Abstract
Background
Gamma radiation sterilization can make cortical bone allograft more brittle, but whether it influences mechanical properties and propensity to form microscopic cracks in structurally intact cancellous bone allograft is unknown.
Questions/purposes
We therefore determined the effects of gamma radiation sterilization on structurally intact cancellous bone mechanical properties and damage formation in both low- and high-density femoral cancellous bone (volume fraction 9%–44%).
Methods
We studied 26 cancellous bone cores from the proximal and distal femurs of 10 human female cadavers (49–82 years of age) submitted to a single compressive load beyond yield. Mechanical properties and the formation of microscopic cracks and other tissue damage (identified through fluorochrome staining) were compared between irradiated and control specimens.
Results
We observed no alterations in mechanical properties with gamma radiation sterilization after taking into account variation in specimen porosity. No differences in microscopic tissue damage were observed between the groups.
Conclusions
Although gamma radiation sterilization influences the mechanical properties and failure processes in cortical bone, it does not appear to influence the performance of cancellous bone under uniaxial loading.
Clinical Relevance
Our observations support the use of radiation sterilization on structurally intact cancellous bone allograft.
Introduction
Dense cancellous bone allograft in the form of blocks or wedges is a useful tissue form for large reconstructions and fusions of metaphyseal or vertebral defects in that it is stiff immediately after surgery yet still has large porous regions that can enable bony ingrowth [4, 18]. Additionally, the pores in dense cancellous bone allograft can potentially be loaded with osteogenic agents to further promote ingrowth and bone remodeling.
A limitation of allograft tissue, however, is the possibility for disease transmission. Gamma radiation sterilization (25–35 kGy) is commonly used to reduce the risk of disease transmission through bone allograft [15]. In cortical bone, gamma radiation at typical sterilization doses causes reductions in ultimate strength [20], bending strength [13], work to fracture [2, 20], fatigue life [1], and resistance to fatigue crack growth [26]. High doses of gamma irradiation (more than 51 kGy) impair cancellous bone strength and elastic modulus (Young’s modulus) [3], whereas lower doses have not been associated with impaired elastic modulus or ultimate strength [3, 5, 11, 12, 14, 19, 31]. Although the density of cancellous bone is the single most important factor influencing cancellous bone strength and stiffness [24], prior studies of the effects of irradiation on cancellous bone have either not accounted for variation in density of the cancellous bone specimens [5, 12, 19, 31] or have studied a relatively small range in cancellous bone density [3, 14]. Because strength and stiffness of cancellous bone are related to the density raised to a power near 2.0 [24], a 10% difference in density among specimens can generate a 20% difference in strength or stiffness, suggesting small differences in density within a group increase the variability in studies, making it difficult to observe an effect of irradiation. Low-density cancellous bone (bone volume fraction < 25%) is believed to fail through large deformation bending and buckling of trabeculae, whereas higher density trabecular bone fails through yielding of regions of the mineralized tissue [8, 16]. If gamma irradiation makes bone tissue more brittle, irradiated trabeculae may have less ability to bend and yield and would instead tend to fracture (trabecular microfracture), a more detrimental failure mode [21]. Previously we showed irradiation of dense bovine cancellous bone did not modify elastic modulus or yield strength in compression but resulted in an increase in microdamage in the form of cross-hatching and microfracture [14]. Because bovine cancellous bone has a different microstructure from human trabecular bone [23] and tends to be much denser (average bone volume fraction 40%) than cancellous bone from humans (bone volume fraction typically 10%–40%), it is unknown if irradiation in human cancellous bone will have the same effects on mechanical properties and microscopic tissue damage.
We asked whether gamma radiation sterilization at 30 kGy (a dose commonly used by allograft providers) (1) reduces yield strain, Young’s modulus, or yield strength or increases the residual strain (permanent deformation); and (2) altered the amount or type of microscopic tissue damage (microscopic cracks, diffuse damage, trabecular microfracture) in cancellous bone submitted to subfailure loading.
Materials and Methods
We obtained 17 fresh-frozen femurs from 10 human female cadavers (49–82 years of age; 74 ± 11, mean ± SD). The proximal and distal ends were cut in 15-mm thick slabs. One slab, aligned in the coronal plane (assuming no anteversion) to include the center of the femoral neck, was collected from each proximal femur. Two or three slabs, each aligned in the sagittal plane, were collected from each distal region. Rectangular regions aligned with primary trabecular orientation were identified in contact radiographs of the center of the femoral neck or metaphyseal distal femur region. Cylindrical cores of cancellous bone aligned with the primary trabecular orientation were collected from the regions using a diamond-tipped coring tool (Starlite, Lancaster, PA, USA). Cores were cut parallel to the plane of each slab and removed from the slab with a perpendicular cut made with a low-speed diamond saw. Cores were examined closely to ensure they were relatively homogeneous and parallel to trabecular alignment. One specimen was collected per femoral neck and three to four were available from each distal femur. We created a total of 33 specimens, 14 from the proximal femur and 19 from the distal femur. Of the 33 specimens, three proximal femur specimens (one control, two irradiated) exceeded ultimate stress outside of the experimental gauge length and four specimens were lost as a result of operator error. Thus, a total of 26 specimens remained for analysis (five control and five irradiated from the proximal femur, nine control and seven irradiated from the distal femur). Specimens were divided into irradiated and control groups distributing tissue from the same donor equally between the two groups where possible.
The study was designed to detect a 20% difference in yield strain, Young’s modulus, and yield strength. Previous studies have shown the coefficients defining the relationship between yield strength and density [29], elastic modulus and density [28] as well as yield strain display standard deviations of 11% of the mean. Using an α = 0.05 level of significance and including specimens from both regions of the femur, the sample size (n = 12 smallest sample size) had a power of 0.99 to detect a difference in yield strain of 1.72*10−3 strain (20% of the expected mean value) with analysis of variance. Using the approximation of statistical power for analysis of covariance proposed by Borm and colleagues [9], the smallest sample size had a power of 0.99 to observe a 20% decrease in the relationship between bone volume fraction and either Young’s modulus or yield strength.
Proximal femur specimens had an average height to diameter ratio of approximately 2.3:1 (length 20.73 ± 2.67 mm; diameter 8.15 ± 0.06 mm, mean ± SD); distal femur specimens had an average height to diameter ratio of approximately 2.5:1 (length 18.87 ± 1.40 mm, diameter 8.24 ± 0.03 mm). We removed marrow from specimens with a low-pressure water jet. The ends of the specimen were polished to ensure parallel alignment. Irradiated specimens were packaged in dry ice and sent to a commercial sterilization facility (Steris Isomedix, Morton Grove, IL, USA) to receive irradiation while frozen (29.45 ± 0.15 kGy, total travel time 48 hours). Nonirradiated specimens were similarly packaged but remained in the laboratory during the time period.
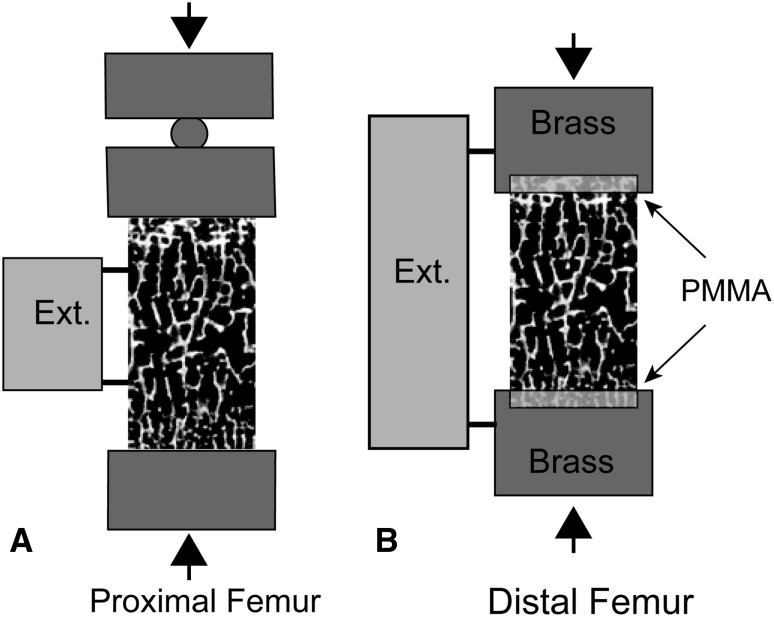
Before mechanical testing, the specimens were stained with xylenol orange (0.5 mM in saline) for 2 hours to label preexisting damage (naturally occurring or caused by specimen preparation). The specimens were then tested in compression. The most commonly used technique for reducing systemic or random error in mechanical testing of cancellous bone specimens is to glue the specimens into metallic end caps (described in [25]). The end cap technique could not be used with these specimens as a result of the short length of the cancellous bone cores. For this reason, we tested proximal femur specimens in compression between two unlubricated stainless steel plates with a 10-mm extensometer with knife edges attached directly to the specimen [14] (Fig. 1). Distal femur specimens were embedded into two brass rods using polymethylmethacrylate (PMMA) bone cement [7] with a 25-mm gauge length extensometer attached to the brass rods (Fig. 1). We adjusted the resulting strain data from the distal femur specimens to account for deflections within the bone cement (see Appendix).
Fig. 1A–B.
(A) Cancellous bone cores from the proximal femur were tested between platens with the extensometer attached directly to the specimen. (B) Cancellous bone cores from the distal femur were embedded in PMMA bone cement with an extensometer spanning the brass ends.
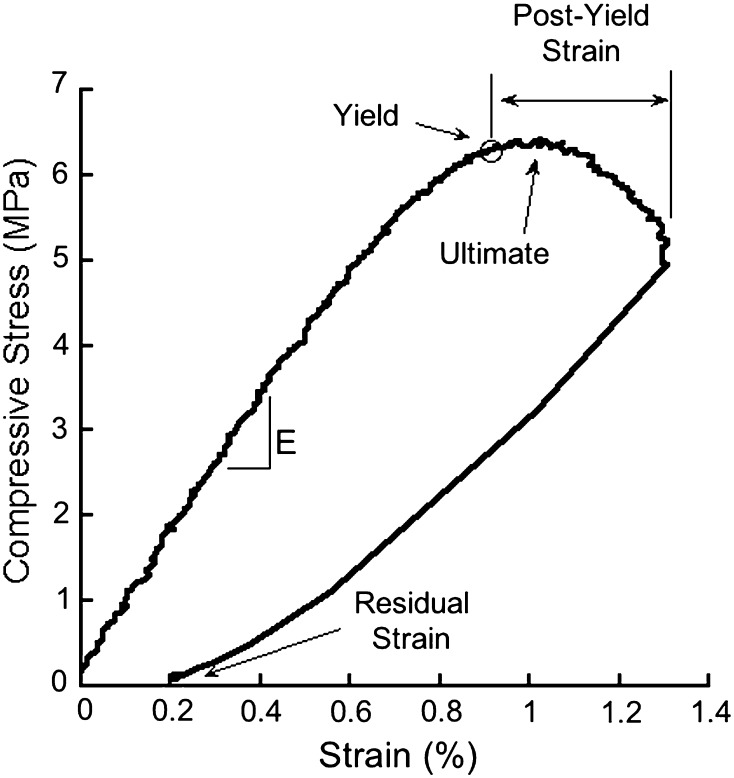
We submitted each specimen to 10 Haversine preconditioning cycles (to 0.2% strain at 1.25 Hz) followed by a final ramp to 1.3% strain at a rate of 0.5% strain/second under displacement control. This applied strain was selected to ensure all specimens exceeded yield strain and a subset would exceed ultimate strain. Specimens were immediately unloaded. Young’s modulus, yield strain, yield stress, and residual strain were determined [14] (Fig. 2). Yield stress was calculated based on the 0.2% offset. Residual strain was defined as the strain at zero load after unloading and represents the amount of permanent deformation caused by the applied load. Although not the primary outcome of the study, ultimate stress, ultimate strain, and postyield strain were also determined [14]. Ultimate stress was determined as the maximum stress during the test.
Fig. 2.
A typical stress strain curve for cancellous bone loaded in compression is shown. The Young’s modulus (E), yield point, ultimate failure point, postyield strain, and residual strains are indicated.
After loading, specimens were removed from the testing device and stained in calcein (0.5 mM in saline) for 2 hours to label damage caused by mechanical loading. Specimens were embedded undecalcified in methylmethacrylate and longitudinal sections were cut, polished to a thickness of 100 μm, and mounted on glass slides. Bone volume fraction was measured in the sections using stereological point counting (100 μm between grid points). Microscopic tissue damage was quantified through direct observation in a microscope at 100× magnification by one observer (DSR, blinded to study group) using the following criteria [14]: Microscopic cracks were identified as linear, thin regions of stain uptake and were distinguished from vessels (Fig. 3A). Microscopic cracks were expressed as crack density (Cr.Dn, cracks/mm2 bone area). Diffuse damage was identified as region of diffuse staining within the bone cross-section without a distinct edge, sometimes associated with cross-hatching, and was measured as a percent of bone area (DV/BV) through point counting (Fig. 3B). Trabecular microfracture was identified as a trabecula with a microscopic crack crossing its entire width and was expressed as number of microfractures (Fig. 3C).
Fig. 3A–C.
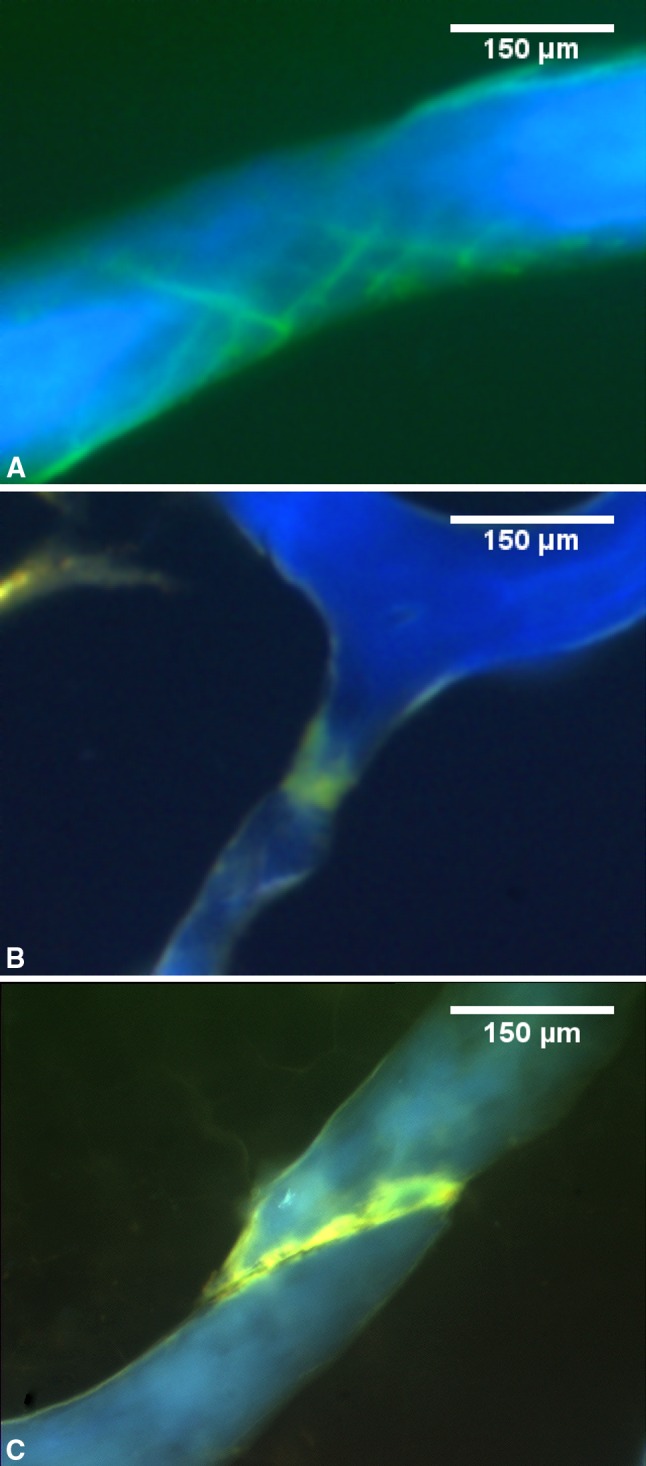
Sections of cancellous bone under ultraviolet illumination are shown. Microscopic tissue damage is detected under fluorescence microscopy based on the green bulk stain (calcein). Three different kinds of microscopic tissue damage measured in the current study are shown, including (A) microscopic cracks in a cross-hatching formation, (B) diffuse damage, and (C) trabecular microfracture.
We used correlation analysis to determine if any mechanical properties were correlated with bone volume fraction. Measures that were not correlated with bone volume fraction (yield strain, ultimate strain, residual strain, microfracture number) were compared among groups using analysis of variance. Measures that were correlated with bone volume fraction (Young’s modulus, yield strength, ultimate stress, postyield strain, crack density, diffuse damage) were examined for differences among study groups using analysis of covariance implemented with a generalized least squares model with bone volume fraction as a covariate [17]. Conceptually such an analysis tests whether the relationship between a mechanical property and bone volume fraction differed among groups (irradiated versus nonirradiated). Additionally, the possibility that region (proximal versus distal femur) influenced mechanical properties was examined by adding region to the generalized least squares model. Statistical analyses were performed with JMP (Version 8.0; SAS Institute, Cary, NC, USA).
Results
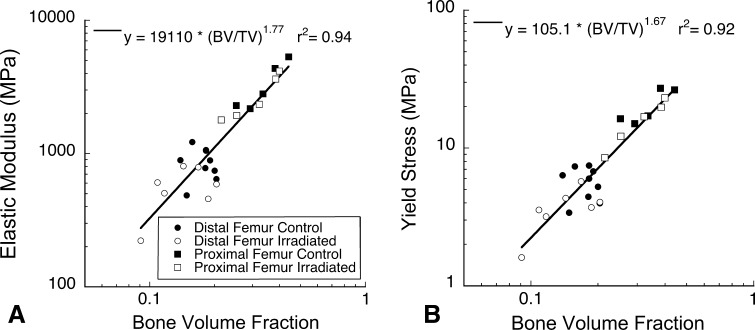
After accounting for bone volume fraction, no differences in Young’s modulus (Fig. 4A) or yield stress (Fig. 4B) were observed between irradiated and control specimens (Table 1). No differences in yield strain or residual strain were associated with gamma radiation sterilization (Table 1). Bone volume fraction in the proximal femur (0.32 ± 0.07) was greater than that in the distal femur (0.16 ± 0.04, p < 0.001), but no differences in bone volume fraction were observed between control and irradiated groups within each region (p = 0.61 proximal femur, p = 0.12 distal femur). No differences in biomechanical parameters were found between proximal and distal femur regions (after accounting for bone volume fraction if appropriate; Table 1). Cross terms between the bone volume fraction and gamma irradiation did not contribute to the prediction of Young’s modulus (p = 0.08) or yield stress (p = 0.17). Gamma radiation sterilization did not influence the other biomechanical measures generated in the study (ultimate strain, ultimate stress, postyield strain; Table 1). Cross terms between bone volume fraction and gamma radiation did not contribute to ultimate stress (p = 0.28) or postyield strain (p = 0.32).
Fig. 4A–B.
No differences in the relationship between bone volume fraction and (A) Young’s modulus or (B) yield strength were observed between the two regions of the femur (proximal or distal) or the irradiated and control groups.
Table 1.
Mechanical parameters measured in the current study are shown*
| Property | Control (n = 14) | Irradiated (n = 12) | Correlation to BV/TV | Effect of irradiation (p value)‡ | Effect of skeletal region (proximal versus distal) (p value)‡ |
|---|---|---|---|---|---|
| Young’s modulus (MPa) | 1764 ± 1482 | 1483 ± 1307 | ρ = 0.95 p < 0.001 |
0.89 | 0.25 |
| Yield strain (%) | 0.84 ± 0.07 | 0.83 ± 0.10 | ρ = −0.32 p = 0.11 |
0.78 | 0.11 |
| Yield stress (MPa) | 10.92 ± 8.10 | 8.88 ± 7.32 | ρ = 0.95 p < 0.001 |
0.52 | 0.06 |
| Residual strain (%) | 0.26 ± 0.05 | 0.26 ± 0.06 | ρ = −0.58 p = 0.21 |
0.78 | 0.29 |
| Ultimate strain (%)† | 1.00 ± 0.12 | 0.95 ± 0.10 | ρ = −0.32 p = 0.39 |
0.46 | 0.90 |
| Ultimate stress (MPa) † | 9.27 ± 8.50 | 6.23 ± 3.48 | ρ = 0.79 p < 0.001 |
0.93 | 0.27 |
| Postyield strain (%) | 0.45 ± 0.08 | 0.46 ± 0.11 | ρ = −0.58 p = 0.047 |
0.56 | 0.32 |
* Data from the proximal and distal femur are pooled because skeletal region did not affect comparisons of mechanical parameters between control and irradiated, as shown in the final column; †only seven per group reached ultimate strain; ‡for parameters not correlated with bone volume fraction (p ≥ 0.05), the difference is tested using analysis of variance. For parameters correlated with bone volume fraction (p < 0.05), the difference is tested using analysis of covariance with bone volume fraction as a covariate.
We observed no differences in microcrack density, diffuse damage, or trabecular microfracture between irradiated and control groups (Table 2). Measures of diffuse damage and crack density were correlated with Young’s modulus and yield stress (Table 2), although the mechanical properties were not predictive of these microdamage measures (r2 < 0.30) and the correlation was not observable after accounting for bone volume fraction suggesting the correlation was secondary to the relationship between the two parameters and bone volume fraction. No other mechanical properties were correlated with measures of microdamage (|ρ| < 0.30, p > 0.25).
Table 2.
Microscopic tissue damage measured in human femoral trabecular bone is reported (mean ± SD)*
| Measure | Control (n = 14) | Irradiated (n = 12) | p value control versus irradiated | Correlation with BV/TV | Correlation with Young’s modulus | Correlation with yield stress |
|---|---|---|---|---|---|---|
| Number of microfractures | 1.00 ± 1.04 | 1.39 ± 1.81 | 0.73 | ρ = −0.35 p = 0.08 |
ρ = −0.31 p = 0.12 |
ρ = −0.30 p = 0.14 |
| Diffuse damage (DV/BV, %) | 0.47 ± 0.26 | 0.63 ± 0.39 | 0.22 | ρ = −0.50 p = 0.01 |
ρ = −0.49 p = 0.01 |
ρ = −0.54 p = 0.005 |
| Crack density (Cr.Dn, 1/mm2) | 0.33 ± 0.28 | 0.38 ± 0.33 | 0.86 | ρ = −0.55 p = 0.004 |
ρ = −0.40 p = 0.05 |
ρ = −0.70 p = 0.04 |
* Specimens from the proximal and distal regions are pooled because no significant differences were observed between the two regions.
Discussion
Gamma radiation sterilization reportedly increases the brittleness of cortical bone allograft [1, 26], but it is not clear if gamma radiation sterilization alters the mechanical performance of structurally intact cancellous bone allograft because prior studies of human cancellous bone tissue have either not accounted for variability in density or included only a small range in density. In the current study we asked if gamma radiation sterilization (30 kGy) alters mechanical properties of cancellous bone (Young’s modulus, yield strength, yield strain) in specimens spanning the range of cancellous bone densities expected in allografts. Additionally, we examined microscopic tissue damage in irradiated and nonirradiated specimens.
Our study also had some limitations. First, for the two different locations (proximal and distal femur), two different boundary conditions (platens with extensometer and PMMA embedding) were used in mechanical testing. The use of two different techniques is not expected to influence our results because the platens loading with attached extensometer is a reportedly accurate approach [29] and the PMMA embedding has been validated ([7] and Appendix). Second, we only examined a single loading event and did not consider other physiological loading modes such as cyclic fatigue loading that are relevant to allograft survival. Additional work is needed to understand the effects of gamma irradiation on fatigue loading of cancellous bone [26].
Many prior studies have compared irradiated and nonirradiated cancellous bone allograft without taking into account variation in bone volume fraction or density among specimens (Table 3). Compared with prior studies, the current study is unique in that it determined the effect of gamma irradiation on cancellous bone biomechanics across a wide range of bone volume fractions (9%–44%). The only prior study of human bone that reported bone volume fraction described a range of 7% to 24% (estimated from apparent density [3]), addressing only the lower range of cancellous bone density that would be used as structurally intact allograft. In the current study, the difference in yield strain and the relationships between bone volume fraction and both Young’s modulus and yield stress between groups was small (the effect of irradiation in the analysis of covariance was less than 5% of that in the control group, as indicated by the coefficient associated with irradiation in the generalized least squares model; Table 3) and a priori power was 0.99 to detect a 20% effect, supporting our conclusion that there is no effect of gamma radiation sterilization on these parameters. Our study confirms that the lack of effect of gamma irradiation on cancellous bone stiffness and strength observed in prior studies [3, 5, 11, 12, 14, 19, 31] was not the result of confounding variability in bone volume fraction or density among specimens [3, 5, 11, 12, 14, 19, 31].
Table 3.
The percent difference in cancellous bone compressive yield strain, Young’s modulus, and yield stress associated with gamma radiation sterilization (25–35 kGy) in prior studies is compared with the current study*
| Study | Mean BV/TV (range) | Percent difference in yield strain | Percent difference in Young’s modulus | Percent difference in yield stress | Accounted for density or BV/TV |
|---|---|---|---|---|---|
| Cornu et al. (2000) [11] | NR | −4% | −4% | −7%† | No |
| Vastel et al. (2004) [31] | NR | NR | −6% | NR | No |
| Grieb et al. (2005)‡ [19] | NR | NR | +5% | NR | No |
| Balsly et al. (2008) [5] | NR | NR | −7% | NR | No |
| Cornu et al. (2010) [12] | NR | −8% | −6% | −8% | No |
| Anderson et al. (1992)§ [3] | 13% (7%–24%) | NR | +8% | NR | Yes |
| Dux et al. (2010) [14] | 40% (26%–61%) | −3% | −2% | −1% | Yes |
| Current study | 22% (9%–44%) | −1% | −1% | −5% | Yes |
* In studies that accounted for density of bone volume fraction, the percent difference represents the difference expressed by an analysis of covariance (ANCOVA) (when ANCOVA is implemented with generalized least squares, this represents the magnitude of the coefficient associated with irradiation relative to that of the regression intercept); †p < 0.05 reported; ‡irradiation dose 50 kGy; §irradiation dose 31 kGy; NR = not reported.
The current study is the first to analyze how gamma radiation sterilization influences microscopic cracks and other tissue damage in human cancellous bone. Measures of microscopic tissue damage have high variability, however. As a result, post hoc power analysis suggests the microdamage measures could only detect effect sizes of 66% to 125% between groups with a power of 0.80 (α = 0.05). The variance in microdamage assays in the current study was comparable to that achieved by other groups [27, 32] and most likely requires more precise measurement approaches. Recent developments in three-dimensional imaging approaches have been reported to be less variable [30]. In a prior study, we examined failure processes in bovine cancellous bone (BV/TV approximately 40%) and found gamma irradiation was associated with increased amounts of trabecular microfracture and cross-hatching type diffuse damage [14]. The current study differs in that it examined lower density human cancellous bone (mean BV/TV = 23%) in which failure processes are likely different and therefore measures of microdamage may also differ.
Our finding that gamma irradiation does not influence the yield properties of the cancellous bone structure is surprising. Subregions of mineralized tissue within the structure are expected to exceed yield and generate microdamage before the entire cancellous structure yields [6]. Because gamma irradiation is expected to modify postyield properties of mineralized tissue, the subregions that exceed yield would be expected to more readily propagate microscopic tissue damage, potentially leading to reductions in yield strength of the entire cancellous bone structure [6]. We speculate that the cancellous microstructure allows for stress to be distributed within the structure and prevents any increase in brittleness caused by irradiation from altering the yield properties of the overall structure. In conclusion, gamma irradiation at commonly used doses (30 kGy) does not appear to have a major effect on the yield strain, Young’s modulus, or yield strength in human cancellous bone ranging in bone volume fraction from 9% to 44%. These observations support the use of gamma radiation sterilization with structurally intact cancellous bone allograft in clinical applications because the process does not appear to alter stiffness or strength [10, 22].
Acknowledgments
Human tissue was received from National Disease Research Interchange (NDRI) and International Institute for the Advancement of Medicine (IIAM). We thank Shangjin Li for assistance with specimen preparation.
Appendix
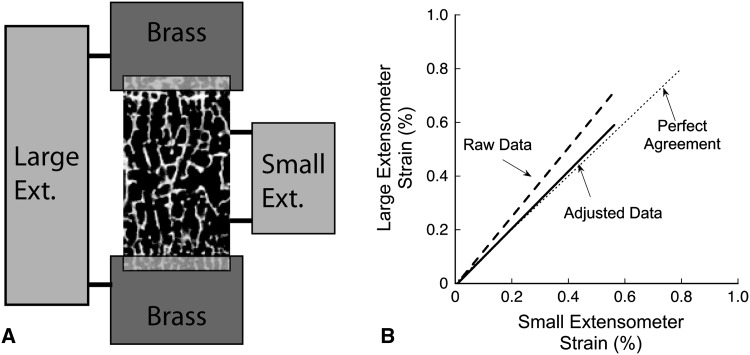
Strain measures made with an extensometer placed on the brass ends will include deflections within the specimen as well as deflections of the polymethylmethacrylate (PMMA) (strain in the brass holders are negligible), generating errors when the gauge length is calculated as the exposed length of the cancellous bone. To adjust the data, we modeled the brass, PMMA, and bone specimen using a simple composite model of the three materials in series assuming a modulus of 3 GPa for PMMA and 110 GPa for brass was used. The model resulted in the following relationship between the strain measured in the large extensometer (εlarge) placed across the brass ends and that measured by a small extensometer (εsmall) directly attached to the bone (Fig. A1-A):
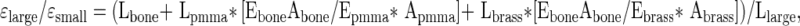 |
where Lbone, Lpmma, and Lbrass are the lengths of bone, PMMA, and brass within the gauge length of the large extensometer; Ebone, Epmma, and Ebrass are the Young’s moduli of the three different materials; and Abone, Apmma, and Abrass are the cross-sectional areas. Three specimens of bovine cancellous bone (BV/TV ~ 40%) were embedded in PMMA in brass ends (Fig. A1-A). The high density of the bovine cancellous bone allowed for placement of a small extensometer directly on the bone (Small Ext.) along with a large extensometer (Large Ext.) as would be used with more porous specimens. As expected, strain measurements made with the large extensometer (using only the exposed bone length as the gauge length) were typically greater than those made by the small extensometer attached directly to the bone (Raw Data, dashed line, Fig. A1-B). After correcting for compliance in the PMMA using the above equation, the adjusted data (solid line) closely matched perfect agreement (dotted line), demonstrating the large extensometer alone can be used to accurately represent strain in cancellous bone embedded in PMMA.
Fig. A1.
(A) The two extensometer technique used to validate the PMMA embedding method is shown. (B) Adjusting the raw data resulted in agreement between the large extensometer and the small extensometer (placed directly on the bone), validating the use of the large extensometer with the PMMA embedding method (Fig. 1).
Footnotes
Two of the authors (CJH, CMR) have received research funding from the Musculoskeletal Transplant Foundation through peer-reviewed grant applications. One author (CJH) received support from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR057362.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at Case Western Reserve University, Cleveland, OH, USA.
References
- 1.Akkus O, Belaney RM. Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude. J Orthop Res. 2005;23:1054–1058. doi: 10.1016/j.orthres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927–934. doi: 10.1016/S0736-0266(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MJ, Keyak JH, Skinner HB. Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg Am. 1992;74:747–752. [PubMed] [Google Scholar]
- 4.Balabhadra RS, Kim DH, Zhang HY. Anterior cervical fusion using dense cancellous allografts and dynamic plating. Neurosurgery. 2004;54:1405–1411. doi: 10.1227/01.NEU.0000125543.38952.87. [DOI] [PubMed] [Google Scholar]
- 5.Balsly CR, Cotter AT, Williams LA, Gaskins BD, Moore MA, Wolfinbarger L., Jr Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008;9:289–298. doi: 10.1007/s10561-008-9069-0. [DOI] [PubMed] [Google Scholar]
- 6.Bayraktar HH, Keaveny TM. Mechanisms of uniformity of yield strains for trabecular bone. J Biomech. 2004;37:1671–1678. doi: 10.1016/j.jbiomech.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Bevill G, Eswaran SK, Farahmand F, Keaveny TM. The influence of boundary conditions and loading mode on high-resolution finite element-computed trabecular tissue properties. Bone. 2009;44:573–578. doi: 10.1016/j.bone.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Bevill G, Eswaran SK, Gupta A, Papadopoulos P, Keaveny TM. Influence of bone volume fraction and architecture on computed large-deformation failure mechanisms in human trabecular bone. Bone. 2006;39:1218–1225. doi: 10.1016/j.bone.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S267–S273. doi: 10.1097/00003086-199810001-00027. [DOI] [PubMed] [Google Scholar]
- 11.Cornu O, Banse X, Docquier PL, Luyckx S, Delloye C. Effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. J Orthop Res. 2000;18:426–431. doi: 10.1002/jor.1100180314. [DOI] [PubMed] [Google Scholar]
- 12.Cornu O, Boquet J, Nonclercq O, Docquier PL, Van Tomme J, Delloye C, Banse X. Synergetic effect of freeze-drying and gamma irradiation on the mechanical properties of human cancellous bone. Cell Tissue Bank. 2010 Aug 12 [Epub ahead of print]. [DOI] [PubMed]
- 13.Currey JD, Foreman J, Laketic I, Mitchell J, Pegg DE, Reilly GC. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res. 1997;15:111–117. doi: 10.1002/jor.1100150116. [DOI] [PubMed] [Google Scholar]
- 14.Dux SJ, Ramsey D, Chu EH, Rimnac CM, Hernandez CJ. Alterations in damage processes in dense cancellous bone following gamma-radiation sterilization. J Biomech. 2010;43:1509–1513. doi: 10.1016/j.jbiomech.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 15.Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005;6:201–219. doi: 10.1007/s10561-005-0338-x. [DOI] [PubMed] [Google Scholar]
- 16.Gibson LJ. The mechanical behavior of cancellous bone. J Biomech. 1985;18:317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 17.Glantz SA, Slinker BK. Primer of Applied Regression & Analysis of Variance. New York: McGraw-Hill; 2001. [Google Scholar]
- 18.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987;225:7–16. [PubMed] [Google Scholar]
- 19.Grieb TA, Forng RY, Stafford RE, Lin J, Almeida J, Bogdansky S, Ronholdt C, Drohan WN, Burgess WH. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials. 2005;26:2033–2042. doi: 10.1016/j.biomaterials.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Hamer AJ, Strachan JR, Black MM, Ibbotson CJ, Stockley I, Elson RA. Biochemical properties of cortical allograft bone using a new method of bone strength measurement. A comparison of fresh, fresh-frozen and irradiated bone. J Bone Joint Surg Br. 1996;78:363–368. [PubMed] [Google Scholar]
- 21.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–832. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z, Ryu W, Ren P, Fasching R, Goodman SB. Controlled release of growth factors on allograft bone in vitro. Clin Orthop Relat Res. 2008;466:1905–1911. doi: 10.1007/s11999-008-0290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keaveny TM. Mechanistic approaches to analysis of trabecular bone. Forma. 1997;12:267–275. [Google Scholar]
- 24.Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu Rev Biomed Eng. 2001;3:307–333. doi: 10.1146/annurev.bioeng.3.1.307. [DOI] [PubMed] [Google Scholar]
- 25.Keaveny TM, Pinilla TP, Crawford RP, Kopperdahl DL, Lou A. Systematic and random errors in compression testing of trabecular bone. J Orthop Res. 1997;15:101–110. doi: 10.1002/jor.1100150115. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell EJ, Stawarz AM, Kayacan R, Rimnac CM. The effect of gamma radiation sterilization on the fatigue crack propagation resistance of human cortical bone. J Bone Joint Surg Am. 2004;86:2648–2657. doi: 10.2106/00004623-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Moore TLA, Gibson LJ. Microdamage accumulation in bovine trabecular bone in uniaxial compression. J Biomech. 2002;124:63–71. doi: 10.1115/1.1428745. [DOI] [PubMed] [Google Scholar]
- 28.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–904. doi: 10.1016/S0021-9290(03)00071-X. [DOI] [PubMed] [Google Scholar]
- 29.Morgan EF, Keaveny TM. Dependence of yield strain of human trabecular bone on anatomic site. J Biomech. 2001;34:569–577. doi: 10.1016/S0021-9290(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 30.Tang SY, Vashishth D. A non-invasive in vitro technique for the three-dimensional quantification of microdamage in trabecular bone. Bone. 2007;40:1259–1264. doi: 10.1016/j.bone.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vastel L, Meunier A, Siney H, Sedel L, Courpied JP. Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials. 2004;25:2105–2110. doi: 10.1016/j.biomaterials.2003.08.067. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Niebur GL. Microdamage propagation in trabecular bone due to changes in loading mode. J Biomech. 2006;39:781–790. doi: 10.1016/j.jbiomech.2005.02.007. [DOI] [PubMed] [Google Scholar]