Abstract
Background
Remodeling of structural bone allografts relies on adequate revascularization, which can theoretically be induced by surgical revascularization. We developed a new orthotopic animal model to determine the technical feasibility of axial arteriovenous bundle implantation and resultant angiogenesis.
Questions/purposes
We asked whether arteriovenous bundles implanted in segmental allografts would increase cortical blood flow and angiogenesis compared to nonrevascularized frozen bone allografts and contralateral femoral controls.
Methods
We performed segmental femoral allotransplantation orthotopically from 10 Brown Norway rats to 20 Lewis rats. Ten rats each received either bone allograft reconstruction alone (Group I) or allograft combined with an intramedullary saphenous arteriovenous flap (Group II). At 16 weeks, we measured cortical blood flow with the hydrogen washout method. We then quantified angiogenesis using capillary density and micro-CT vessel volume measurements.
Results
All arteriovenous bundles were patent. Group II had higher mean blood flow (0.12 mL/minute/100 g versus 0.05 mL/minute/100 g), mean capillary density (23.6% versus 2.8%), and micro-CT vessel volume (0.37 mm3 versus 0.07 mm3) than Group I. Revascularized allografts had higher capillary density than untreated contralateral femora, while vessel volume did not differ and blood flow was lower.
Conclusions
Axial surgical revascularization in orthotopic allotransplants can achieve strong angiogenesis and increases cortical bone blood flow.
Clinical Relevance
Poor allograft revascularization results in frequent complications of nonunion, infection, and late stress fracture. The presented technique of surgical revascularization could therefore offer a beneficial adjunct to clinical segmental bone allografting.
Introduction
Structural bone allografts have been widely used to replace large bone defects resulting from infection, trauma, or tumor. Revascularization of conventional frozen allografts is limited: histologic studies of host-graft junctions described incomplete vascular ingrowth reaching no more than 10 mm from the graft-host junction and 2 mm below the periosteal surface [6]. Enneking et al. [13, 14] also reported minimal revascularization in their observations on retrieved bone allografts, with rarely more than a few millimeters of revascularization per year. Preclinical and clinical research shows revascularization of the largely necrotic bone is required for allografts to heal at the graft-host junction and to remodel [6, 18, 20, 24, 25, 43]. However, allografts remain functional over time despite limited revascularization and remodeling, suggesting full biologic incorporation is not obligatory for necrotic grafts to retain their mechanical properties during load bearing [7, 13, 22, 24]. However, restricted blood circulation and limited remodeling make structural allografts prone to nonunion (11%–44%) [11, 16, 20, 52], infection (6%–21%) [7, 8, 10, 11, 16, 31–33], and late stress fractures (10%–42%) [3, 11, 16, 31, 39, 48], the latter presumably from accumulated microfractures [39, 42, 49, 53].
Blood circulation in normal bone is maintained largely by the intramedullary vasculature [2, 51]. In cryopreserved conventional allografts, no such vasculature is preserved. We proposed using surgical angiogenesis to generate a new or neoangiogenic bone circulation in these large structural bone segments by implantation of an arteriovenous bundle. Experimentally, this method has been successful in improving circulation in autologous bone [9, 19, 55], living (vascularized) allotransplants [27], and avascular carpal bone [46]. We presumed bone circulation could be similarly reconstructed in cryopreserved structural bone allografts.
We therefore developed a model to place a femoral allograft orthotopically within a segmental femoral defect and implant an arteriovenous bundle within the femoral medullary canal. We asked whether the construct increased capillary proliferation (angiogenesis) and cortical bone blood flow compared to nonrevascularized orthotopic allografts.
Materials and Methods
Femoral diaphyseal grafts, harvested from 10 female Brown Norway rats, were frozen at −80°C. The grafts were orthotopically transplanted into segmental bone defects of 20 male Lewis rats using rigid internal fixation. Ten rats received a bone allograft reconstruction alone (Group I). Another 10 animals were simultaneously revascularized by intramedullary implantation of a saphenous arteriovenous flap at the time of structural bone grafting (Group II). We used contralateral untreated femora as controls. This study was approved by the Institutional Animal Care and Use Committee.
Power calculations were based on the primary biologic outcome of interest: capillary density. The power calculation was based on a two-sample t-test at a significance level of α = 0.05, performed using nQuery Advisor® v6.01 (Statistical Solutions, Saugus, MA, USA). With 10 rats/group, we had 80% power to detect an effect size of at least 1.33, where effect size is defined as the difference in group means divided by the common SD. We considered an effect size of 0.8 or more as large. A previous study from our laboratory estimated a mean capillary density of 29% and SD of 17% at 21 weeks in a group of 10 arteriovenous bundle-patent, immunosuppressed rats. Assuming a similar SD would be seen in the present study, this translated to 80% power of detecting a difference in means of 22% or greater between any two groups [29, 54].
To harvest the grafts, we anesthetized 10 female brown Norway (RT1n) rats (weight, 200–250 g) with pentobarbital sodium at a dose of 35 mg/kg intraperitoneally. The femoral middiaphyseal segment, measuring 10 mm, was removed bilaterally under sterile conditions. We then euthanized donor rats with pentobarbital sodium at a dose of 200 mg/kg intraperitoneally. The grafts were reamed with a 2-mm hand drill to allow arteriovenous bundle implantation, rinsed with sterile saline, and stored at −80°C for at least 1 month before transplantation.
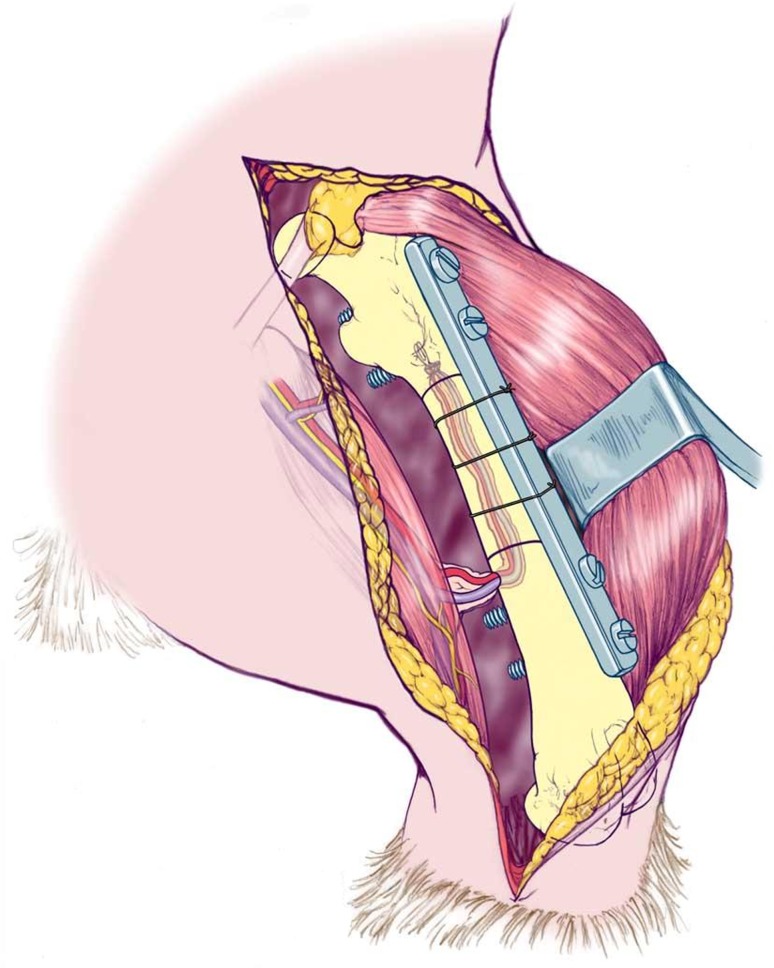
For the graft transplantation procedure, male Lewis (RT1l) rats (weight, 250–300 g) served as recipients, representing a major histocompatibility mismatch. We anesthetized recipient rats with ketamine (90 mg/kg intramuscular) and xylazine (10 mg/kg intramuscular). If necessary, we injected additional ketamine (20 mg/kg intramuscular) during surgery. A subcutaneous injection of 10 IU fragmin was given preoperatively and once daily for 5 days postoperatively. A longitudinal skin incision was made at the lateral aspect of the femur and separated the muscles from the femoral diaphysis. A 10-mm segment was exposed and removed with a minisaw, creating a large recipient bone defect. The proximal and distal osteotomies were made at a 15° angle. The graft was then thawed in sterile saline at room temperature and cut proximally and distally to match. This oblique osteotomy improved rotational stability of the construct. We anterolaterally fixed a custom-made 28-mm-long miniplate with two stainless steel screws (1.2 by 8 mm; McMaster-Carr, Los Angeles, CA, USA) in the recipient proximal femur and two identical screws in the distal recipient femur (Fig. 1). No screws were used in the graft, allowing the intramedullary canal of the graft to remain accessible for placement of the saphenous arteriovenous bundle to be implanted within the bone. The saphenous vessels with perivascular tissue were dissected from their femoral origin distally to the ankle. In Group II, the arteriovenous bundle was pulled through the allograft with an 8-0 suture via a small opening in the distal femur (Fig. 1). In Group I, we left the arteriovenous bundle in situ. Once we inserted the screws, we used three 4-0 nylon monofilament sutures as cerclage fixation to secure the graft to the plate. Throughout surgery, we took care to preserve nutrient vessels. The fascia was closed with 4-0 Vicryl® sutures (Ethicon, Inc, Somerville, NJ, USA) and the skin with 4-0 Ethilon® sutures (Ethicon, Inc).
Fig. 1.
A drawing shows the rat model with orthotopic segmental allograft transplantation with retrograde saphenous arteriovenous bundle implantation. Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved.
Buprenorphine (0.05–0.1 mg/kg subcutaneously) was given postoperatively and once daily for 2 days, and we allowed all rats to move freely. Survival time was 16 weeks from the day of surgery.
Cortical bone blood flow was measured at 16 weeks in the allograft and in the contralateral femur using the hydrogen washout method as previously described and validated in our laboratory [28, 35]. Anesthesia was induced in a nonsurvival procedure using ketamine (90 mg/kg intramuscular) and xylazine (10 mg/kg intramuscular), with additional ketamine (20 mg/kg intramuscularly) as needed. The allograft and contralateral femur were exposed, and a hydrogen-sensing electrode with microtip (H2-50; Unisense, Aarhus, Denmark) was placed within a 0.36-mm superficial cortical drill hole placed at the junction of the proximal 1/3 and the middle 1/3 of the graft. The electrode was advanced just enough to allow the tip of the sensor to enter the cortex but not the medullary canal. While breathing a 30% oxygen, 70% hydrogen mixture, hydrogen concentration rose and reached equilibrium in the bone, as measured with a picoammeter (Picoammeter 2000; Unisense) and plotted using LabVIEW™ software (National Instruments Corp, Austin, TX, USA). At equilibrium, we stopped hydrogen inhalation. The resulting rate of the hydrogen washout from bone, proportional to cortical bone blood flow, was calculated as previously described [28]. The hydrogen washout data were calculated using the LabVIEW™ software [35] and expressed as milliliter per minute per 100 g tissue.
Before sacrifice, the aorta and vena cava were cannulated, and the vasculature of the lower extremity was irrigated with 50 mL heparinized saline. Microangiography was performed with a polymerizing contrast agent (Microfil®; Flow Tech Inc, Carver, MA, USA), injected under physiologic pressure [29, 30]. We euthanized the rat with pentobarbital sodium 200 mg/kg intravenously. When present in the arteriovenous bundle, blue contrast verified its patency. The femur was removed and fixed in 10% buffered formalin for 24 hours and then decalcified in 14% ethylenediaminetetraacetic acid for 7 hours in a laboratory microwave at 750 watts (Pelco Biowave® 3450 Laboratory Microwave; Ted Pella Inc, Redding, CA, USA).
To measure angiogenesis by capillary density determination, we used a modified Spalteholz method to obtain optically clear bone: after decalcification in 14% EDTA, the bone was immersed in 50% alcohol, followed by bleaching with 10% H2O2. Next, dehydration was achieved with increasing concentrations of alcohol (50%, 75%, 95%, 100%), and bones were embedded in methyl salicylate [5, 29, 40, 54]. Digital images were taken in AP and lateral positions of each graft and analyzed with image analysis software (Scion® Imaging for Windows® Version 4.03; Scion, Frederick, MD, USA). Colored Microfil® was easily detected and distinguishable from the rest of the bone, so that a fixed level of color registration was set, and consistent calculation was achieved. Two authors (WFW, TK) measured capillary density as the ratio of vessel (Microfil®) pixels to total bone pixels for each image, using the average of AP and lateral images to calculate the surface of vessels as compared to the complete bone surface. We measured the capillary density of the contralateral femur in the same fashion.
Because the blue Microfil® polymer is radiopaque, it could be detected by CT. To determine vessel volume, we scanned both femora in each animal using a micro-CT system (MicroCT40; Scanco Medical, Basserdorf, Switzerland). The femur was placed in a polyethylimide holder with saline, and the graft was scanned at 70 KvP and 114 μA with 20-μm-thickness axial cut slices (500 in total, equaling 10 mm). Two authors (WFW, TK) set these parameters to ensure maximum detection of polymer solution and exclusion of background scatter. MicroCT40 software (Scanco Medical) was used to process the three-dimensional reconstructed micro-CT data and acquire the vessel volume.
We obtained bilateral measurements (grafted and contralateral untreated femora) for bone blood flow, capillary density, and micro-CT vessel volume. When comparing results of the grafts to results of the contralateral femora, the ratio obtained was an expression of the variance of the allograft values from those of the undisturbed femur. We analyzed both the absolute (experimental side only) and adjusted data (the ratio of ipsilateral graft/contralateral femur). To compare Groups I and II (absolute and adjusted data), we analyzed the data using the Mann-Whitney U test. The Wilcoxon signed-rank test was used to detect differences between ipsilateral and contralateral sides within each group. Data are presented as mean ± SD. We analyzed all data using the GraphPad Prism™ Version 5.0 software (GraphPad Software, La Jolla, CA, USA).
Results
In all rats, the arteriovenous bundle and the proximal and distal nutrient vessels were patent, as confirmed by filling of the vessels with Microfil®.
Mean capillary density was higher (p < 0.001) in Group II than in Group I (Table 1). Mean cortical bone blood flow in Group II was higher (p = 0.04) than in Group I (0.12 mL/minute/100 g versus 0.05 mL/minute/100 g; ratios: 0.66 versus 0.30). Mean vessel volume (Fig. 2) was higher in Group II than in Group I, as calculated with absolute values (p < 0.001) and adjusted values (p = 0.01): 0.37 mm3 versus 0.07 mm3 and 1.74 mm3 versus 0.62 mm3, respectively.
Table 1.
Capillary density, bone blood flow, and vessel volume for Groups I and II
| Variable | Absolute or adjusted value* | Group I | Group II | p value |
|---|---|---|---|---|
| Capillary density (%) | Absolute | 2.8 ± 2.2 | 23.6 ± 3.5 | < 0.001 |
| Adjusted | 0.4 ± 0.3 | 3.7 ± 1.7 | < 0.001 | |
| Bone blood flow (mL/minute/100 g) | Absolute | 0.05 ± 0.02 | 0.12 ± 0.09 | 0.04 |
| Adjusted | 0.30 ± 0.19 | 0.66 ± 0.40 | 0.03 | |
| Vessel volume (mm3) | Absolute | 0.07 ± 0.06 | 0.37 ± 0.16 | < 0.001 |
| Adjusted | 0.62 ± 0.71 | 1.74 ± 1.69 | 0.01 |
Values are expressed as mean ± SD; * absolute values = values for experimental sides (grafts); adjusted values = ratios of values for grafts normalized to values for untreated contralateral femurs, displayed to illustrate graft vascularization as compared to normal femoral vascularization; Group I = grafts without arteriovenous bundle; Group II = grafts with arteriovenous bundle.
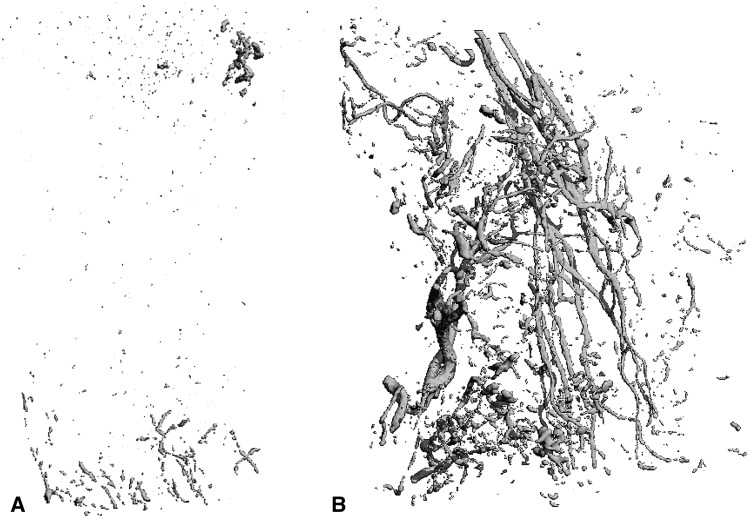
Fig. 2A–B.
(A) A representative micro-CT image of a sample from Group I shows limited angiogenesis. (B) A representative micro-CT image of a sample from Group II shows strong angiogenesis.
When comparing grafts with untreated contralateral femora, in Group I, conventional allografts had lower mean capillary density (p = 0.004) as compared to contralateral untreated femora (2.8% ± 2.21% versus 7.3% ± 3.19%, respectively). Conversely, Group II (surgically revascularized bone allografts) had greater (p = 0.008) mean capillary density than contralateral untreated femora (23.6 ± 3.5 % versus 7.7 ± 3.3 %). In Group I, mean bone blood flow in the allografts was lower (p = 0.009) than the untreated contralateral femora (0.05 ± 0.02 mL/minute/100 g versus 0.19 ± 0.05 mL/minute/100 g). Revascularized allografts had less mean cortical bone blood flow (p = 0.02) than the contralateral untreated femora (0.12 ± 0.09 mL/minute/100 g versus 0.19 ± 0.09 mL/minute/100 g). Within Group I, bone grafts had lower (p = 0.049) mean vessel volume as compared to the contralateral femora (0.07 ± 0.06 mm3 versus 0.19 ± 0.17 mm3). In Group II, the mean vessel volume of the surgical revascularized graft tended to be higher (p = 0.74) than the contralateral femur (0.37 ± 0.16 mm3 versus 0.30 ± 0.17 mm3). Comparing contralateral untreated femora (internal control) between groups, we observed no differences in mean capillary density (p = 0.76), bone blood flow (p = 0.96), or vessel volume (p = 0.19).
Discussion
After conventional bone allotransplantation, revascularization of the allograft is a slow process, with only small portions of the graft being invaded by fibrovascular tissue at the host-graft junctions [13]. Studies have reported multiple factors that influence graft revascularization and remodeling, including fixation technique [1], adjuvant therapy [20, 42], perigraft environment [42], comorbidity [31], and weightbearing [41]. Enneking and Campanacci [13], in their extensive review of 73 retrieved allografts, found remodeling was limited to 20% of the complete graft at 5 years. This does not necessarily mean all grafts will fail. Allografts that have remained largely necrotic over time could still have sufficient functionality and sustain loading forces, provided host-graft union was achieved [6]. However, incomplete revascularization and sparse remodeling in conventional allografts have been related to a high incidence of complications [11, 16, 42]. Therefore, we designed a model of a large segmental allograft in which an arteriovenous bundle could be implanted. We then determined whether capillary density and blood circulation were greater in surgical revascularized grafts compared to nonrevascularized allografts and contralateral untreated femoral bone.
This study had a number of limitations. First, the survival time of 16 weeks was only one time point in the dynamic process of graft revascularization. To understand its exact course over time, multiple shorter and longer time points need to be analyzed. Second, in this rat model, the saphenous arteriovenous bundle was transposed. In humans, the saphenous vein is not accompanied by an artery. To revascularize a large segmental allograft in the human femur, smaller arteriovenous bundles, such as the descending genicular vessels (retrograde transposition) or descending branch of the lateral circumflex vessels (antegrade transposition), could be used. Additionally, transposition of an arteriovenous bundle could have compromised the blood supply to its original tissue and evoked donor site morbidity. In our study, we saw no signs of wound problems, venous stasis, or arterial insufficiency of the leg. Third, including an additional study group in which a thrombosed (ligated) arteriovenous bundle was transposed intramedullary would have clarified to what extent the presence of the arteriovenous tissue by itself, without patent blood circulation, would induce angiogenesis. In this preclinical study, we investigated primarily whether a patent arteriovenous bundle would induce angiogenesis and bone blood flow as opposed to conventional grafting, which was a necessary step toward clinical implementation. Fourth, surgical revascularization of a graft could have led to longer surgical procedures. Therefore, a beneficial effect of increased bone blood flow on osteogenesis, host graft union, and risk of fracture should be confirmed or refuted by long-term analysis of biomechanical properties of revascularized allografts in larger animal models before clinical implementation. These are objectives of future research in our laboratory.
The importance of adequate vascularization for bone graft vitality has been the subject of research for decades [9, 36, 44]. Two studies published in 1963, Woodhouse [55] and Dickerson and Duthie [9], described implantation of arteriovenous bundles in autogenous bone grafts with the intent to augment vascularization. Later, Nagi [34] found vascular bundle implantation within autogenous grafts to improve their revascularization, and Hori et al. [19] described a proliferation of blood vessels from the implanted arteriovenous bundles when placed into necrotic heterotopic autografts. In the last two decades, various techniques of surgical revascularization have been studied in autografts [38, 56], heterotopic allografts [4, 27], xenografts [5, 15], and prefabricated heterotopic bone flaps [17, 26, 37, 50], as well as in avascular necrotic bone [46] and nonunited bone [47]. However, surgical revascularization of large segmental orthotopic allografts by providing an axial (longitudinal) blood supply has not been studied. These data are of importance since strain and axial loading forces have a considerable effect on bone allograft biology, including revascularization and incorporation [12, 21, 23, 42, 45]. In our orthotopic model, arteriovenous bundles were patent in all animals, which proved surgical revascularization in orthotopic allotransplantation was not disrupted throughout the 16-week survival period, and a new intramedullary blood circulation was sustained in the allograft.
Angiogenesis, as measured with capillary density and vessel volume, as well as cortical bone blood flow, were superior in Group II when compared to conventional grafts, as analyzed with absolute values and with values adjusted for the contralateral femora. These findings indicated surgical revascularization by arteriovenous bundle implantation had a beneficial effect on overall graft revascularization in segmental orthotopic allotransplantation. Previous data on implantation of arteriovenous bundles in rat femora were mainly derived from heterotopically transplanted autografts with basic histologic and angiographic descriptive data of angiogenesis [9, 19, 55]. Kumta et al. [27] inserted a femoral vascular bundle in heterotopically transplanted rat allografts and compared these with conventional allografts. The bundle patency rate was 50% at 6 and 12 weeks. At 24 weeks, only one of six rats had a patent vascular bundle. The authors observed vascular proliferation in the medullary canal histologic observations, but they performed no quantitative analysis of angiogenesis or bone blood flow. Carneiro and Malinin [4] reported revascularization in heterotopically transplanted canine allografts with arteriovenous bundle implantation; however, these observations were not quantified, and no control groups with conventional allografts or untreated femora were included.
In Group I, we found conventional allografts showed inferior angiogenesis and bone blood flow as compared to untreated contralateral femora. These findings were supported by preclinical and clinical observations of very limited angiogenesis in conventional allografts, only located at the periphery of the grafts [14, 27, 43]. In Group II, bone blood flow in revascularized allografts had not reached normal values as found in the untreated femora. Angiogenesis, as measured with capillary density, was higher in revascularized grafts than in untreated femora, while vessel volume was equal. Thus, at 16 weeks, angiogenesis from the arteriovenous bundle resulted in capillary density exceeding that found in normal femora, and resultant cortical blood flow, while greater than untreated allografts, had not reached normal values. Whether cortical blood flow in surgically revascularized allografts would equal that of normal bone with a longer survival time will require additional study.
Clinical reconstruction of segmental bone loss with cryopreserved structural allografts results in incomplete revascularization of transplanted grafts. While many function despite this handicap, there is a substantial risk of nonunion, infection, and late stress fractures. We reconstructed rat femoral defects using similar methods but used surgical angiogenesis from implanted arteriovenous bundles to generate a neocirculation within the bone and improve cortical blood flow. We demonstrated all arteriovenous bundles were patent, resulting in increased angiogenesis and improved cortical blood flow when compared to otherwise identical allografts without the arteriovenous bundles. Further study is needed to confirm improved blood flow results in improved graft healing, improved bone material properties, and sufficient bone remodeling to improve the extent of cortical bone viability.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res. 2012;470:728–734. doi: 10.1007/s11999-011-1952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baadsgaard K. Transplantation of pedicle bone grafts to fresh skeletal defects and defect pseudarthroses: an experimental study. Acta Orthop Scand. 1970;41:261–271. doi: 10.3109/17453677008991513. [DOI] [PubMed] [Google Scholar]
- 3.Berrey BH, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts: frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72:825–833. [PubMed] [Google Scholar]
- 4.Carneiro R, Malinin T. Vascularized bone allografts: an experimental study in dogs. J Reconstr Microsurg. 1991;7:101–103. doi: 10.1055/s-2007-1006767. [DOI] [PubMed] [Google Scholar]
- 5.Chung YG, Bishop AT, Giessler GA, Suzuki O, Platt JL, Pelzer M, Friedrich PF, Kremer T. Surgical angiogenesis: a new approach to maintain osseous viability in xenotransplantation. Xenotransplantation. 2010;17:38–47. doi: 10.1111/j.1399-3089.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 6.Davy TD. Biomechanical issues in bone transplantation. Orthop Clin North Am. 1999;30:553–563. doi: 10.1016/S0030-5898(05)70108-5. [DOI] [PubMed] [Google Scholar]
- 7.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: what they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–579. doi: 10.2106/JBJS.E.00943. [DOI] [PubMed] [Google Scholar]
- 8.Delloye C, De Halleux J, Cornu O, Wegmann E, Buccafusca GC, Gigi J. Organizational and investigational aspects of bone banking in Belgium. Acta Orthop Belg. 1991;57:27–34. [PubMed] [Google Scholar]
- 9.Dickerson RC, Duthie RB. The diversion of arterial blood flow to bone. J Bone Joint Surg Am. 1963;45:356–364. [PubMed] [Google Scholar]
- 10.Donati D, Di Bella C, Colangeli M, Bianchi G, Mercuri M. The use of massive bone allografts in bone tumour surgery of the limb. Curr Orthop. 2005;19:393–399. doi: 10.1016/j.cuor.2005.08.001. [DOI] [Google Scholar]
- 11.Donati D, Giacomini S, Gozzi E, Salphale Y, Mercuri M, Mankin HJ, Springfield DS, Gebhardt MC. Allograft arthrodesis treatment of bone tumors: a two-center study. Clin Orthop Relat Res. 2002;400:217–224. doi: 10.1097/00003086-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich PJ, Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporos Int. 2002;13:688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- 13.Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83:971–986. [PubMed] [Google Scholar]
- 14.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed] [Google Scholar]
- 15.Erkin UR, Kerem M, Tug M, Orbay H, Sensöz O. Prefabrication of a conjoint flap containing xenogenic tissues: a preliminary report on an experimental model. J Craniofac Surg. 2007;18:1451–1456. doi: 10.1097/scs.0b013e31814e0553. [DOI] [PubMed] [Google Scholar]
- 16.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Gill DR, Ireland DC, Hurley JV, Morrison WA. The prefabrication of a bone graft in a rat model. J Hand Surg Am. 1998;23:312–321. doi: 10.1016/S0363-5023(98)80133-0. [DOI] [PubMed] [Google Scholar]
- 18.Graham SM, Leonidou A, Aslam-Pervez N, Hamza A, Panteliadis P, Heliotis M, Mantalaris A, Tsiridis E. Biological therapy of bone defects: the immunology of bone allo-transplantation. Expert Opin Biol Ther. 2010;10:885–901. doi: 10.1517/14712598.2010.481669. [DOI] [PubMed] [Google Scholar]
- 19.Hori Y, Tamai S, Okuda H, Sakamoto H, Takita T, Masuhara K. Blood vessel transplantation to bone. J Hand Surg Am. 1979;4:23–33. doi: 10.1016/s0363-5023(79)80101-x. [DOI] [PubMed] [Google Scholar]
- 20.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Zhao J, Rosen C, Geusens P, Genant HK. Perspectives on bone mechanical properties and adaptive response to mechanical challenge. J Clin Densitom. 1999;2:423–433. doi: 10.1016/S1094-6950(06)60408-3. [DOI] [PubMed] [Google Scholar]
- 22.Kandel RA, Pritzker KP, Langer F, Gross AE. The pathologic features of massive osseous grafts. Hum Pathol. 1984;15:141–146. doi: 10.1016/S0046-8177(84)80054-4. [DOI] [PubMed] [Google Scholar]
- 23.Kasashima T, Minami A, Kato H, Kaneda K. Experimental study of vascularized bone grafts: hypertrophy of the grafted bone. J Reconstr Microsurg. 2000;16:121–128. doi: 10.1055/s-2000-7546. [DOI] [PubMed] [Google Scholar]
- 24.Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77–86. [PubMed] [Google Scholar]
- 25.Kleinheinz J, Stratmann U, Joos U, Wiesmann HP. VEGF-activated angiogenesis during bone regeneration. J Oral Maxillofac Surg. 2005;63:1310–1316. doi: 10.1016/j.joms.2005.05.303. [DOI] [PubMed] [Google Scholar]
- 26.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon’s point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumta S, Yip K, Roy N, Lee SK, Leung PC. Revascularisation of bone allografts following vascular bundle implantation: an experimental study in rats. Arch Orthop Trauma Surg. 1996;115:206–210. doi: 10.1007/BF00434555. [DOI] [PubMed] [Google Scholar]
- 28.Larsen M, Pelzer M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique. Part II. Validation by comparison to microsphere entrapment. J Orthop Res. 2008;26:746–752. doi: 10.1002/jor.20561. [DOI] [PubMed] [Google Scholar]
- 29.Larsen M, Willems WF, Pelzer M, Friedrich PF, Yaszemski MJ, Bishop AT. Augmentation of surgical angiogenesis in vascularized bone allotransplants with host-derived a/v bundle implantation, fibroblast growth factor-2, and vascular endothelial growth factor administration. J Orthop Res. 2010;28:1015–1021. doi: 10.1002/jor.21098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopez-Curto JA, Bassingthwaighte JB, Kelly PJ. Anatomy of the microvasculature of the tibial diaphysis of the adult dog. J Bone Joint Surg Am. 1980;62:1362–1369. [PMC free article] [PubMed] [Google Scholar]
- 31.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. doi: 10.1097/00003086-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 33.Matejovsky Z, Jr, Matejovsky Z, Kofranek I. Massive allografts in tumour surgery. Int Orthop. 2006;30:478–483. doi: 10.1007/s00264-006-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagi ON. Revascularization of diaphyseal bone segments by vascular bundle implantation. Clin Orthop Relat Res. 2005;440:233–241. doi: 10.1097/01.blo.0000176451.22891.65. [DOI] [PubMed] [Google Scholar]
- 35.Pelzer M, Larsen M, Friedrich PF, Bishop AT. Measurement of bone blood flow using the hydrogen washout technique. Part I. Quantitative evaluation of tissue perfusion in the laboratory rat. J Orthop Res. 2008;26:741–745. doi: 10.1002/jor.20562. [DOI] [PubMed] [Google Scholar]
- 36.Phemister D. Changes in bones and joints resulting from interruption of circulation. Arch Surg. 1940;41:436–472. doi: 10.1001/archsurg.1940.01210020234023. [DOI] [Google Scholar]
- 37.Polykandriotis E, Arkudas A, Beier JP, Hess A, Greil P, Papadopoulos T, Kopp J, Bach AD, Horch RE, Kneser U. Intrinsic axial vascularization of an osteoconductive bone matrix by means of an arteriovenous vascular bundle. Plast Reconstr Surg. 2007;120:855–868. doi: 10.1097/01.prs.0000277664.89467.14. [DOI] [PubMed] [Google Scholar]
- 38.Safak T, Akyürek M, Ozcan G, Keçik A, Aydin M. Osteocutaneous flap prefabrication based on the principle of vascular induction: an experimental and clinical study. Plast Reconstr Surg. 2000;105:1304–1313. doi: 10.1097/00006534-200004040-00008. [DOI] [PubMed] [Google Scholar]
- 39.San-Julian M, Cañadell J. Fractures of allografts used in limb preserving operations. Int Orthop. 1998;22:32–36. doi: 10.1007/s002640050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spalteholz K. Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen. Nebst Anhang: Über Knochenfärbung. Leipzig, Germany: S Hirzel; 1914.
- 41.Stevenson S. Biology of bone grafts. Orthop Clin North Am. 1999;30:543–552. doi: 10.1016/S0030-5898(05)70107-3. [DOI] [PubMed] [Google Scholar]
- 42.Stevenson S, Emery SE, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;324:66–74. doi: 10.1097/00003086-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson S, Li XQ, Davy DT, Klein L, Goldberg VM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts: quantification of a complex process and structure. J Bone Joint Surg Am. 1997;79:1–16. doi: 10.1302/0301-620X.79B1.7020. [DOI] [PubMed] [Google Scholar]
- 44.Stringa G. Studies of the vascularisation of bone grafts. J Bone Joint Surg Br. 1957;39:395–420. doi: 10.1302/0301-620X.39B2.395. [DOI] [PubMed] [Google Scholar]
- 45.Tamai S. Experimental vascularized bone transplantations. Microsurgery. 1995;16:179–185. doi: 10.1002/micr.1920160404. [DOI] [PubMed] [Google Scholar]
- 46.Tamai S. Bone revascularization by vessel implantation for the treatment of Kienböck disease. Tech Hand Up Extrem Surg. 1999;3:154–161. doi: 10.1097/00130911-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Tang P, Fischer CR. A new volar vascularization technique using the superficial palmar branch of the radial artery for the collapsed scaphoid nonunion. Tech Hand Up Extrem Surg. 2010;14:160–172. doi: 10.1097/BTH.0b013e3181d4871f. [DOI] [PubMed] [Google Scholar]
- 48.Thompson RC, Jr, Garg A, Clohisy DR, Cheng EY. Fractures in large-segment allografts. Clin Orthop Relat Res. 2000;370:227–235. doi: 10.1097/00003086-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 49.Thompson RC, Jr, Pickvance EA, Garry D. Fractures in large segment allografts. J Bone Joint Surg Am. 1993;75:1663–1673. doi: 10.2106/00004623-199311000-00011. [DOI] [PubMed] [Google Scholar]
- 50.Top H, Aygit C, Sarikaya A, Cakir B, Cakir B, Unlu E. Bone flap prefabrication: an experimental study in rabbits. Ann Plast Surg. 2005;54:428–434. doi: 10.1097/01.sap.0000151463.82495.84. [DOI] [PubMed] [Google Scholar]
- 51.Trueta J. The role of vessels in osteogenesis. J Bone Joint Surg Br. 1963;45:402–418. [PubMed] [Google Scholar]
- 52.Vander Griend RA. The effect of internal fixation on the healing of large allografts. J Bone Joint Surg Am. 1994;76:657–663. [DOI] [PubMed]
- 53.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 54.Willems WF, Larsen M, Giusti G, Friedrich PF, Bishop AT. Revascularization and bone remodeling of frozen allografts stimulated by intramedullary sustained delivery of FGF-2 and VEGF. J Orthop Res. 2011;29:1431–1436. doi: 10.1002/jor.21338. [DOI] [PubMed] [Google Scholar]
- 55.Woodhouse CF. The transplantation of patent arteries to bone. J Int Coll Surg. 1963;39:437–446. [PubMed] [Google Scholar]
- 56.Yao Y, Hua C, Tang X, Wang Y, Zhang F, Xiang Z. Angiogenesis and osteogenesis of non-vascularised autogenous bone graft with arterial pedicle implantation. J Plast Reconstr Aesthet Surg. 2010;63:467–473. doi: 10.1016/j.bjps.2008.11.053. [DOI] [PubMed] [Google Scholar]