Abstract
Background
Scaffold devices are used to augment rotator cuff repairs in humans. While the strength of a novel poly-L-lactic acid-reinforced (human) fascia patch has been documented, it is unclear whether such patches will enhance the strength or likelihood of healing of rotator cuff repairs.
Questions/purposes
In a canine shoulder model, we asked: Do tendon repairs augmented with a reinforced fascia patch have (1) increased biomechanical properties at Time 0 and (2) less tendon retraction and increased cross-sectional area and biomechanical properties after 12 weeks of healing compared to repairs without augmentation? (3) Do the biomechanical properties of tendon repairs reach normal values by 12 weeks of healing? And (4) is the host response associated with use of the reinforced fascia patch biocompatible?
Methods
Eleven dogs underwent bilateral shoulder surgery with partial release and acute repair of the infraspinatus tendon, one shoulder with augmentation and one without augmentation. Repair retraction, cross-sectional area, biomechanical properties, and biocompatibility were assessed at 12 weeks.
Results
At Time 0, the mean ± SD ultimate load of augmented repairs was 296 ± 130 N (46% ± 25%) more than nonaugmented repairs, with no difference in stiffness between groups. At 12 weeks, the ultimate load of augmented repairs averaged 192 ± 213 N (15% ± 16%) less than nonaugmented repairs, with no difference in stiffness between groups. At the tendon repair site at 12 weeks, the fascia patch showed a biocompatible host tissue response.
Conclusions
The biomechanical properties of repairs augmented with a reinforced fascia patch demonstrated greater ultimate load at Time 0 than nonaugmented repairs but remained essentially unchanged after 12 weeks of healing, despite improvements in the ultimate load of nonaugmented controls in the same time frame.
Clinical Relevance
Together with our previous work, these findings support the possibility that reinforced fascia patches would incorporate and provide (at least early) mechanical augmentation to rotator cuff repair in human patients.
Introduction
Rotator cuff tears affect 40% or more of those older than 60 years and are thought to be a common cause of debilitating pain, reduced shoulder function, and weakness. In the United States, 30,000 to 75,000 rotator cuff repairs are performed annually [36, 37]. Although surgical treatment and rehabilitation strategies for rotator cuff repair continue to evolve, repair failure rates of 20% to 70% for medium to large tears remain a major clinical challenge [7–9, 11, 20, 21, 33, 35, 40]. Hence, strategies to mechanically augment the repair and biologically enhance its healing potential are needed [1, 29].
Currently, scaffolds derived from extracellular matrix (ECM), poly(urethane urea), and poly-L-lactic acid (PLLA) are commercially available and cleared by the FDA to augment rotator cuff repair in humans [13]. The rationale for using a scaffold may include mechanical augmentation by off-loading the repair and/or biologic augmentation by improving the rate and quality of healing. The material structural and biologic properties of the scaffold and how the scaffold is used are likely to define its mechanism of action and efficacy. Several reports have reported reduced pain and improvement in activities of daily living, satisfaction, and strength in patients with rotator cuff repair receiving scaffold augmentation compared to their preoperative condition [4, 10, 18, 19, 24, 30, 38], but only one [5] found a better healing rate (by MR arthrogram) in patients with rotator cuff repair receiving scaffold augmentation compared to a nonaugmented repair control group. Numerous questions related to the indication, surgical application, safety, mechanism of action, and efficacy of scaffold devices remain to be clarified or addressed.
Unique to scaffolds derived from ECM, only fascia lata has material structural and biochemical properties similar to those of tendon [15, 16]. However, native fascia lata has poor suture retention properties (~ 10 N) [3], which limits its usefulness as a tendonlike scaffold for rotator cuff repair augmentation. We recently reinforced fascia ECM with PLLA polymer braids to engineer the suture retention properties of fascia to meet the needs of musculoskeletal applications [3]. We found the reinforced fascia patch has and maintains its suture retention properties on the order of human rotator cuff tendon before and after implantation in a rat subcutaneous model. Further, we found augmentation with a reinforced fascia patch decreased cyclic gap formation compared to nonaugmented repairs in a human cadaver model [23]. These studies demonstrate the potential for a reinforced fascia patch to provide mechanical augmentation, minimize tendon retraction, and possibly reduce the incidence of rotator cuff repair failure; however, the biocompatibility and biomechanical utility of this patch in the context of rotator cuff repair healing remain to be investigated.
Therefore, we evaluated augmentation of acute rotator cuff tendon repair with a reinforced fascia patch in a canine model. We asked the following questions: Do augmented repairs have (1) increased biomechanical properties at Time 0 and (2) less tendon retraction and increased cross-sectional area and biomechanical properties after 12 weeks of healing compared to repairs without augmentation? (3) Do the biomechanical properties of tendon repairs reach normal values by 12 weeks of healing? And (4) is the host response associated with use of the reinforced fascia patch biocompatible?
Materials and Methods
Eleven male, mongrel dogs (age, 9–13 months; weight, 23–28 kg) underwent bilateral shoulder surgery. Both shoulders received partial release of the superior 8 to 9 mm of the infraspinatus tendon, which is approximately 2/3 of the tendon width [17]. One shoulder underwent tendon release and repair only, and the contralateral shoulder was subjected to release and repair followed by augmentation with a reinforced (human) fascia patch. Tendon retraction, cross-sectional area, stiffness, ultimate load, and biocompatibility of the repair site were evaluated at 12 weeks after surgery. In addition, seven pairs of canine cadaver shoulders underwent infraspinatus injury and repair with and without augmentation and served as Time 0 biomechanical controls. Historical biomechanical data from eight unpaired, canine cadaver shoulders were referenced as normal controls [17]. The study received prior approval of our Institutional Animal Care and Use Committee.
This study was powered primarily to detect differences in mechanical properties between nonaugmented and augmented repairs at Time 0 and after 12 weeks of healing. We chose to detect a mean difference of 165 N in ultimate load based on the rationale that a 25% change in the properties of nonaugmented tendon repairs at Time 0 could be clinically important [17]. Previously, the mean ± SD ultimate load of nonaugmented tendon repairs at Time 0 was 668 ± 146 N (n = 8) [17]. A sample size of 11 allowed us to detect an effect size of 1.1 (165/146 N) with α = 0.05 and power = 0.9.
We prepared reinforced fascia patches by stitching lyophilized human fascia lata from the iliotibial tract of three human donors aged 18 to 55 years (Musculoskeletal Transplant Foundation, Edison, NJ, USA), with custom 100% PLLA fiber as described [3]. However, given the size constraints of the canine shoulder, we scaled down the patches to 18 × 34 mm, from our previously described 50 × 50 mm [3, 23]. The use of smaller and rectangular-shaped patches required the stitch pattern to be modified as well. The Time 0 failure load of the patches used in the canine study was assessed by fixation of the patch to a wooden block using four simple FiberWire® sutures (Arthrex, Inc, Naples, FL, USA) on its lateral (two), superior (one), and inferior sides (one) and pulling its medial end to failure using three simple FiberWire® sutures. The Time 0 failure load of patches designed as in this canine study averaged 153 ± 27 N (n = 6).
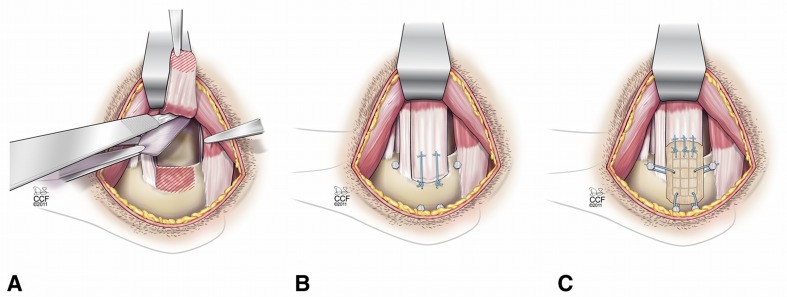
Surgical methods were as described in our previous study with this animal model [17]. Briefly, dogs were anesthetized with intravenous sodium methohexital (10 mg/kg), intubated, and maintained on isoflurane in oxygen (3%). The infraspinatus tendon was approached and the superior 2/3 was detached from its insertion. A portion of the joint capsule was excised to model an intraarticular injury (Fig. 1A). The tendon was repaired back to its insertion with two transosseous Number 0 FiberWire® sutures in a modified Mason-Allen configuration (Fig. 1B). For augmentation, a fascia patch was laid over the tendon repair and attached to the tendon medially using three Number 0 FiberWire® Mason-Allen sutures and tensioned across the tendon repair with four Number 0 FiberWire® simple sutures (Fig. 1C). The wounds were irrigated with normal saline and closed in layers.
Fig. 1A–C.
Diagrams illustrate bilateral rotator cuff injury and repair with and without patch augmentation in a canine model. (A) The superior 2/3 of the infraspinatus tendon was sharply detached from its insertion at the greater tuberosity, and a 1.5- × 2-cm portion of the underlying joint capsule was excised. (B) The infraspinatus tendon was immediately repaired back to its insertion using two transosseous modified Mason-Allen sutures. Bone anchors for patch fixation were included in the nonaugmented shoulders. (C) For augmentation, a fascia patch was laid over the tendon repair and attached to the tendon medially with use of three Number 0 FiberWire® Mason-Allen sutures. The device was tensioned by advancing the lateral edge approximately 2 mm laterally for osseous attachment to two customized stainless steel machine screw anchors (2.2 mm in diameter) using Number 0 FiberWire® simple sutures. Finally, the patch was tensioned superiorly and inferiorly to osseous anchors because of the absence of a soft tissue rotator cuff in this animal model. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2009–2011. All rights reserved.
Postoperatively, dogs were housed individually in 2.13- × 0.91-m cages with restricted ceilings. Dogs were given subcutaneous injections of buprenorphine (0.02 mg/kg body weight) twice daily for 3 to 5 days postoperatively for analgesia and 500 mg cephalexin orally twice daily for 7 days as a prophylactic antibiotic.
At 12 weeks, we euthanized the dogs using a lethal injection of barbiturate (1 mL/4.5 kg; Beuthanasia-D®; WA Butler, Dublin, OH, USA). We harvested the tendon repair construct, including the infraspinatus muscle and 20 cm of the proximal humerus. It was not possible to reproducibly separate the intact portion of the tendon from the repaired portion, so the entire tendon (and reinforced fascia patch in augmented samples) constituted the repair construct. Samples were stored in saline-soaked gauze at −20° C up to 8 months until tested. This delay in testing allowed all samples to be collected and biomechanically tested in one analysis. Since both the experimental and control samples from any given dog were frozen for an identical amount of time, any effects of frozen storage, though expected to be minor [25, 27, 28, 34, 39], would have affected each group similarly.
To assess tendon retraction distance at 12 weeks, we used visual inspection and palpation to approximate the position of the tendon stump within the fibrous tissue at the repair site [17]. We used calipers to measure the distance between the retracted stump and osseous repair site. The somewhat subjective nature of identifying the position of the retracted tendon stump led us to report the tendon retraction data categorically in four groups: (1) 5 mm or less; (2) 5 to 10 mm; (3) 10 to 15 mm; or (4) 15 mm or more.
At mechanical testing, the humeri were potted and the muscle belly was gripped in a custom cryoclamp as described previously [17]. We estimated the cross-sectional area of the repair construct from caliper measurements of the width and thickness. Suture markers of 4-0 braided silk were stitched through the tendon and overlying soft tissue on the repair construct 20 mm medial to the insertion site, which was medial to the reinforced fascia patch attachment to tendon. A 1.6-mm tantalum bead was placed as a bone marker at the insertion site. Samples were tested in tension along their anatomic direction of pull. Testing was conducted in air at room temperature, and samples were kept moist by spraying with saline solution. Samples underwent 100 prefailure loading cycles from 5 to 100 N and were immediately tested to failure at 30 mm/minute (MTS Systems Corp, Eden Prairie, MN, USA). Load was recorded with a 5000-N load cell (Honeywell Sensotec, Columbus, OH, USA). A custom optical system, synchronized with the load data and sampling at 30 Hz, was used to track the optical markers and calculate the local displacements across the tendon-bone repair site using custom texture correlation software [6]. Repair stiffness was defined from failure testing as the slope of the load-displacement curve from 50 to 400 N. Ultimate load was defined as the maximum load the sample reached.
After mechanical testing, we processed the tendon-bone repair sites of three augmented repairs, which included the reinforced fascia patch, for histologic evaluation of biocompatibility. Samples were fixed in 10% neutral buffered formalin for 3 to 7 days, decalcified in 5% trichloroacetic acid solution for 1 to 2 weeks, processed routinely, and embedded in paraffin. From each specimen, 6-μm-thick sections were cut and stained with hematoxylin and eosin. One section from within the repaired region was visually inspected by a board certified pathologist (CDT) so that qualitative comments regarding fascia patch morphology, overall cellular infiltration, inflammatory cells, and neovascularization could be made.
We compared stiffness, ultimate load, and cross-sectional area between paired shoulders using a Wilcoxon signed-rank test and tendon retraction distance using a sign test. Within the nonaugmented and augmented repair groups, we compared stiffness and ultimate load with the respective Time 0 and normal controls using a Kruskal-Wallis one-way ANOVA on ranks, with pairwise Wilcoxon rank-sum post tests for significance. Since at least one data set failed the test for normality, nonparametric tests were used for all statistical comparisons. SigmaPlot® 10.0 (Systat Software, Inc, Chicago, IL, USA) was used for statistical analysis.
Results
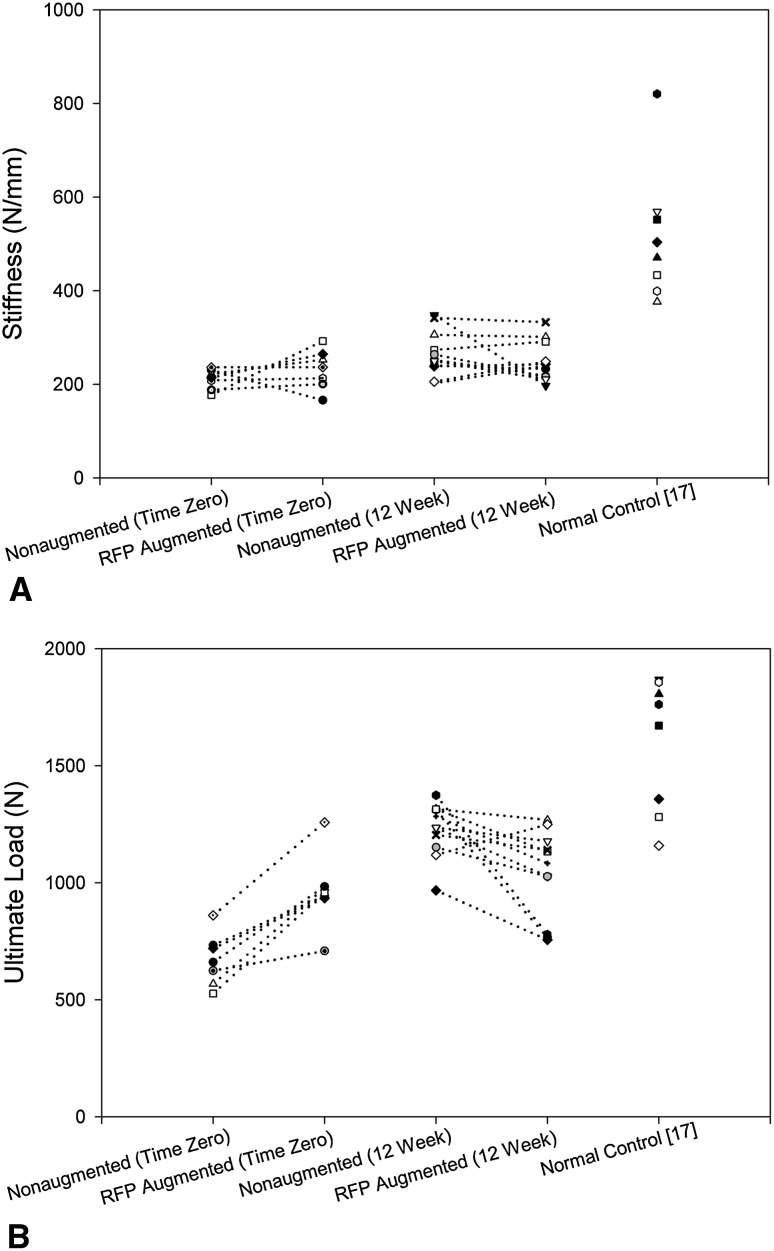
When the group means for the biomechanical properties (stiffness, ultimate load) (Table 1) were paired and analyzed, at Time 0, stiffness was similar (p = 0.219) between nonaugmented and augmented repairs (Fig. 2A). However, the ultimate load of augmented repairs averaged 296 ± 130 N (46% ± 25%) more (p = 0.016) than that of nonaugmented repairs (Fig. 2B). The predominant mode of failure for both nonaugmented and augmented repairs was suture cut-through of the tendon (Table 2).
Table 1.
Cross-sectional area and biomechanical properties of repair constructs
| Variable | Normal controls* (n = 8) | Nonaugmented repairs | Augmented repairs | ||
|---|---|---|---|---|---|
| Time 0 (n = 7) | 12 weeks (n = 11) | Time 0 (n = 7) | 12 weeks (n = 11) | ||
| Cross-sectional area (mm2) | 32 ± 2 | NA | 70 ± 19† | NA | 94 ± 10†,§ |
| Stiffness (N/mm) | 515 ± 141 | 211 ± 22† | 265 ± 49†,‡ | 232 ± 43† | 248 ± 43† |
| Ultimate load (N) | 1595 ± 285 | 670 ± 112† | 1228 ± 115†,‡ | 966 ± 160†,‡ | 1037 ± 189†,‖ |
Values are expressed as mean ± SD; * previously published data [17]; †compared with normal controls, the difference was significant (p < 0.001); ‡compared with Time 0 nonaugmented repairs, the difference was significant (p ≤ 0.016); §compared with 12-week nonaugmented repairs, the difference was significant (p = 0.019); ‖compared with 12-week nonaugmented repairs, the difference was significant (p = 0.01); NA = not applicable (cross-sectional areas not obtained for the Time 0 repairs).
Fig. 2A–B.
Graphs show the biomechanical properties of the paired nonaugmented and reinforced fascia patch (RFP) augmented repairs at Time 0 and 12 weeks. (A) There was no difference in stiffness between nonaugmented and augmented repairs at Time 0 (p = 0.219) or 12 weeks (p = 0.365). (B) At Time 0, the ultimate load of augmented repairs averaged 296 ± 130 N (46% ± 25%) more (p = 0.016) than that of nonaugmented repairs. At 12 weeks, the ultimate load of augmented repairs averaged 192 ± 213 N (15% ± 16%) less (p = 0.01) than that of nonaugmented repairs.
Table 2.
Ex vivo failure modes for mechanical testing of repair constructs
| Failure mode | Normal controls* (n = 8) | Time 0 | 12 weeks | ||
|---|---|---|---|---|---|
| Nonaugmented (n = 7) | Augmented (n = 7) | Nonaugmented (n = 11) | Augmented (n = 11) | ||
| Soft tissue† | 0 | 5 | 6 | 8 | 9 |
| Bone avulsion of intact tendon strut | 0 | 2 | 1 | 1 | 1 |
| Bone avulsion of humeral head‡ | 8 | 0 | 0 | 2 | 1 |
* Previously published data [17]; †failure by suture pulling through tendon for Time 0 samples; failure by rupture of the repair and/or intact portion of the tendon, at or away from the bone, for 12-week samples; ‡humerus fractured before the repair failing; failure load reported is therefore underestimated.
Tendon retraction was similar (p = 0.72) between nonaugmented and augmented repairs. During the 12-week healing period, all repairs retracted to some extent (Table 3). Four of 11 repairs in each group retracted less than 5 mm during the 12 weeks of healing, whereas three of 11 nonaugmented repairs and four of 11 augmented repairs retracted greater than 15 mm during the same time period. After 12 weeks of healing, the cross-sectional area of augmented repairs averaged 24 ± 24 mm2 (46% ± 50%) more (p = 0.019) than paired nonaugmented repairs (Table 1). At 12 weeks, we found no difference (p = 0.365) in construct stiffness between augmented and nonaugmented repairs (Fig. 2A). However, the ultimate load of augmented repairs averaged 192 ± 213 N (15% ± 16%) less than (p = 0.01) paired nonaugmented repairs (Fig. 2B). The predominant failure mode of both groups started in soft tissue by rupture of the repair and/or intact portion of the tendon, at or away from the bone (Table 2).
Table 3.
In vivo tendon retraction at 12 weeks
| Tendon retraction distance (mm) | Number of tendons | |
|---|---|---|
| Nonaugmented repairs (n = 11) | Augmented repairs (n = 11) | |
| ≤ 5 | 4 | 4 |
| 5–10 | 3 | 1 |
| 10–15 | 1 | 2 |
| ≥ 15 | 3 | 4 |
The stiffness (p = 0.011) and ultimate load (p < 0.001) of nonaugmented repairs increased between Time 0 and 12 weeks of healing, but both parameters remained less (p < 0.02) than normal controls at 12 weeks (Table 1). Neither the stiffness (p = 0.65) nor the ultimate load (p = 0.24) of augmented repairs changed between Time 0 and 12 weeks of healing, and both parameters remained less (p < 0.001) than normal controls at 12 weeks (Table 1).
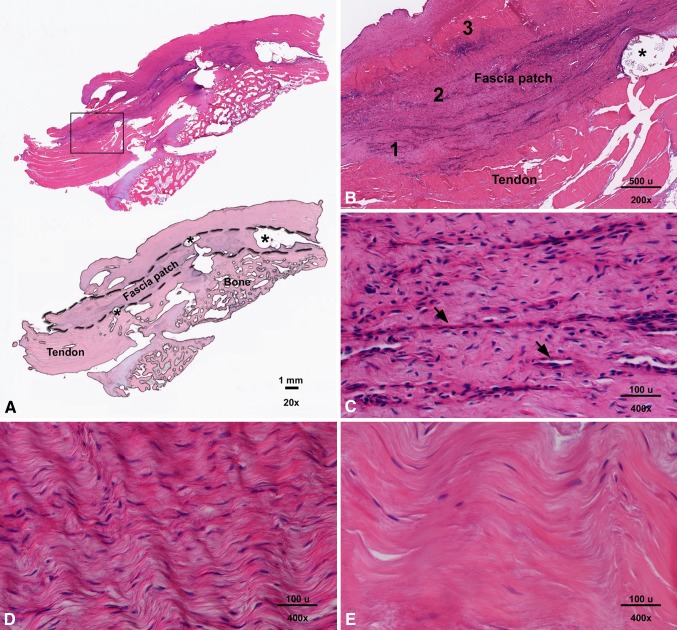
Visual inspection suggested the fascia patch was appreciably resorbed at its medial suture connection to tendon in three of 11 samples. At the tendon repair sites of the three samples examined histologically, the fascia matrix was discernable and intimately associated with surrounding host tissues (Fig. 3A). Neither the fascia matrix nor the PLLA reinforcing fiber appeared to be resorbed in this region (Fig. 3B). The interface between the reinforced fascia patch and host tissue showed cellularized fibrous tissue with mild neovascularization (Fig. 3C). Grafts exhibited variable regions of cellularity and most of the cellular infiltrates were accounted for by spindle-shaped cells infiltrating within and between the fascicles of the native fascia architecture (high cellular density: Fig. 3D; low cellular density: Fig. 3E). There was no evidence of an inflammatory cell infiltrate, except in regions immediately adjacent to the PLLA reinforcing fiber where rare giant cells were noted.
Fig. 3A–E.
Images show the reinforced fascia augmented repair at 12 weeks. (A) In a representative longitudinal section of an augmented repair site (upper image), the reinforced fascia patch overlying the bone and tendon is discernable and intimately associated with surrounding host tissues (stain, hematoxylin and eosin; original magnification, ×20). In the lower image, a masked representation of the upper image outlines the fascia patch, tendon, and bone at the tendon repair site. The asterisk indicates a PLLA reinforcement fiber. (B) An inset box from (A) demonstrates neither the fascia matrix nor the PLLA reinforcing fiber appear to be resorbed in this region (stain, hematoxylin and eosin; original magnification, ×200). (C) In Region 1 from (B), the interface between the reinforced fascia patch and host tissue shows cellularized fibrous tissue with mild neovascularization (arrows; stain, hematoxylin and eosin; original magnification, ×400); (D) Region 2 from (B) demonstrates a region of high cellular density within the fascia patch (stain, hematoxylin and eosin; original magnification, ×400). (E) Region 3 from (B) demonstrates a region of low cellular density within the fascia patch (stain, hematoxylin and eosin; original magnification, ×400). Most of the cellular infiltrates within the patch were accounted for by spindle-shaped cells. There was no evidence of an inflammatory cell infiltrate, except in regions immediately adjacent to the PLLA reinforcing fiber where rare giant cells were noted.
Discussion
Although numerous patch devices derived from ECM or synthetic polymers are available to augment rotator cuff repair in humans [13], the efficacy of any device in reducing the incidence of retear compared to nonaugmented primary repair has not yet been demonstrated. Our group has developed a polymer-reinforced fascia ECM patch and demonstrated its biomechanical properties and utility, as well as initial biocompatibility, through a series of bench, cadaveric, and small-animal investigations [3, 23]. The current study builds on our previous work by investigating the biomechanical utility and biocompatibility of this patch in the context of large-animal rotator cuff repair healing.
Limitations of our canine shoulder model include the following. First, the animals were relatively young and the injury model was acute. While these healing conditions were consistent for both repair groups, they likely do not closely reflect those of chronic rotator cuff repairs in an older human population. Second, we used a bilateral injury model. While a bilateral injury model allows each animal to be its own control, it likely provides an overly rigorous test of the repair construct as postoperative rehabilitation cannot be controlled and the animal cannot favor either limb during the healing process. Third, there were anatomic and size dissimilarities compared to the human rotator cuff condition [14]. As a consequence, the fascia patch used in this canine study was scaled down from its well-characterized and optimum design for human application [3, 23]. Fourth, bone anchors were needed to secure the patch superiorly and inferiorly over the repair because dogs lack the soft tissue anatomy of a human rotator cuff. In addition, patch fixation with seven FiberWire® sutures over approximately 1 × 3 cm was necessary but exceeds the typical suture density for securing an augmentation patch onto the human rotator cuff tendon. In particular, the three medial sutures used to attach the patch to the tendon were densely spaced across a 1-cm tendon width and could introduce mechanical irritation, wear, and/or local inflammation. Furthermore, their Mason-Allen configuration could act to strangulate the tendon and introduce necrosis [26], which could reduce the capacity of the tendon to integrate with the patch or heal to the bone. Finally, we did not perform a thorough histologic survey of the tendon repair and patch in a large number of samples or in samples devoted exclusively to histology.
We found repair augmentation increased the ultimate load but not the stiffness of the canine repairs at Time 0. Similarly, repair augmentation with a PLLA patch (X-Repair®; Synthasome Inc, San Diego, CA, USA) increased the ultimate load but not the repair stiffness in canine [17] and human [22] cadaveric models. The potential for patch augmentation to increase construct stiffness may be mitigated to a large extent by setting of the repair sutures during the early loading of the repair. The 46% increased ultimate load achieved with device augmentation at Time 0 is likely the result of having five points of tendon fixation (two sutures between tendon and bone and three sutures between patch and tendon) rather than two points.
Repair augmentation did not reduce the amount of tendon retraction at the repair site or increase the biomechanical properties of the repair at 12 weeks compared to nonaugmented controls. These findings are similar to a previous study that showed rotator cuff repairs augmented with a small intestinal submucosa (SIS) patch (Restore®; DePuy Orthopaedics, Inc, Warsaw, IN, USA) had no increase in failure load compared to nonaugmented repairs at 12 weeks of healing in the ovine model [32]. However, our findings are in contrast to previous studies that showed rotator cuff repair augmentation with a PLLA patch (X-Repair®) reduced tendon retraction and improved the stiffness (26%) and ultimate load (35%) of the repair at 12 weeks in the canine model [17]. In addition, infraspinatus tendon repairs augmented with a polycarbonate poly(urethane) urea (PCPU) patch (RCR Patch™; Biomerix Corp, Fremont, CA, USA) showed an increase in failure load compared to nonaugmented repairs at 12 weeks in the ovine model [31].
One explanation for improved biomechanical outcomes at 12 weeks with the use of synthetic patches but not with biologics, at least in these animal models, could be related to patch resorption. Patch fixation using nonresorbable suture may induce local inflammation that results in accelerated enzymatic resorption, particularly at the medial end of the patch where the suture density is high in the animal model. This mechanism is supported by our observation that the reinforced fascia patch in our study was appreciably resorbed at its medial suture connection to the tendon in three of 11 samples. Medial patch resorption could abrogate the initially strong Time 0 attachment of the patch to the tendon, leaving the augmented repairs to perform much like the nonaugmented repairs after 12 weeks of healing. Although three FiberWire® sutures were also used for medial fixation of the PLLA patch previously [17], PLLA would be less susceptible than a biologic patch to enzymatic resorption resulting from local inflammation induced by suture fixation. Hence, the Time 0 biomechanical benefit of augmentation with a synthetic patch could be maintained over the 12-week study period by way of the same all-mechanical mechanism as at Time 0, and any additional tendon healing or fibrous tissue formation would then serve to increase the overall construct properties. Given the possibility that medial patch resorption is exacerbated by use of a animal model that requires excessively dense suture fixation, the extent to which our findings reflect those expected with use of the reinforced fascia patch in the context of human rotator cuff repair is uncertain at this time. However, it is certainly possible that biologic and synthetic patches could act by way of different mechanisms and thus serve different roles in the context of human rotator cuff repair augmentation.
We found repairs augmented with reinforced fascia had lower stiffness (48%) and ultimate load (65%) than normal tendon at 12 weeks. Similarly, rotator cuff repairs augmented with an SIS patch had lower stiffness (40%) and ultimate load (24%) than normal tendon at 12 weeks in an ovine model [32], rotator cuff repairs augmented with a PLLA patch had lower stiffness (47%) and ultimate load (77%) than normal tendon at 12 weeks in a canine model [17], and rotator cuff repairs augmented with a PCPU patch had lower stiffness (41%) and ultimate load (38%) than normal tendon at 12 weeks in an ovine model [31]. The extent to which the biomechanical properties of these repair constructs would approach normal tendon after a period of time sufficient for either mature engraftment and/or remodeling (in the case of a biologic patch) or complete hydrolytic resorption (in the case of a biodegradable synthetic patch) is unknown and should be addressed with longer-term studies.
The reinforced fascia patch demonstrated a biocompatible histologic response at the tendon repair site, as evidenced by a noninflammatory (fibroblastlike) cell infiltrate, intimate association with host tissues, and minimal resorption at 12 weeks. Similarly, noncrosslinked biologic scaffolds derived from dermis [2] and SIS [12, 32] and synthetic scaffolds derived from PLLA [17] have shown a biocompatible host response at 12 weeks in large-animal rotator cuff models. A thorough histologic survey of these augmented repair constructs from the bone to the medial tendon attachment of the patch has not been previously performed and would allow investigation of region-specific cellular infiltration and vascularization, patch integration, and patch resorption.
In summary, the biomechanical properties of rotator cuff repairs augmented with a reinforced fascia patch in the canine model demonstrated greater ultimate load at Time 0 than nonaugmented repairs but remained essentially unchanged after 12 weeks of healing, despite improvements in the ultimate load of the nonaugmented controls in the same time frame. At the tendon repair site, the fascia patch showed a biocompatible host tissue response. Together with our previous body of work demonstrating the biomechanical utility of the reinforced fascia patch in a variety of in vitro and in vivo model systems [3, 23], our observations support the possibility that reinforced fascia patches would incorporate and provide (at least early) mechanical augmentation to rotator cuff repair in human patients. Future studies investigating the use of the reinforced (human) fascia patch for augmentation of rotator cuff repair in human patients will help to more conclusively define the efficacy of this patch for improving repair outcomes in humans.
Acknowledgments
We thank Amit Aurora PhD for adaptation and production of the reinforced fascia patches used in this study; Esteban Walker PhD for consultation on the statistical analysis; and Ryan Milks MS for mechanical testing assistance. We also thank the Musculoskeletal Transplant Foundation for donating the human fascia lata used to make the reinforced fascia patches for this study.
Footnotes
One or more of the authors (KAD) have received funding from a research grant from the Musculoskeletal Transplant Foundation (Edison, NJ, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Cleveland Clinic Foundation, Cleveland, OH, USA.
References
- 1.Accousti KJ, Flatow EL. Technical pearls on how to maximize healing of the rotator cuff. Instr Course Lect. 2007;56:3–12. [PubMed] [Google Scholar]
- 2.Adams JE, Zobitz ME, Reach JS, Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Aurora A, Mesiha M, Tan CD, Walker E, Sahoo S, Iannotti JP, McCarron JA, Derwin KA. Mechanical characterization and biocompatibility of a novel reinforced fascia patch for rotator cuff repair. J Biomed Mater Res A. 2011;99:221–230. doi: 10.1002/jbm.a.33179. [DOI] [PubMed] [Google Scholar]
- 4.Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17(1 suppl):35S–39S. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28:8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. A noncontact, nondestructive method for quantifying intratissue deformations and strains. J Biomech Eng. 2002;124:253–258. doi: 10.1115/1.1449917. [DOI] [PubMed] [Google Scholar]
- 7.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15:290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsson HC, Norlin R, Johansson K, Adolfsson LE. The influence of age, delay of repair, and tendon involvement in acute rotator cuff tears. Acta Orthop. 2011;82:187–192. doi: 10.3109/17453674.2011.566144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 10.Burkhead WZ, Schiffern SC, Krishnan SG. Use of GraftJacket as an augmentation for massive rotator cuff tears. Semin Arthroplasty. 2007;18:11–18. doi: 10.1053/j.sart.2006.11.017. [DOI] [Google Scholar]
- 11.Cho NS, Yi JW, Lee BG, Rhee YG. Retear patterns after arthroscopic rotator cuff repair single-row versus suture bridge technique. Am J Sports Med. 2010;38:664–671. doi: 10.1177/0363546509350081. [DOI] [PubMed] [Google Scholar]
- 12.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa: histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 13.Derwin KA, Badylak SF, Steinmann SP, Iannotti JP. Extracellular matrix scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2010;19:467–476. doi: 10.1016/j.jse.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16:21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derwin KA, Baker AR, Spragg RK, Leigh DR, Farhat W, Iannotti JP. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. J Biomed Mater Res A. 2008;84:500–507. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 16.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair: biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 17.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91:1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Intl J Shoulder Surg. 2007;1:7–15. doi: 10.4103/0973-6042.30673. [DOI] [Google Scholar]
- 19.Encalada-Diaz I, Cole BJ, MacGillivray JD, Ruiz-Suarez M, Kercher JS, Friel NA, Valero-Gonzalez F. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Shoulder Elbow Surg. 2011;20:788–794. doi: 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keener JD, Wei AS, Kim HM, Paxton ES, Teefey SA, Galatz LM, Yamaguchi K. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92:590–598. doi: 10.2106/JBJS.I.00267. [DOI] [PubMed] [Google Scholar]
- 21.Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27:453–462. doi: 10.1016/j.arthro.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 22.McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:688–696. doi: 10.1016/j.jse.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 23.McCarron JA, Milks RA, Mesiha M, Aurora A, Walker E, Iannotti JP, Derwin KA. Reinforced fascia patch limits cyclic gapping of rotator cuff repairs in a human cadaver model. J Shoulder Elbow Surg. 2012 February 21 [Epub ahead of print]. [DOI] [PubMed]
- 24.Metcalf MH, Savoie FH, 3rd, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper Tech Orthop. 2002;12:204–208. doi: 10.1053/otor.2002.36298. [DOI] [Google Scholar]
- 25.Moon DK, Woo SL, Takakura Y, Gabriel MT, Abramowitch SD. The effects of refreezing on the viscoelastic and tensile properties of ligaments. J Biomech. 2006;39:1153–1157. doi: 10.1016/j.jbiomech.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson GP, Breur GJ, Van SD, Yao JQ, Kim J, Blanchard CR. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder Elbow Surg. 2007;16(5 suppl):S184–S190. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH., 3rd Anterior cruciate ligament allograft transplantation: long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14:348–360. doi: 10.1177/036354658601400502. [DOI] [PubMed] [Google Scholar]
- 28.Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58:1074–1082. [PubMed] [Google Scholar]
- 29.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 30.Rotini R, Marinelli A, Guerra E, Bettelli G, Castagna A, Fini M, Bondioli E, Busacca M. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet Surg. 2011;95(suppl 1):S13–S23. doi: 10.1007/s12306-011-0141-8. [DOI] [PubMed] [Google Scholar]
- 31.Santoni BG, McGilvray KC, Lyons AS, Bansal M, Turner AS, MacGillivray JD, Coleman SH, Puttlitz CM. Biomechanical analysis of an ovine rotator cuff repair via porous patch augmentation in a chronic rupture model. Am J Sports Med. 2010;38:679–686. doi: 10.1177/0363546510366866. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. Am J Sports Med. 2006;34:275–280. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 33.Tashjian RZ, Hollins AM, Kim HM, Teefey SA, Middleton WD, Steger-May K, Galatz LM, Yamaguchi K. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38:2435–2442. doi: 10.1177/0363546510382835. [DOI] [PubMed] [Google Scholar]
- 34.Thomas ED, Gresham RB. Comparative tensile strength study of fresh, frozen, and freeze-dried human fascia lata. Surg Form. 1963;14:442–443. [PubMed] [Google Scholar]
- 35.Toussaint B, Schnaser E, Bosley J, Lefebvre Y, Gobezie R. Early structural and functional outcomes for arthroscopic double-row transosseous-equivalent rotator cuff repair. Am J Sports Med. 2011;39:1217–1225. doi: 10.1177/0363546510397725. [DOI] [PubMed] [Google Scholar]
- 36.Turkelson CM, Zhao G. Musculoskeletal conditions and disorders: occurrence and healthcare use in the United States. Available at: http://www.aaos.org/research/stats/patientstats.asp. Accessed June 11, 2008.
- 37.Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16:181–187. doi: 10.1016/j.jse.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. J Shoulder Elbow Surg. 2010;19:104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Woo SL, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19:399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- 40.Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]