Abstract
Background
The evidence supporting continued use of shelf acetabuloplasty in Legg-Calvé-Perthes disease (LCPD) is not well-defined, and there is controversy regarding the long-term benefits related to clinical and functional improvement.
Questions/purposes
Our goals were to determine whether shelf arthroplasty for LCPD (1) prevents the onset of early osteoarthritis; (2) improves pain, ROM, activity, and functional outcomes; (3) maintains or improves femoral head containment, sphericity, and congruency; (4) changes the acetabular index; and (5) is associated with a low rate of complications.
Methods
We performed a systematic review of the medical literature from 1966 to 2009 using the search terms Perthes, shelf procedure, and acetabuloplasty. We excluded reports using multiple/combined treatment methods and those not clearly stratifying outcomes. Thirteen studies met the criteria. There were no Level I studies, one Level II prognostic study, five Level III therapeutic studies, and seven Level IV studies. Mean followup ranged from 2.6 to 17.9 years.
Results
Only one study reported progression to early osteoarthritis in one patient. We found no evidence for improvement in ROM and continued pain relief at long-term followup. Mean decrease in lateral subluxation ratio was 13% to 30%, demonstrating an improvement in femoral head containment. Mean acetabular cover percentage improved 16% to 38%, and mean acetabular and center-edge angles improved 4° to 14° and 8° to 33°, respectively. There were no reports of any major complications after the procedure.
Conclusions
While radiographic measurements indicate improved coverage of the femoral head after shelf acetabuloplasty for LCPD, available evidence does not document the procedure prevents early onset of osteoarthritis or improves long-term function.
Introduction
Legg-Calvé-Perthes disease (LCPD) is predominantly thought to involve an unexplained vascular insult to the capital femoral epiphysis with resulting avascular necrosis [1, 26, 54]. The exact etiology behind LCPD remains largely unknown [3, 22, 24, 62]. Biologic sequelae include a chain of events with eventual revascularization leading to biologic plasticity of the femoral head usually followed by femoral head shape change, flattening, and even subluxation in severe cases. These morphologic changes can be a precursor to premature hip osteoarthritis [2, 7]. Although considered primarily a problem of the femoral head, acetabular dysplasia or retroversion has also been reported [12].
The strategy of treating LCPD includes containment of the femoral epiphysis within the acetabulum [15, 16]. The concept of containment is to center the femoral head within the acetabulum during the fragmentation and reossification phase. This allows the acetabulum to serve as a mold during the healing (revascularization) phase when the biologically plastic femoral head [46] is at risk for subluxation, hinge abduction, and permanent femoral head deformation. Severity of femoral head deformity and joint incongruity at skeletal maturity increase the risk of loss of function long-term [51]. Containment can be achieved nonoperatively or operatively. Nonoperative measures, using either abduction casts or bracing, attempt to maintain weightbearing and ROM in the contained, abducted position [25, 41, 42, 45], but these orthoses require prolonged treatment times and may not be tolerated well by the patient, both physically and psychologically.
Surgical containment is intended to contain the femoral head and promote a spherical femoral head at skeletal maturity. Proposed methods include proximal femoral varus osteotomy [19, 33, 37, 49, 55], innominate osteotomy [19, 33, 37, 39, 47, 49, 55], and shelf acetabuloplasty with or without proximal femoral osteotomy [27]. Innominate osteotomy combined with a proximal femoral varus osteotomy [8, 58] or triple pelvic osteotomy [28, 38] can provide greater femoral head containment in patients with more severe femoral head and/or acetabular deformity.
The shelf procedure is an acetabuloplasty intended to promote eventual long-term congruency between the uncovered femoral head and the opposing acetabulum. This procedure involves increasing the superolateral support or coverage of the femoral head by extending the acetabular roof [50, 60]. The anatomy of the original acetabular roof, and thus the intraarticular hyaline cartilage, remains unchanged [53]. However, unlike redirectional pelvic osteotomies in which the femoral head is centralized within the original acetabulum and its hyaline cartilage, the shelf acetabuloplasty increases femoral head coverage by placement of a bone block, which does not contain hyaline cartilage, in the ilium. Those supporting the procedure argue addition of bone to the lateral rim supports the labrum in abduction and prevents subluxation and abutment of the femoral head against the superolateral margin of the acetabulum with opening of the medial joint space, so-called hinged abduction [13]. Those opposing the procedure question the basis of the surgery, including potential to damage the lateral acetabular epiphysis [9], which contributes a substantial amount to acetabular growth [31, 48], inadequacy of the small bony ledge to provide any meaningful coverage, and lack of evidence that substantiates improvement in functional outcomes or delay in the onset of osteoarthritis. Thus, both the rationale for and interpretation of the data reporting the effects of the procedure are controversial.
We therefore evaluated the literature related to shelf acetabuloplasty in the treatment of LCPD to determine whether the procedure (1) prevents the onset of early osteoarthritis; (2) improves pain, ROM, activity, and functional outcomes as determined by patient-centered outcome measures; (3) maintains or improves femoral head containment (with improved acetabular coverage and center-edge [CE] angle), femoral head sphericity, and joint congruency; (4) improves the acetabular index; and (5) is associated with a low rate of complications.
Search Strategy and Criteria
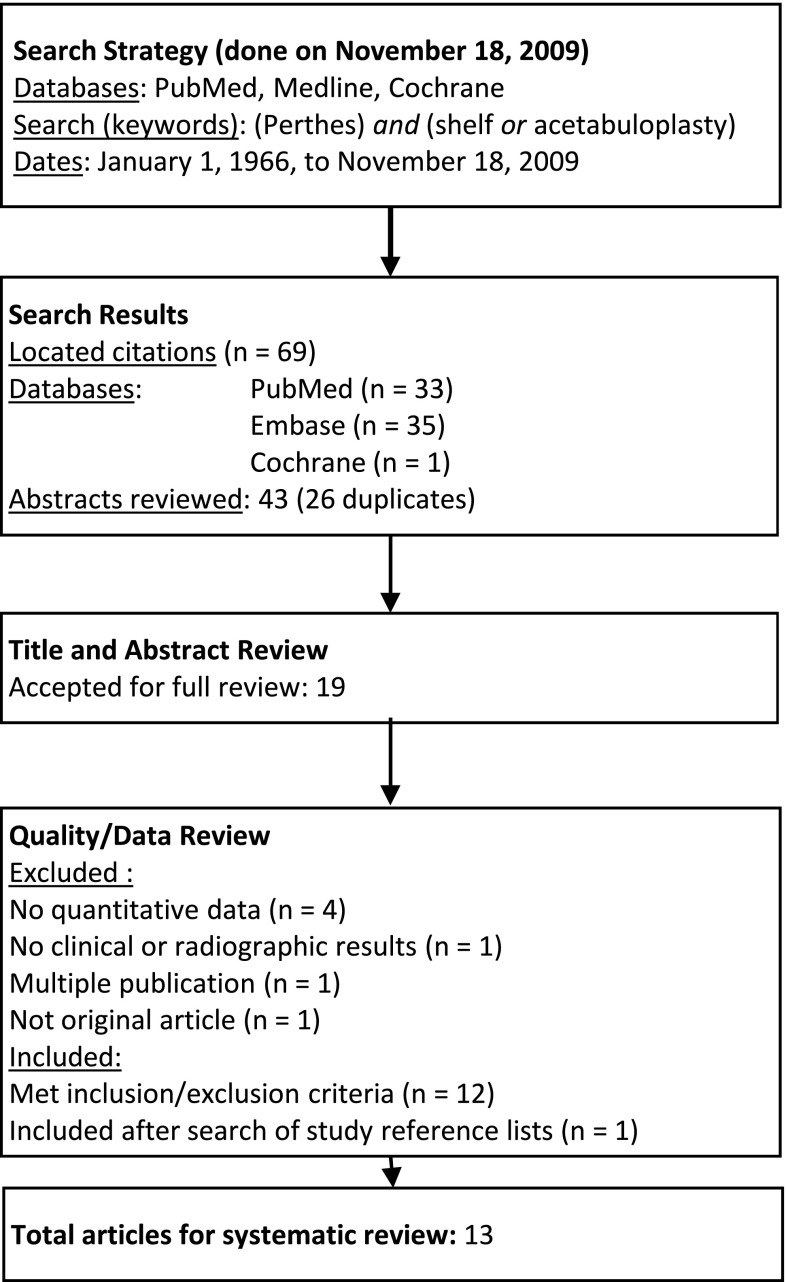
We performed a systematic review of the literature to assess the results of the shelf acetabuloplasty procedure for LCPD using the PubMed, Embase, and Cochrane databases (Fig. 1). The literature was searched utilizing the following search terms: Perthes AND shelf OR acetabuloplasty. We included only articles published between January 1966 and November 2009. This search yielded 69 articles. The initial search was performed by one author (JEH), results imported into EndNote, and duplicates discarded. Two of the authors (JEH, KDB) independently reviewed each title and abstract of the search results and selected studies for full review based on predefined inclusion and exclusion criteria. In addition, references of all selected articles were manually reviewed to ensure all possible articles were considered.
Fig. 1.
A flowchart shows the procedure for selecting the studies included in this review.
Inclusion criteria were specified before starting the search: (1) patients had a diagnosis of LCPD; (2) subjects were between 2 and 16 years old; (3) patients were treated with a shelf acetabuloplasty procedure; (4) the study had a minimum of 10 patients; (5) the study included quantitative clinical outcomes or radiographic outcomes or classifications (described below); (6) the study had Level I, II, III, or IV evidence; and (7) the study was in English. Studies were excluded if patients had previous surgeries or multiple procedures/concomitant bony procedure(s) to the ipsilateral lower extremity unrelated to the shelf acetabuloplasty or the study included various treatment methods and did not clearly stratify outcomes of the shelf acetabuloplasty group. Of the 69 abstracts reviewed in our initial screening (Fig. 1), 26 were discarded as duplicates, and the abstracts of the remaining 43 studies were reviewed. Based on the abstracts alone, 19 studies were included for full review. Six of these 19 studies were excluded, including four studies reporting qualitative rather than quantitative data, one that did not explicitly report any of the quantitative clinical or radiographic results described below, and one that was not an original research article. This left 13 studies that met inclusion and exclusion criteria. After review of the reference lists from the selected articles, one additional study by Kuwajima et al. [29] was identified for inclusion for a total of 14 studies [4, 9–11, 13, 14, 20, 23, 27, 29, 56, 57, 61, 63]. Of these, the two studies by Domzalski et al. in 2006 [10] and 2007 [11] appeared to use the same population, as did the studies by van der Haven et al. [57] and van der Geest et al. [56]. The data from both studies by Domzalski et al. [10, 11] were included, as they reported different quantitative measurements from the same group. However, the study by van der Haven et al. [57] was not included in the review, as it appeared to be a subset of the same population described by van der Geest et al. [56] without reporting different outcomes. The remaining 13 studies [4, 9–11, 13, 14, 20, 23, 27, 29, 56, 61, 63] were performed at separate institutions without overlapping patient populations. Although the study by Ghanem et al. [14] included 16 of 30 patients that had undergone concomitant femoral varus osteotomy, the remaining 14 patients did not have any concomitant procedures and were included in this review.
We compiled a database for the 13 studies. Patient demographics collected included dates of data collection, number of patients, number of shelf acetabuloplasties performed, proportion of male and female patients, age at disease onset, age at operation, and length of followup. Surgical techniques and variables included preoperative arthrography, additional soft tissue procedures performed, and postoperative rehabilitation protocol. All clinical patient-centered outcome measures were recorded, which included the Iowa hip score and the modified Sundt criteria. The Iowa hip score employs a 100-point rating scale with points allotted for freedom from pain, function, gait, freedom from deformity, and motion [30]. The modified Sundt criteria [52] rate clinical results as good (no symptoms, full ROM), fair (mild symptoms, hip motion slightly restricted), and poor (symptomatic, hip motion markedly restricted). Five standard radiographic measurements based on biplanar plain radiographs were recorded: percent acetabular coverage, acetabular angle, CE angle, lateral subluxation ratio (width of the medial joint space of the affected to unaffected hip), and femoral head size ratio between the involved and normal hips. These radiographic measures were reported at varying time points of disease and treatment. Most studies reported pre- and postoperative radiographic values (including femoral head containment/acetabular coverage or extrusion index, acetabular index, and CE angle). Radiographic classification systems were also recorded and included the classifications of Catterall [6], lateral pillar [17], and Stulberg et al. [51].
Data collection periods ranged from 1940 to 2005, with a total of 348 shelf acetabuloplasties performed (Table 1). The majority of patients (84.7%) were male. The average age at time of operation ranged from 8.0 to 10.2 years. Length of followup was reported in all studies, with the average ranging from 1.8 to 15 years; three of the 13 studies reported results with followup averaging longer than 10 years [11, 27, 56]. Patients lost to followup were not commonly reported in the studies included.
Table 1.
Demographics
| Study | Year published | Study period | Level of evidence | Number of patients/hips | Number of males/females | Age at onset (years)* | Age at operation (years)* | Followup (years)* |
|---|---|---|---|---|---|---|---|---|
| Ghanem et al.† [14] | 2009 | 1996–2002 | IV | 14/14 | NA | 7.0 (6.2–9.4) | 8.2 (7.1–11.1) | NA |
| Yoo et al. [63] | 2009 | 1999–2005 | IV | 25/25 | 22/3 | NA | 8.9 (7.0–12.3) | 6.7 (3.2–9.0) |
| Freeman et al. [13] | 2008 | 1995–2003 | IV | 27/27 | 21/6 | 6.9 (3–14) | 8.3 (4.6–14.6) | 5.2 (2.2–10.4) |
| Domzalski et al. [11] | 2007 | 1944–1998 | II | 69/69 | 59/10 | 7.5 (5–13) | 9.1 (6–14.1) | 15 (2–44) |
| Domzalski et al.‡ [10] | 2006 | NA | III | 49/49 | 44/5 | 7.2 | 8.8 | 5+ |
| Bursal and Erkula [4] | 2004 | 1993–2000 | IV | 18/19 | 13/5 | NA | 9.2 | 2.6 |
| Jacobs et al. [23] | 2004 | 1992–1998 | IV | 43/43 | 36/7 | 6.4 (2.8–11.4) | 8.3 (3.5–11.7) | 3.7 (1.3–6.2) |
| Kuwajima et al. [29] | 2002 | 1983–1992 | III | 40/40 | 36/4 | 6.4 (4–10) | NA | 4.7 (2.0–10.0) |
| van der Geest et al. [56] | 2001 | 1980–1992 | IV | 30/30 | 23/7 | 6.6 (3.0–9.4) | 8.0 (4.7–11.1) | 12 (5.6–18.4) |
| Huang and Huang [20] | 1999 | 1990–1993 | III | 14/14 | 12/2 | NA | 10.2 (4.5–15.7) | 3.7 (2.6–5) |
| Daly et al. [9] | 1999 | 1986–1992 | IV | 26/27 | 24/3 | 9.8 (8–12.1) | 10.0 (SD, 1.5) | 5.9 (2.6–9.9) |
| Willett et al. [61] | 1992 | 1985–1989 | III | 20/20 | 17/3 | 9.2 (8–13) | NA | 1.8 (0.7–3.5) |
| Kruse et al. [27] | 1991 | 1940–1987 | III | 19/20 | 19/1 | 8 (6–14) | 10 (7–14) | 17.9 (2–46) |
| Total | 1991–2009 | 1940–2005 | 1 Level II 5 Level III 7 Level IV |
345/348 | 282/51 |
* Values are expressed as mean, with range in parentheses; †14 of 30 patients included in this study; 16 patients in which proximal femoral varus osteotomy was performed were excluded from study totals; ‡patients in this study appear to be included in the other study of Domzalski et al. [11]; therefore, numbers from this study were excluded from study totals; NA = not available.
We identified no Level I studies of this technique. There was one Level II prognostic study [11]. Five were Level III therapeutic studies, comparing shelf acetabuloplasty to proximal femoral varus osteotomy [10], Salter/innominate osteotomy [20, 29], and nonoperative treatment [27, 61]. The remaining seven studies were Level IV case series ranging from 18 to 43 patients.
A systematic quality assessment for articles was undertaken as described by Zaza et al. [65]. This method of assessing study quality entails a checklist encompassing five major areas of study design: a description of the population and intervention, sampling, measurement, data analysis, and interpretation of results. No summary score is generated for this tool. Five of the 13 articles we reviewed had some issue regarding their description of the population or intervention used. The two most common problems were descriptions of population. These studies either did not provide enough information about inclusion or exclusion criteria to decide whether the cases were consecutive or the studies did not disclose the indications for surgical intervention with a shelf arthroplasty. Sampling bias was a problem in 11 of the articles; the entire available population was not used in the majority of the studies. In five of the studies, the selection and screening process was not described at all. Six studies had an adequate description of the exclusion criteria but still did not use all available patients. Eight studies used solely or mostly radiographic criteria to determine outcome. The surgical procedure was adequately described in all studies. Only three studies controlled for multilevel data by adjusting for confounders or stratifying data, and less than ½ controlled for multiple tests using statistical adjustment of Type I error rate. One study reported a p value but did not report what statistical test was used. Three-quarters of the studies employed statistical testing and adequately described their statistical methods. Eleven (84.6%) had greater than 80% followup, and the units of measurement were similar pre- and postoperatively.
Results
We found no evidence the shelf procedure delayed onset of osteoarthritis: the presence of osteoarthritis was reported in only one study [27]. In that study, one patient required THA at the age of 50 years for pain from osteoarthritis.
Residual pain was reported in 0% to 14% of patients, and full ROM was achieved in 14% to 67% of patients at 3.7 to 5.2 years postoperatively [13, 20, 23] (Table 2). Two studies that reported on restriction of activities reported all patients resumed activities without difficulty [20, 61]. Limited abduction at last followup was found in 33% to 45% of patients in two studies [13, 61], and residual hinge abduction was reported in 0% to 16% of patients in two other studies [23, 27].
Table 2.
Surgical protocols and clinical outcomes
| Study | Catterall | Pillar | Preoperative arthrography | Additional procedures | Postoperative protocol | Clinical outcome | Followup (years)* | Complications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scale | Score* | |||||||||||||
| I | II | III | IV | A | B | C | ||||||||
| Ghanem et al. [14] | 14/14 | Adductor tenotomy (100%) | Hip spica for 4 weeks | Iowa | 88.6 (SD, 5.4) | 9.5 (5.2–12) | 4 patients required additional surgery | |||||||
| Yoo et al. [63] | 0 | 0 | 10 | 15 | 0 | 11 | 14 | 25/25 | Adductor + psoas tenotomy (48%), adductor only (4%), psoas only (4%) | Bilateral short leg casts, connecting bar for 3–6 weeks | NA | NA | 6.7 (3.2–9.0) | NA |
| Freeman et al. [13] | 0 | 0 | 12 | 15 | 0 | 12 | 15 | 27/27 | None | Hip spica for 6 weeks (7/27); no immobilization, TTWB (20/27) | NA | NA | 5.2 (2.2–10.4) | 1 patient required VGEO |
| Bursal and Erkula [4] | 0 | 7 | 12 | No | NA | NA | Iowa | 97.2 | 2.6 | 1 partial graft resorption | ||||
| 1 fracture through graft | ||||||||||||||
| Jacobs et al. [23] | 0 | 5 | 21 | 17 | 0 | 23 | 20 | NA | Adductor tenotomy (16/43) | Hip spica for 3 weeks, TTWB for 3 weeks; hip spica for 6 weeks if tenotomy | NA | NA | 3.7 (1.3–6.2) | NA |
| Kuwajima et al. [29] | 0 | 0 | 17 | 6 | 23/40 | NA | NA | NA | NA | 4.7 (2.0–10.0) | NA | |||
| van der Geest et al. [56] | 0 | 0 | 12 | 18 | 0 | 11 | 19 | NA | NA | Hip spica for 6–12 weeks | Iowa | 96 (74–100) | 12 (5.6–18.4) | NA |
| Huang and Huang [20] | 0 | 0 | 5 | 9 | 0 | 5 | 9 | NA | NA | NA | Sundt | 2 good 12 fair |
3.7 (2.6–5) | NA |
| Daly et al. [9] | 0 | 3 | 17 | 7 | 27/27 | NA | Hip spica for 9 weeks | NA | NA | 5.9 (2.6–9.9) | NA | |||
| Willett et al. [61] | 0 | 4 | 16 | 0 | NA | NA | Hip spica for 8 weeks | NA | NA | 1.8 (0.7–3.5) | NA | |||
| Kruse et al. [27] | 0 | 0 | 3 | 17 | 13/20 | NA | NA | Iowa | 90.6 (63–99) | 17.9 (2–46) | 2 graft resorption | |||
* Values are expressed as mean, with range in parentheses; NA = not available; TTWB = toe touch weightbearing; VGEO = valgus extension osteotomy.
Iowa hip scores at last followup ranged from 88.6 to 97.2 at an average of 2.6 to 17.9 years postoperatively [4, 14, 27, 56]. One study using Sundt criteria reported 14% good and 86% fair results [20].
The radiographic data based on frontal plane imaging suggested improvement in acetabular coverage of the femoral head (Table 3). The mean change in acetabular cover percentage improved between 16% and 38%. Similarly, the mean change in acetabular angle improved between 4° and 14°, and the CE angle improved between 8° and 33°. The lateral subluxation ratio decreased on average between 13% and 30%. The femoral head size ratio changed from −1% to 10%. In four studies [9, 14, 23, 56], comparison of these five radiographic parameters between early postoperative and latest followup time points was performed to assess incorporation or resorption of the graft. These data showed an improvement in each of the parameters; there were, however, negative changes in acetabular angle and CE angle (from postoperative measurement to latest followup) in two studies likely related to graft resorption [14, 29]. Radiographic occurrence of secondary osteoarthritis was not reported in any of the included studies.
Table 3.
Radiographic outcomes
| Study | Stulberg | Mean change in acetabular cover percentage | Mean improvement in acetabular angle (°) | Mean improvement in center-edge angle (°) | Mean improvement in lateral subluxation ratio | % change in femoral head size ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Ghanem et al. [14] | 3 | 6 | 3 | 2 | 0 | 38 | 0 | 10 | −3 | 32 | −4 | −9 | 3 | −1 | 6 |
| Yoo et al. [63] | 1 | 8 | 13 | 3 | 0 | 4 | 8 | 30 | 10 | ||||||
| Freeman et al. [13] | 1 | 13 | 10 | 2 | 1 | 16 | 26 | 8 | |||||||
| Bursal and Erkula [4] | 18 | ||||||||||||||
| Jacobs et al. [23] | 5 | 3 | 20 | 10 | |||||||||||
| Kuwajima et al. [29] | 9 | 15 | 3 | −9 | |||||||||||
| van der Geest et al. [56] | 6 | 10 | 2 | 19 | −6 | 15 | 3 | ||||||||
| Huang and Huang [20] | 0 | 2 | 8 | 4 | 0 | 35 | 10 | 33 | 29 | 8 | |||||
| Daly et al. [9] | 2 | 12 | 8 | 3 | 2 | 22 | 14 | 26 | 7 | ||||||
| Willett et al. [61] | 7 | 11 | 2 | 31 | 13 | 13 | 1 | ||||||||
Pre = change between preoperative and latest followup; Post = change between early postoperative and latest followup.
Clinical complications were reported in four studies and happened to be minor complications (Table 2) [4, 13, 14, 27]. Two of the studies reported a total of three cases of graft resorption as a complication [4, 27], and one reported a fracture through the graft. Additional subsequent surgery for persistent symptoms included distal transfer of the greater trochanter in two patients [14], epiphysiodesis of the greater trochanter in one patient [14], femoral lengthening in one patient [14], and valgus extension osteotomy in one patient [13]. There were no major complications reported.
Discussion
LCPD of the hip was described more than 100 years ago [5, 32, 40, 59], and while we have furthered our understanding about the morphologic variations in the sequelae of LCPD, the etiology of the condition continues to be an enigma. Surgical containment is an accepted modality of treatment intended to promote a spherical femoral head at skeletal maturity. These containment methods, including proximal femoral osteotomy with innominate osteotomy or triple periacetabular osteotomy, have been advocated even in severe stages (greater head involvement) of LCPD. While the shelf acetabuloplasty with or without proximal femoral osteotomy also continues to be used as a salvage procedure in many centers, there is controversy regarding the true utility of the shelf acetabuloplasty, particularly related to its long-term benefits toward clinical and functional outcomes. Our objectives were to determine whether the shelf procedure (1) prevents the onset of early osteoarthritis; (2) improves pain, ROM, activity, and functional outcomes as determined by patient-centered outcome measures; (3) maintains or improves femoral head containment (with improved acetabular coverage and CE angle), femoral head sphericity, and joint congruency; (4) improves the acetabular index; and (5) is associated with a low rate of complications.
There are limitations with the literature and specific to our study. First, the level of evidence regarding shelf acetabuloplasty is low, with no prospective studies. This means the studies were subject to considerable selection bias without allowing for comparisons between alternative treatments. Second, the quality of the studies was generally low, with the majority of studies having sampling bias and lacking adequate clinical outcome measurements and data analysis measures. Third, we found no long-term studies assessing the rate of development of arthritis. While treatment of LCPD is aimed at containment of the femoral head, the long-term goal of containment is to reduce the progression to debilitating arthritis. The literature contains no studies to show the procedure achieves this aim. It is therefore important to recognize the perceived ability of the shelf acetabuloplasty to prevent early arthritis is unfounded. Fourth, the methods for pre- and postoperative clinical assessment of shelf acetabuloplasty lacked consistency across studies. More specifically, common clinical variables such as pain, presence of a limp, ROM, and leg length discrepancy were lacking in most studies. Again, it is important to recognize the expectation of predictable pain relief, improvement of ROM, and possible limb length equalization may not be met after the shelf acetabuloplasty. Fifth, while radiographic measurements such as acetabular coverage, acetabular slope, CE angle, medial joint subluxation, and femoral head size were more commonly reported than clinical measures, they were still inconsistently reported. The presumption that shelf procedure always improved the radiographic outcomes in patients with LCPD is not always true. Sixth, some studies analyzing radiographic outcomes had no preoperative values [9, 23]. Despite these limitations, available evidence (Table 3) suggests improvement of indexes calculated on biplanar radiographs after shelf acetabuloplasty. It is important to point out this is only an improvement of the osseous coverage while the actual coverage and orientation of the hyaline cartilage may remain unchanged. The association between these radiographic results after shelf acetabuloplasty and clinically important variables such as resorption of the graft or premature arthritis is not clarified by any of the studies included in this review. Seventh, the included studies were heterogeneous in that they included different outcome measures, sometimes none at all, various lengths of followup, various postoperative protocols, and surgeons whose techniques may vary. Our criteria only required a 12-month followup, and studies with any quantitative clinical and radiographic outcome data were included, resulting in a heterogeneous but comprehensive review. Lastly, our review is systematic, and thus, every study we picked was by predefined inclusion and exclusion criteria; however, it is limited by its use of only studies in the English language and by use of only studies represented in the selected databases we used. As such, our review had quality control inherent in the rigorous reviews of journals represented by these groups but may not have the breadth of information had we included other languages and “gray” literature.
The long-term goal of surgical intervention in the treatment of LCPD is to prevent the development of early arthritis. Whether this goal is achieved by the shelf procedure is unknown, as our systematic review reveals a paucity of long-term data. Only two studies [27, 56] had followup of longer than 10 years, and only one study reported on the development of arthritis in a single patient requiring THA. The remainder of the studies had a followup that was inadequate to assess the effect of this procedure on the development of arthritis [14, 27, 56]. Studies on the natural history of LCPD describe the development of arthritis decades after initial presentation, and children are often asymptomatic despite asphericity of the femoral head on radiographs [18, 21, 35, 36, 64]. Therefore, followup decades after skeletal maturity would likely be required to deem the procedure a potential improvement over other modalities of treatment or nonoperative methods.
While one long-term goal of operative intervention in LCPD is to prevent the onset of early arthritis, short-term goals include minimization of hip irritability and restoration of ROM. When the femoral head becomes deformed to the point where it is not contained within the acetabulum, abduction can lead to hinging on the lateral edge of the acetabulum, also called hinge abduction [7, 43, 44]. While our review revealed a paucity of studies that reported clinical data, those that did report on clinical outcomes described an improvement in pain, ROM, and return to daily activities without difficulty. Full ROM may not always be obtained, however, and limited abduction may be a residual problem [13, 61]. Whether this postoperative limited ROM in the abduction plane can lead to further deformity of both the proximal femur and the acetabulum and subsequent development of early degenerative changes is unknown.
Radiographically, femoral head coverage improved after shelf arthroplasty. The radiographic indexes described in all studies were based on two-dimensional plain films. These radiographic indexes describing acetabular coverage of the femoral head suggest the shelf arthroplasty improves containment of the femoral head. There were no data to suggest any improvement in the sphericity or congruency of the femoral head within the acetabulum, and no study included any three-dimensional imaging. The radiographic data suggest shelf acetabuloplasty improves acetabular coverage of the femoral head in a frontal plane image obtained via plain radiography.
Complications after shelf acetabuloplasty are relatively uncommon. Graft resorption or fracture through the graft are concerns after the shelf procedure, but the overall incidence of these complications [4, 27] was relatively low (about 1%) in the studies included. The graft must be placed at the correct level to contribute to stability of the hip and prevent resorption or fracture of the graft; if placed too high, the graft may resorb with time, while placement of the graft in too low of a position may lead to damage to the femoral head [34]. With correct surgical technique in which the shelf is optimally positioned and properly stabilized in continuity with the subchondral bone of the acetabular roof, these complications should be rare. In two of the studies [13, 14], patients required subsequent procedures after the shelf acetabuloplasty to address restriction in abduction.
In conclusion, shelf acetabuloplasty for LCPD is a procedure that improves femoral containment as determined by two-dimensional plain radiography and is associated with low complication rates. The long-term benefit of shelf acetabuloplasty in preventing progression to early osteoarthritis, however, is uncertain, and improvements in pain and ROM are not well documented in the literature. There is no literature to support or reject the use of this surgical technique to prevent early development of arthritis. More prospective comparative studies are needed to determine whether this procedure provides a long-term benefit to patients by creation of a congruent joint space, continued containment of the femoral head, and prevention of early osteoarthritis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
This is a systematic review and as such was conducted at all of the authors’ institutions.
References
- 1.Atsumi T, Yoshihara S, Hiranuma Y. Revascularization of the artery of the ligamentum teres in Perthes disease. Clin Orthop Relat Res. 2001;386:210–217. doi: 10.1097/00003086-200105000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Bowen JR, Foster BK, Hartzell CR. Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 1984;185:97–108. [PubMed] [Google Scholar]
- 3.Broder H. The late results in Legg-Perthes’ disease and factors influencing them: a study of one hundred and two cases. Bull Hosp Joint Dis. 1953;14:194–216. [PubMed] [Google Scholar]
- 4.Bursal A, Erkula G. Lateral shelf acetabuloplasty in the treatment of Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2004;13:150–152. doi: 10.1097/00009957-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Calvé J. Sur une forme particuliere de coxalgie greffe sur des deformations caracteristiques de 1′extremite superieure de femur. Rev Chir. 1910;42:54. [Google Scholar]
- 6.Catterall A. The natural history of Perthes’ disease. J Bone Joint Surg Br. 1971;53:37–53. [PubMed] [Google Scholar]
- 7.Catterall A. Legg-Calvé-Perthes syndrome. Clin Orthop Relat Res. 1981;158:41–52. [PubMed] [Google Scholar]
- 8.Crutcher JP, Staheli LT. Combined osteotomy as a salvage procedure for severe Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:151–156. doi: 10.1097/01241398-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Daly K, Bruce C, Catterall A. Lateral shelf acetabuloplasty in Perthes’ disease: a review of the end of growth. J Bone Joint Surg Br. 1999;81:380–384. doi: 10.1302/0301-620X.81B3.9405. [DOI] [PubMed] [Google Scholar]
- 10.Domzalski ME, Glutting J, Bowen JR, Littleton AG. Lateral acetabular growth stimulation following a labral support procedure in Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 2006;88:1458–1466. doi: 10.2106/JBJS.E.00689. [DOI] [PubMed] [Google Scholar]
- 11.Domzalski ME, Inan M, Guille JT, Glutting J, Kumar SJ. The proximal femoral growth plate in Perthes disease. Clin Orthop Relat Res. 2007;458:150–158. doi: 10.1097/BLO.0b013e3180380ef2. [DOI] [PubMed] [Google Scholar]
- 12.Ezoe M, Naito M, Inoue T. The prevalence of acetabular retroversion among various disorders of the hip. J Bone Joint Surg Am. 2006;88:372–379. doi: 10.2106/JBJS.D.02385. [DOI] [PubMed] [Google Scholar]
- 13.Freeman RT, Wainwright AM, Theologis TN, Benson MK. The outcome of patients with hinge abduction in severe Perthes disease treated by shelf acetabuloplasty. J Pediatr Orthop. 2008;28:619–625. doi: 10.1097/BPO.0b013e3181804be0. [DOI] [PubMed] [Google Scholar]
- 14.Ghanem I, Haddad E, Haidar R, Haddad-Zebouni S, Aoun N, Dagher F, Kharrat K. Lateral shelf acetabuloplasty in the treatment of Legg-Calvé-Perthes disease: improving mid-term outcome in severely deformed hips. J Child Orthop. 2009 November 13 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 15.Grzegorzewski A, Bowen JR, Guille JT, Glutting J. Treatment of the collapsed femoral head by containment in Legg-Calvé-Perthes disease. J Pediatr Orthop. 2003;23:15–19. doi: 10.1097/00004694-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Herring JA. Legg-Calvé-Perthes disease. In: Herring JA, editor. Tachdjian’s Pediatric Orthopaedics. 3. Philadelphia: WB Saunders; 2001. pp. 655–704. [Google Scholar]
- 17.Herring JA, Neustadt JB, Williams JJ, Early JS, Browne RH. The lateral pillar classification of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1992;12:143–150. doi: 10.1097/01241398-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Herring JA, Williams JJ, Neustadt JN, Early JS. Evolution of femoral head deformity during the healing phase of Legg-Calvé-Perthes disease. J Pediatr Orthop. 1993;13:41–45. doi: 10.1097/01241398-199301000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Hoikka V, Poussa M, Yrjonen T, Osterman K. Intertrochanteric varus osteotomy for Perthes’ disease: radiographic changes after 2–16-year follow-up of 126 hips. Acta Orthop Scand. 1991;62:549–553. doi: 10.3109/17453679108994494. [DOI] [PubMed] [Google Scholar]
- 20.Huang MJ, Huang SC. Surgical treatment of severe Perthes disease: comparison of triple osteotomy and shelf augmentation. J Formos Med Assoc. 1999;98:183–189. [PubMed] [Google Scholar]
- 21.Ippolito E, Tudisco C, Farsetti P. The long-term prognosis of unilateral Perthes’ disease. J Bone Joint Surg Br. 1987;69:243–250. doi: 10.1302/0301-620X.69B2.3818755. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs BW. Early recognition of osteochondrosis of capital epiphysis of femur. J Am Med Assoc. 1960;172:527–531. doi: 10.1001/jama.1960.03020060017005. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs R, Moens P, Fabry G. Lateral shelf acetabuloplasty in the early stage of Legg-Calvé-Perthes disease with special emphasis on the remaining growth of the acetabulum: a preliminary report. J Pediatr Orthop B. 2004;13:21–28. doi: 10.1097/00009957-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kallio P, Ryoppy S, Kunnamo I. Transient synovitis and Perthes’ disease: is there an aetiological connection? J Bone Joint Surg Br. 1986;68:808–811. doi: 10.1302/0301-620X.68B5.3782251. [DOI] [PubMed] [Google Scholar]
- 25.King EW, Fisher RL, Gage JR, Gossling HR. Ambulation-abduction treatment in Legg-Calvé-Perthes disease (LCPD) Clin Orthop Relat Res. 1980;150:43–48. [PubMed] [Google Scholar]
- 26.Kleinman RG, Bleck EE. Increased blood viscosity in patients with Legg-Perthes disease: a preliminary report. J Pediatr Orthop. 1981;1:131–136. doi: 10.1097/01241398-198110000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Kruse RW, Guille JT, Bowen JR. Shelf arthroplasty in patients who have Legg-Calvé-Perthes disease: a study of long-term results. J Bone Joint Surg Am. 1991;73:1338–1347. [PubMed] [Google Scholar]
- 28.Kumar D, Bache CE, O’Hara JN. Interlocking triple pelvic osteotomy in severe Legg-Calvé-Perthes disease. J Pediatr Orthop. 2002;22:464–470. doi: 10.1097/00004694-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kuwajima SS, Crawford AH, Ishida A, Roy DR, Filho JL, Milani C. Comparison between Salter’s innominate osteotomy and augmented acetabuloplasty in the treatment of patients with severe Legg-Calvé-Perthes disease: analysis of 90 hips with special reference to roentgenographic sphericity and coverage of the femoral head. J Pediatr Orthop B. 2002;11:15–28. doi: 10.1097/00009957-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Larson CB. Rating scale for hip disabilities. Clin Orthop Relat Res. 1963;31:85–93. doi: 10.1097/00003086-196300310-00011. [DOI] [PubMed] [Google Scholar]
- 31.Lee MC, Eberson CP. Growth and development of the child’s hip. Orthop Clin North Am. 2006;37:119–132. doi: 10.1016/j.ocl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Legg AT. An obscure affection of the hip joint. Boston Med Surg. 1910;162:202. doi: 10.1056/NEJM191002171620702. [DOI] [Google Scholar]
- 33.Lloyd-Roberts GC, Catterall A, Salamon PB. A controlled study of the indications for and the results of femoral osteotomy in Perthes’ disease. J Bone Joint Surg Br. 1976;58:31–36. doi: 10.1302/0301-620X.58B1.1270493. [DOI] [PubMed] [Google Scholar]
- 34.Love BR, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62:321–325. doi: 10.1302/0301-620X.62B3.7410463. [DOI] [PubMed] [Google Scholar]
- 35.McAndrew MP, Weinstein SL. A long-term follow-up of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1984;66:860–869. doi: 10.2106/00004623-198466060-00006. [DOI] [PubMed] [Google Scholar]
- 36.Mose K. Methods of measuring in Legg-Calvé-Perthes disease with special regard to the prognosis. Clin Orthop Relat Res. 1980;150:103–109. [PubMed] [Google Scholar]
- 37.Noonan KJ, Price CT, Kupiszewski SJ, Pyevich M. Results of femoral varus osteotomy in children older than 9 years of age with Perthes disease. J Pediatr Orthop. 2001;21:198–204. doi: 10.1097/00004694-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor PA, Mulhall KJ, Kearns SR, Sheehan E, McCormack D. Triple pelvic osteotomy in Legg-Calvé-Perthes disease using a single anterolateral incision. J Pediatr Orthop B. 2003;12:387–389. doi: 10.1097/00009957-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DC, Leitch JM, Foster BK. Results of innominate osteotomy in the treatment of Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 1991;266:96–103. [PubMed] [Google Scholar]
- 40.Perthes G. Uber Arthritis Deformans Juvenilis. Dtsch Z Chir. 1910;10:111. doi: 10.1007/BF02816154. [DOI] [Google Scholar]
- 41.Petrie JG, Bitenc I. The abduction weight-bearing treatment in Legg-Perthes’ disease. J Bone Joint Surg Br. 1971;53:54–62. [PubMed] [Google Scholar]
- 42.Purvis JM, Dimon JH, 3rd, Meehan PL, Lovell WW. Preliminary experience with the Scottish Rite Hospital abduction orthosis for Legg-Perthes disease. Clin Orthop Relat Res. 1980;150:49–53. [PubMed] [Google Scholar]
- 43.Quain S, Catterall A. Hinge abduction of the hip: diagnosis and treatment. J Bone Joint Surg Br. 1986;68:61–64. doi: 10.1302/0301-620X.68B1.3941142. [DOI] [PubMed] [Google Scholar]
- 44.Reinker KA. Early diagnosis and treatment of hinge abduction in Legg-Perthes disease. J Pediatr Orthop. 1996;16:3–9. doi: 10.1097/01241398-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Richards BS, Coleman SS. Subluxation of the femoral head in coxa plana. J Bone Joint Surg Am. 1987;69:1312–1318. [PubMed] [Google Scholar]
- 46.Salter RB. The present status of surgical treatment for Legg-Perthes disease. J Bone Joint Surg Am. 1984;66:961–966. doi: 10.2106/00004623-198466060-00021. [DOI] [PubMed] [Google Scholar]
- 47.Salter RB, Thompson GH. Legg-Calvé-Perthes disease: the prognostic significance of the subchondral fracture and a two-group classification of the femoral head involvement. J Bone Joint Surg Am. 1984;66:479–489. [PubMed] [Google Scholar]
- 48.Singh S, Hee HT, Low YP. Significance of the lateral epiphysis of the acetabulum to hip joint stability. J Pediatr Orthop. 2000;20:344–348. doi: 10.1097/00004694-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Sponseller PD, Desai SS, Millis MB. Comparison of femoral and innominate osteotomies for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1988;70:1131–1139. [PubMed] [Google Scholar]
- 50.Staheli LT, Chew DE. Slotted acetabular augmentation in childhood and adolescence. J Pediatr Orthop. 1992;12:569–580. [PubMed] [Google Scholar]
- 51.Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981;63:1095–1108. [PubMed] [Google Scholar]
- 52.Sundt H. Further investigation respecting malum coxae Calvé-Legg-Perthes’ disease with special regard to the prognosis and treatment. Acta Chir Scand. 1949;suppl 148:1–101.
- 53.Tachdjian MO. Tachdjian Pediatric Orthopedics. Philadelphia, PA: WB Saunders; 1990. [Google Scholar]
- 54.Theron J. Angiography in Legg-Calvé-Perthes disease. Radiology. 1980;135:81–92. doi: 10.1148/radiology.135.1.7360984. [DOI] [PubMed] [Google Scholar]
- 55.Thompson GH, Salter RB. Legg-Calvé-Perthes disease: current concepts and controversies. Orthop Clin North Am. 1987;18:617–635. [PubMed] [Google Scholar]
- 56.van der Geest IC, Kooijman MA, Spruit M, Anderson PG, De Smet PM. Shelf acetabuloplasty for Perthes’ disease: 12-year follow-up. Acta Orthop Belg. 2001;67:126–131. [PubMed] [Google Scholar]
- 57.van der Haven I, Kooijman MA, Havinga ME, van der Geest IC, Jacobs W, Anderson PG. Teardrop-femoral head distance after shelf acetabuloplasty for Perthes’ disease. Acta Orthop Belg. 2003;69:157–161. [PubMed] [Google Scholar]
- 58.Vukasinovic Z, Slavkovic S, Milickovic S, Siqeca A. Combined Salter innominate osteotomy with femoral shortening versus other methods of treatment for Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2000;9:28–33. doi: 10.1097/01202412-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Waldenstrom H. Der obere Tuberkulose collumnerd. Z Orthop Chir. 1909;24:487. [Google Scholar]
- 60.Wiberg G. Shelf operation in congenital dysplasia of the acetabulum and in subluxation and dislocation of the hip. J Bone Joint Surg Am. 1953;35:65–80. [PubMed] [Google Scholar]
- 61.Willett K, Hudson I, Catterall A. Lateral shelf acetabuloplasty: an operation for older children with Perthes’ disease. J Pediatr Orthop. 1992;12:563–568. [PubMed] [Google Scholar]
- 62.Wynne-Davies R, Gormley J. The aetiology of Perthes’ disease: genetic, epidemiological and growth factors in 310 Edinburgh and Glasgow patients. J Bone Joint Surg Br. 1978;60:6–14. doi: 10.1302/0301-620X.60B1.564352. [DOI] [PubMed] [Google Scholar]
- 63.Yoo WJ, Choi IH, Cho TJ, Chung CY, Shin YW, Shin SJ. Shelf acetabuloplasty for children with Perthes’ disease and reducible subluxation of the hip: prognostic factors related to hip remodelling. J Bone Joint Surg Br. 2009;91:1383–1387. doi: 10.2106/JBJS.H.00995. [DOI] [PubMed] [Google Scholar]
- 64.Yrjonen T. Prognosis in Perthes’ disease after noncontainment treatment: 106 hips followed for 28–47 years. Acta Orthop Scand. 1992;63:523–526. doi: 10.3109/17453679209154728. [DOI] [PubMed] [Google Scholar]
- 65.Zaza S, Wright-De Aguero LK, Briss PA, Truman BI, Hopkins DP, Hennessy MH, Sosin DM, Anderson L, Carande-Kulis VG, Teutsch SM, Pappaioanou M. Data collection instrument and procedure for systematic reviews in the Guide to Community Preventive Services. Task Force on Community Preventive Services. Am J Prev Med. 2000;18:44–74. doi: 10.1016/S0749-3797(99)00122-1. [DOI] [PubMed] [Google Scholar]