 | 0.764 | 0.828 | |||
| LA | 0.001 | 0.971 | 0.766 | 0.694 | 0.071 | ||
| Day 2 drain loss (mL)† | 134 (62.1) | 168 (89.0) | 147 (93.8) | 164 (105.7) | 143 (72.2) | 142 (72.0) | 2.4792 |
* Using ANOVA for comparisons among groups; †values are expressed as mean, with SD in parentheses; ‡using Scheffe’s test for pairwise comparisons; IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
The total blood loss was lower in each study group than in the control group (Table 5). The difference was significant in the POIO, POIOPO, and LA groups versus the control group. In the study groups, the mean total blood loss was least (688 mL) in the POIOPO group and greatest (864 mL) in the IOPO group, but the difference between them was not significant. Comparing the effect of single LA versus single intravenous dose (IO), the LA group had greater reduction of drain loss and total blood loss compared with the IO group, but the difference was not significant.
Table 5.
Total blood loss compared among groups
| Variable | Control | IO | IOPO | POIO | POIOPO | LA | p value* |
|---|---|---|---|---|---|---|---|
| Total blood loss (mL)† | 1097 (674.2) | 824 (226.8) | 864 (315.0) | 782 (233.1) | 688 (308.2) | 809 (341.1) | 0.0012 |
| p value‡ | |||||||
| IO | 0.073 | ||||||
| IOPO | 0.194 | 0.999 | |||||
| POIO | 0.020 | 0.999 | 0.968 | ||||
| POIOPO | 0.001 | 0.772 | 0.516 | 0.944 | |||
| LA | 0.048 | 1.000 | 0.995 | 1.000 | 0.846 |
* Using ANOVA for comparison among groups; †values are expressed as mean, with SD in parentheses; ‡using Scheffe’s test for pairwise comparisons; IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
The number of patients advised regarding blood transfusions and the number who actually received them (Table 6) were not the same. No difference was found among the groups regarding the mean number of transfusions given or with respect to the mean volume of blood transfused. Also, there was no difference in the day of ambulation or the day of discharge in all groups.
Table 6.
Comparison of groups for transfusions
| Group | Number of patients receiving transfusion | Number of patients refusing transfusions | Total number of transfusions indicated as per protocol |
|---|---|---|---|
| Control | 7 | 3 | 10 |
| IO | 5 | 3 | 8 |
| IOPO | 7 | 0 | 7 |
| POIO | 1 | 3 | 4 |
| POIOPO | 3 | 1 | 4 |
| LA | 3 | 2 | 5 |
IO = intraoperative intravenous dose; POIO = intraoperative intravenous dose with an additional preoperative dose; IOPO = intraoperative intravenous dose with an additional postoperative dose; POIOPO = all three doses; LA = single local application.
No difference was observed regarding the occurrence of adverse effects in any particular group. Three patients had clinical suspicion of DVT and all were from the single-dose groups: two from the IO group and one from the LA group. The duplex Doppler study of each of these patients was negative.
Discussion
The antifibrinolytic tranexamic acid reduces surgical blood loss, but studies have not identified any preferred regimen. We performed this study to identify the most effective regimen of tranexamic acid by comparing drain loss and total blood loss between five administration regimens of tranexamic acid (four intravenous, one local application) and a control group (no tranexamic acid). In addition, we studied the incidence of thromboembolic events in the postoperative period and compared them among all groups.
This study has some limitations. One is that the control group was retrospective. We started using tranexamic acid in March 2009 and by then its efficacy in reducing blood loss in TKA was well established. Our experience with tranexamic acid revealed a dramatic reduction in the amount of drain collection and number of transfusions needed. We performed this study not to confirm the efficacy of tranexamic acid, an already established fact, but to identify its most effective regimen for administration. In light of these observations, we thought it inappropriate to deprive patients in a prospective untreated control group of the benefits of tranexamic acid and subject them to increased blood loss and probably more transfusions. To avoid bias, we studied the general demographics (Table 1) and the tourniquet time and pressure (Table 2) in the control group and confirmed they were statistically similar to the other groups; therefore, the comparisons were justified. Second, no cost benefit analysis was done for this study. Finally, the study was powered mainly to detect a difference between control and active groups based on certain assumptions that have been explained. It was not powered to detect any difference among the five active groups because there were no grounds on which assumptions could be made regarding the size and variability of the effect of different active regimens. This study would provide some estimates of these, and if any of the differences were clinically large, but not statistically significant, an additional study would be required to test their significance. Even if some assumptions were to be made for the effect size and variability of the different active regimens, it would involve 15 possible comparisons as there were six groups. For a study with so many comparisons, the formal significance level would need to be proportionately low and this would increase the sample size per group to more than double for even the largest difference, which would be impractical.
In addition to measuring drain loss, we calculated the total blood loss using the hemoglobin balance method. One study suggested the latter method is more accurate because the hemoglobin concentration of drains can be variable and because hidden surgical blood loss and intraoperative blood loss should be accounted for [12].
The combined results with both parameters showed the POIO and POIOPO groups had reduced blood loss as compared with the control group. This would suggest a preoperative dose made a difference and should be part of any intravenous regimen of tranexamic acid. Fibrinolysis activation begins with surgical trauma and is further enhanced by tourniquet inflation. Giving tranexamic acid preoperatively 15 minutes before tourniquet inflation so that it reaches its peak plasma concentration in the surgically treated limb before tourniquet inflation would deactivate fibrinolysis as soon as it starts. Fibrinolysis, being a cascade reaction, is best inhibited in the initial stages [15], and this is best achieved with a preoperative dose. Tanaka et al. [29] came to the same conclusion regarding the preoperative dose.
A single intraoperative intravenous dose decreased the drain loss and total blood loss as compared with the control group, but the difference was not significant. A single intraoperative local application decreased the total blood loss, which was significant, but not the drain loss. Wong et al. [31] reported the same findings. Therefore, a single-dose regimen, which is widely used, is not the most effective regimen. If however one elects to use just a single dose, then local application is recommended rather than an intravenous dose. Local tranexamic acid application would avoid problems associated with systemic absorption [31] and it would take care of the reported fibrinolysis that predominantly is activated locally in the surgically treated tissue [26]. Our study also showed the total blood loss was reduced with local application.
Adding either a preoperative or a postoperative dose to the intraoperative dose in the POIO and IOPO groups did cause an additional reduction, but the differences in the POIO versus IO groups and the IOPO versus IO groups were not significant. Addition of a preoperative and postoperative dose to the intraoperative dose in the POIOPO group brought about maximum reduction in drain loss (with significance) and total blood loss compared with the single intraoperative dose.
A few studies have shown, if the blood loss in the control group is 1 L or less [9, 10, 18], the effect of tranexamic acid will not be appreciable. Despite the loss in the control group being approximately 1 L in our patients, the POIO and POIOPO regimens still showed a reduction in drain loss and total blood loss.
No major thromboembolic complication occurred in our patients, corroborating earlier studies reporting the same [29, 32].
A single-dose regimen of tranexamic acid cannot be recommended as the most effective regimen. For a single dose, local intraoperative application produced reduction in total blood loss when compared with the control whereas a single intravenous intraoperative dose did not. A two-dose regimen of a preoperative dose and intraoperative dose is the least necessary regimen for effective reduction in drain loss and total blood loss. When compared against the control, the two-dose POIO regimen produced a reduction in drain loss and total blood loss whereas the two-dose IOPO regimen did not. The POIOPO regimen was the most effective regimen in our study, with maximum reduction in drain loss and total blood loss, but further studies with a larger sample size are required to prove whether it is better than the POIO regimen. Based on our findings, we now use a regimen of three doses but the intraoperative dose is a local application (POLAPO). The study of this new regimen is ongoing.
Acknowledgments
We thank Arun Nanivadekar MD MSc, Medical Research Consultant, Lilavati Hospital, for assistance with statistical analysis of our data.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was conducted at Lilavati Hospital and Research Centre.
References
- 1.Benoni G, Carlsson A, Petersson C, Fredin H. Does tranexamic acid reduce blood loss in knee arthroplasty? Am J Knee Surg. 1995;8:88–92. [PubMed] [Google Scholar]
- 2.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomized, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–440. [PubMed] [Google Scholar]
- 3.Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 1997;85:195–206. doi: 10.1016/S0049-3848(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 4.Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, Roger SL, Glynn MF. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85:1043–1048. doi: 10.1097/00000542-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Burkart BC, Bourne RB, Rorabeck CH, Kirk PG, Nott L. The efficacy of tourniquet release in blood conservation after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:147–152. [PubMed] [Google Scholar]
- 6.Camarasa MA, Olle G, Serra-Prat M, Martin A, Sanchez M, Ricos P, Perez A, Opisso L. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–582. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 7.Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clin Orthop Relat Res. 1991;269:98–101. [PubMed] [Google Scholar]
- 8.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 9.Engel JM, Hohaus T, Ruwoldt R, Menges T, Jürgensen I, Hempelmann G. Regional hemostatic status and blood requirements after total knee arthroplasty with and without tranexamic acid or aprotinin. Anesth Analg. 2001;92:775–780. doi: 10.1213/00000539-200103000-00041. [DOI] [PubMed] [Google Scholar]
- 10.Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18:132–138. [PubMed] [Google Scholar]
- 11.Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63:461–465. [PubMed] [Google Scholar]
- 12.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 13.Hiippala S, Strid L, Wennerstrand M, Arvela V, Mäntylä S, Ylinen J, Niemelä H. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534–537. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 14.Hiippala ST, Strid LJ, Wennerstrand MI, Arvela JV, Niemelä HM, Mäntylä SK, Kuisma RP, Ylinen JE. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–844. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Jansen AJ, Andreica S, Claeys M. D”Haese J, Camu F, Jochmans K. Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth. 1999;83:596–601. doi: 10.1093/bja/83.4.596. [DOI] [PubMed] [Google Scholar]
- 16.Kambayashi J, Sakon M, Yokota M, Shiba E, Kawasaki T, Mori T. Activation of coagulation and fibrinolysis during surgery, analyzed by molecular markers. Thromb Res. 1990;60:157–167. doi: 10.1016/0049-3848(90)90294-M. [DOI] [PubMed] [Google Scholar]
- 17.Klenerman L, Chakrabarti R, Mackie I, Brozovic M, Stirling Y. Changes in haemostatic system after application of a tourniquet. Lancet. 1977;1:970–972. doi: 10.1016/S0140-6736(77)92276-0. [DOI] [PubMed] [Google Scholar]
- 18.Langdown AJ, Field J, Grote J, Himayat H. Aprotinin (Trasylol) does not reduce bleeding in primary total hip arthroplasty. J Arthroplasty. 2000;15:1009–1012. doi: 10.1054/arth.2000.8102. [DOI] [PubMed] [Google Scholar]
- 19.Lisander B, Ivarsson I, Jacobsson SA. Intraoperative autotransfusion is associated with modest reduction of allogeneic transfusion in prosthetic hip surgery. Acta Anaesthesiol Scand. 1998;42:707–712. doi: 10.1111/j.1399-6576.1998.tb05305.x. [DOI] [PubMed] [Google Scholar]
- 20.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement: effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am. 1991;73:1037–1040. [PubMed] [Google Scholar]
- 21.Mangano DT, Tudor IC. Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 22.Mongan PD, Brown RS, Thwaites BK. Tranexamic acid and aprotinin reduce postoperative bleeding and transfusions during primary coronary revascularization. Anaesth Analg. 1998;87:258–265. doi: 10.1097/00000539-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 24.Nakahara M, Sakahashi H. Effect of application of a tourniquet on bleeding factors in dogs. J Bone Joint Surg Am. 1967;49:1345–1351. [PubMed] [Google Scholar]
- 25.Nilsson IM. Clinical pharmacology of aminocaproic and tranexamic acids. J Clin Pathol Suppl (R Coll Pathol). 1980;14:41–47. doi: 10.1136/jcp.s3-14.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petaja J, Myllynen P, Myllyla G, Vahtera E. Fibrinolysis after application of a pneumatic tourniquet. Acta Chir Scand. 1987;153:647–651. [PubMed] [Google Scholar]
- 27.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7:151–155. doi: 10.1016/S0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 28.Slaughter TF, Greenberg CS. Antifibrinolytic drugs and perioperative hemostasis. Am J Hematol. 1997;56:32–36. doi: 10.1002/(SICI)1096-8652(199709)56:1<32::AID-AJH7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Timing of the administration of tranexamic acid for maximum reduction in blood loss in arthroplasty of the knee. J Bone Joint Surg Br. 2001;83:702–705. doi: 10.1302/0301-620X.83B5.11745. [DOI] [PubMed] [Google Scholar]
- 30.Wellington K, Wagstaff AJ. Tranexamic acid: a review of its use in the management of menorrhagia. Drugs. 2003;63:1417–1433. doi: 10.2165/00003495-200363130-00008. [DOI] [PubMed] [Google Scholar]
- 31.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 32.Zohar E, Fredman B, Ellis M, Luban I, Stern A, Jedeikin R. A comparative study of the postoperative allogeneic blood-sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382–1387. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]