Abstract
Motilitone® (DA-9701) is a new herbal drug that was launched for the treatment of functional dyspepsia in December 2011 in Korea. The heterogeneous symptom pattern and multiple causes of functional dyspepsia have resulted in multiple drug target strategies for its treatment. DA-9701, a compound consisting of a combination of Corydalis Tuber and Pharbitidis Semen, has being developed for treatment of functional dyspepsia. It has multiple mechanisms of action such as fundus relaxation, visceral analgesia, and prokinetic effects. Furthermore, it was found to significantly enhance meal-induced gastric accommodation and increase gastric compliance in dogs. DA-9701 also showed an analgesic effect in rats with colorectal distension induced visceral hypersensitivity and an antinociceptive effect in beagle dogs with gastric distension-induced nociception. The pharmacological effects of DA-9701 also include conventional effects, such as enhanced gastric emptying and gastrointestinal transit. The safety profi le of DA-9701 is also preferable to that of other treatments.
Keywords: DA-9701, Functional dyspepsia, Pharmacology, Gastric accommodation, Visceral hypersensitivity, Prokinetics
INTRODUCTION
DA-9701, a new herbal drug for the treatment of functional dyspepsia (FD), was developed by Dong-A Pharmaceutical in Korea. The drug received New Drug Application (NDA) approval in May 2011 from the KFDA (Korea Food and Drug Administration). It is formulated as a 50% ethanol extract from Corydalis Tuber and Pharbitidis Semen.
FD is a complex of dyspeptic symptoms including upper abdominal pain, heartburn, bloating, and discomfort (Stanghellini et al., 2003). These symptoms are thought to originate from the upper abdomen but their structural and organic causes are not known (Halder and Talley, 2007). The pathophysiology of FD is not simple, and is known to have multiple causes including delayed gastric emptying, impaired gastric accommodation, visceral hypersensitivity, small bowel dysmotility, genetic factors, social stress, and inflammation (Brun and Kuo, 2010). Among them, impaired gastric accommodation, visceral hypersensitivity and delayed gastric emptying are known to be the major pathophysiological causes of FD (Brun and Kuo, 2010). Fig. 1 shows pathophysiology of FD and drug target. Delayed gastric emptying is a traditional therapeutic target of gastroprokinetics (Stanghellini et al., 2004). Visceral hypersensitivity is a common cause of functional gastrointestinal diseases and there is no established treatment for visceral hypersensitivity (Talley et al., 2012). However, off-label antidepressants have been frequently prescribed for visceral pain management (Talley et al., 2012). Impaired gastric accommodation was reported to be related to early satiety and a decrease of weight gain. Previously, there have been no treatments termed fundic relaxants (Tack and Janssen, 2011b).
Fig. 1. Major drug targets and main ideal therapies discussed for correction of the pathophysiological causes of functional dyspepsia [modified from (Moro et al., 2004)].
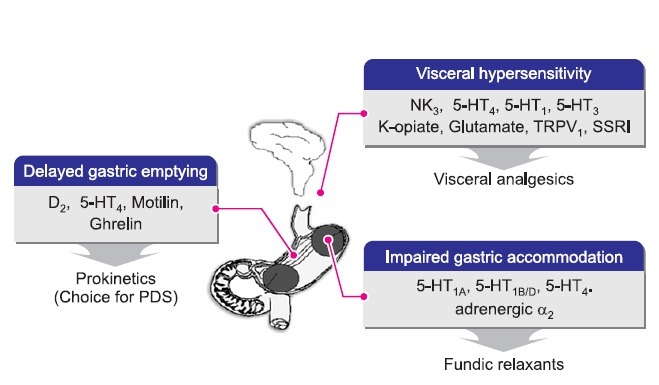
In Rome II criteria (Drossman, 1999), FD was defined as recurrent upper abdominal pain and discomfort for at least 12 weeks during the preceding 1 year. The Rome II classification divided patients having a wide range of dyspepsia symptoms into 4 groups on the basis of the major symptomatic pattern: reflux-like, ulcer-like, dysmotility-like, or nonspecific FD. The criteria excluded patients with predominant heartburn (refl ux-like pattern) from the dyspepsia spectrum (Suzuki et al., 2006). In Rome III criteria, the definition of FD was less restrictive and had a shorter time frame (3 months of symptoms in the previous 6 months). The symptomatic categorization of Rome II was not reliable because of frequent overlap among irritable bowel syndrome (IBS), gastroesophageal reflux disease (GERD), postprandial distress syndrome (PDS), and epigastric pain syndrome (EPS) (Suzuki et al., 2006). Due to this reason, amongst others, the Rome III committee decided to categorize FD into 2 groups for the purpose of therapeutic research and clinicalutility: PDS and EPS (Tack et al., 2006). Major symptoms of FD include postprandial fullness, early satiety, epigastric pain, and epigastric burning. Nausea, vomiting, and heartburn, which were included under FD symptoms under the Rome II criteria, were moved to other categories.
The prevalence of FD was reported to be about 20% in western countries (Talley et al., 1992; Agreus et al., 1995; Piessevaux et al., 2009). In Korea the prevalence of FD was reported to be 13.4% on using the Rome II criteria, with the dysmotility type being dominant (Rhie et al., 2007). On using the Rome III criteria, the prevalence was reported to be 8.1% (EPS, 4.6% and PDS, 6.5%) (Noh et al., 2010). FD significantly decreases patients’ quality of life and increases social cost.
The multiple mechanisms involved in the pathophysiology of FD seem to make it difficult for a new FD drug to reach a satisfactory efficacy level, or even for it to be appropriately evaluated. Further, opinion leaders worldwide have said that an agent that is able to modulate multiple mechanisms will be more promising than an agent highly selective for a single mechanism and that a new drug for FD should target all or many pathophysiological targets (Camilleri et al., 2006). DA- 9701 was developed to have multiple action mechanisms by combining herbs, and finally, Pharbitidis Semen and Corydalis Tuber were selected. The pharmacology concerning the multiple action mechanisms of DA-9701 and some associated safety issues are reviewed here.
CURRENT STATUS OF DRUG DEVELOPMENT FOR TREATMENT OF FD
Currently, major drugs prescribed for treatment of FD are gastrointestinal prokinetics. After withdrawal of cisapride due to its cardiovascular side effects, several prokinetic agents were developed to have lower safety concerns. There were numerous attempts to develop new agent for treatment of functional dyspepsia and their clinical study results are summarized in Table 1. Major end point of clinical trial were symptomatic relief and correction of pathophysiological cause such as delayed gastric emptying, impaired gastric accommodation and visceral hypersensitivity. Some of them succeeded in showing efficacy and launched into the market. They are tegaserod, itopride, mosapride, domperidone, metoclopramide, levosulpiride and so on. However, the need for good efficacy and safety is stillunmet. Besides, Tegaserod was withdrawn from the market due to cardiovascular side effect and D2 antagonists were known to induce hyperprolactinemia. The efficacy of these agents is narrowed to prokinetic effect. Recently acotiamide and STW 5 were launched into market. Acotiamide, a muscarinic antagonist was developed as prokinetics but failed to correct delayed gastric emptying in clinical trial while showing symptomatic relief and lack of dose response (Tack and Janssen, 2011a). Besides, the dose of symptomatic relief and efficacy for increasing accommodation didn’t match (Tack and Janssen, 2011a). STW 5 (Iberogast®) is a phytomedicine consisted of nine herbs. It as a leading phytomedicine has been used for more than 40 years (Rosch et al., 2006) and studied abundantly. Interestingly acotiamide has been known to increase gastric accommodation and STW 5 also has multiple action mechanism including fundus relaxation (Schemann et al., 2006). Moreover, DA-9701 has been known to increase gastric accommodation in vivo (Kim et al., 2012) beyond prokinetic effect and visceral analgesic effect. These new agents and the efficacy on fundus relaxation seem to refl ect increasing importance of gastric accommodation in pathophysiology of FD. Multiple action mechanism including fundus relaxation efficacy may be more suitable for chronic complex disease than single action, which will be verified before long.
Table 1.
Overview of studied prokinetics, fundic relaxants and visceral analgesics in functional dyspepsia
| Drug class | Drug name | Clinical trial results |
|---|---|---|
|
| ||
| Prokinetics | ||
| 5-HT4 receptor | Cisapride | Accelerated gastric emptying of patients with functional dyspepsia (Degryse et al., 1993) |
| agonists | Enhanced perception of gastric distension and the gastric accommodation to a meal in health (Tack et al., 1998a) | |
| Tegaserod | Accelerates gastric emptying and gastrointestinal transit in healthy subject (Degen et al., 2001) | |
| Enhanced gastric accommodation in health and in FD (Tack et al., 2003b) | ||
| No significant effect on gastric motor and sensory function in healthy individuals (Talley et al., 2006) | ||
| Renzapride | Enhances gastric emptying in health and in diabetic gastroparesis (Mackie et al., 1991) | |
| Mosapride | Enhances gastric emptying in health (Kanaizumi et al., 1991) | |
| Phase 2 in FD: no benefit over placebo (Hallerback et al., 2002) | ||
| Dopamine 2 | Itopride | Decreases gastric accommodation in health (Choung et al., 2007) |
| receptor antagonists | Acceleration of gastric emptying: benefit shown by a placebo-controlled trial in diabetic patients (Stevens et al., 2008) | |
| Phase 2 in FD significant benefit (Choung et al., 2007) | ||
| Phase 3 in FD no benefit over placebo (Talley et al., 2008) | ||
| Levosulpiride | Acceleration of gastric emptying: benefit shown by a randomized trial to be similar to cisapride (Mansi et al., 2000) | |
| Domperidone | Acceleration of gastric emptying: benefit shown by meta-analysis of a small number of trials (Veldhuyzen van Zanten et al., 2001) | |
| Metoclopramide | Accelerated gastric emptying in dysmotility-like dyspepsia (Banani et al., 2008) | |
| Accelerated antral emptying in FD (Dumitrascu et al., 1998) | ||
| Metoclopramide significantly improved delayed gastric emptying (Hancock et al., 1974) | ||
| Motilin receptor | Mitemcinal | Enhances gastric emptying in gastroparesis (McCallum and Cynshi, 2007b) |
| agonists | Phase 2 in diabetic gastroparesis: no benefit over placebo; post hoc potential benefit in subgroups (McCallum and Cynshi, 2007a) | |
| Ghrelin receptor agonists | TZP-101 | Enhances gastric emptying in diabetic gastroparesis patients (Ejskjaer et al., 2009) |
| Fundic relaxants | ||
| Nitrates | Nitroglycerine | Enhances gastric accommodation in health and in FD (Gilja et al., 1997) |
| PDE inhibitors | Sildenafil | Enhances gastric accommodation in health and delays gastric emptying in health (Sarnelli et al., 2004) |
| SSRI | Paroxetine | Enhances gastric accommodation in health (Tack et al., 2003a) |
| 5-HT1B/D receptor agonists | Sumatriptan | Enhances gastric accommodation in health and have no influence in antral contraction (Sekino et al., 2012) |
| Alpha 2 adrenergic agonists | Clonidine | Relaxes stomach and reduces gastric sensation without inhibiting gastric accommodation and gastric emptying (Thumshirn et al., 1999) |
| 5-HT1A receptor agonists | Buspirone | Relaxes the proximal stomach in the fasting state health and delays gastric emptying in healthy volunteers (Van Oudenhove et al., 2008) |
| R137696 | Relaxes the proximal stomach in health (Boeckxstaens et al., 2006) | |
| Phase 2 in FD: no benefit over placebo (Tack et al., 2009b) | ||
| M1/M2 muscarinic | Acotiamide | May enhance accommodation in FD |
| Phase 2a in FD: potential benefit (Tack et al., 2009a) | ||
| Phase 3 in FD: over 4 weeks, significantly improved symptom severity and eliminated meal- Related symptoms in patients with FD (Matsueda et al., 2012) | ||
| Visceral analgesics | ||
| 5-HT3 antagonist | Alosetron | Reduction of visceral hypersensitivity (Delvaux et al., 1998) |
| Benefit shown by a placebo-controlled trial (Dukes et al., 2000) | ||
| Opioid k agonist | Fedotozine | Reduction of visceral hypersensitivity in healthy (Coffin et al., 1996) |
| Benefit shown by a placebo-controlled trial (Read et al., 1997) | ||
| NK-1 antagonist | Aprepitant | No influence on gastrointestinal motility in healthy volunteers (Madsen and Fuglsang, 2008) |
| NK-3 antagonist | Talnetant | No effect on rectal compliance or distension-induced rectal sensation in healthy participants (Houghton et al., 2007) |
DA-9701: A HERBAL COMBINATION
DA-9701 is formulated with Pharbitidis Semen and Corydalis Tuber. Corydalis Tuber had been used in traditional medicine for the treatment of gastric (Soji et al., 1969) and duodenal ulcers (Kamigauchi and Iwasa, 1994) and dysmenorrhea (Jia et al., 2006). Extracts from Corydalis Tuber have been used as antispasmodic agents for the gastrointestinal tract and as analgesics (Ding et al., 2007). Pharbitidis Semen is the seed of Pharbitis nil Choisy of the Convolvulaceae family and has been used as a folk medicine for its analgesic effects on the abdomen (Kumar et al., 2009).
Compared to non-botanical drugs, extracts of herbal material mixtures require more complicated quality control. Various analyses were performed for quality certification. The tests included identification of herbal material and extracts, loss on drying, inorganic impurities (like heavy metals), microbial limits, and pesticides, and chemical assays. Botanical materials are complex mixtures of numerous chemical constituents. Highperformance liquid chromatography (HPLC) performed for batch-to-batch control revealed corydaline and chlorogenic acid as the major constituents.
THE PHARMACOLOGY OF DA-9701
The in vivo pharmacological study results of DA-9701 were summarized in Table 2 and in vitro receptor affinities to major receptor related to gastrointestinal function were summarized in Table 3 including comparison with other prokinetics.
Table 2.
Pharmacological profi le summary of DA-9701
| Experimental pharmacology | ||||
|---|---|---|---|---|
|
| ||||
| Study type | Effect studied | Experimental | Result | Reference |
|
| ||||
| In vitro | Modulation of pacemaker activity | Whole cell patch clamp | DA-9701 affect GI motility by the modulation of pacemaker activity in the ICC | (Choi et al., 2009) |
| In vivo | D2 antagonistic activity | Inhibition of apomorphineinduced delayed gastric emptying in rats | Antagonism of D2 agonist apomorphine inhibited gastric emptying (3 mg/kg po) | (Lee et al., 2008) |
| In vivo | 5-HT1A antagonist activity | Restraint stress-induced feeding inhibition in rats | The stimulatory effects of DA-9701 (3 mg/kg po) were blocked by the 5-HT1A antagonist WAY 100635 | Not reported. |
| In vivo | 5-HT1A antagonist activity | Restraint stress-induced impaired fundic relaxation in rats | The fundic relaxing effects of DA-9701 (3 mg/kg po) were blocked by the 5-HT1A antagonist WAY 100635 | Not reported |
| In vivo | Fundus-relaxing activity | Canine gastric compliance with barostat | Active at 0.3 mg/kg po | (Lee et al., 2008) |
| In vivo | Fundus-relaxing activity | Meal-induced gastric accommodation with barostat in dogs | Active at 0.3 mg/kg po | (Kim et al., 2012) |
| In vivo | Gastroprokinetic activity | Gastric emptying in rats | Active at 0.3 and 3 mg/kg po | (Lee et al., 2008) |
| In vivo | Improvement of delayed gastric emptying | Rat gastric emptying delayed by cisplatin treatment | Active at 3 mg/kg po | (Lee et al., 2008) |
| In vivo | Imrovement of delayed gastric emptying induced by stress and inhibition of stress related hormones | Rat, stress induced delayed gastric emptying | Active at 3 mg/kg | (Jung et al., 2013) |
| In vivo | Antinociceptive effect against Colorectal distension induced visceral pain in visceral hypersensitivity rat | Neonatal Colorectal distension induced visceral hypersensitivity | Active at 0.3~3 | Not reported |
| In vivo | Antinociceptive effect in gastric distension induced nociception | Gastric distension induced nociception by barostat | Active 0.3~1 | Not reported |
Table 3.
Receptor affi nities of DA-9701 and related visceral functions
| Receptor/Affinity# | Related GI** function | DA-9701 (function)** | Cisapride* | Mosapride* | Itopride## | Domperidone* |
|---|---|---|---|---|---|---|
|
| ||||||
| 5-HT1A | Fundus relaxation(1) | 6.87 (agonist) | - | - | - | - |
| Adrenergic α2 | Visceral hypersensitivity(2) | 4.81 (agonist) | - | - | - | - |
| D2 | GI motility(3) | 0.38 (antagonist) | 0.11 | - | 0.434 | 0.008 |
| 5-HT4 | GI motility(4) | 13.2 (agonist) | 0.019 | 0.057 | - | - |
| Visceral hypersensitivity(5) | ||||||
GI: gastrointestinal.
*Data from Thomson Reuters Integrity, **Values from Eurofins Panlabs assays results, #Ki value (μg/ml), ##(Kessler et al., 1991).
Fundus relaxation effect
Impaired gastric accommodation is one of major therapeutic targets in FD. There are 2 kinds of responses in proximal gastric relaxation. One is “receptive relaxation” (Cannon and Lieb, 1911), and the other is “adaptive relaxation” (Jahnberg et al., 1975), a synonym of accommodation. Receptive relaxation occurs when swallowing foods, and adaptive relaxation initiates with entrance of the chyme to the duodenum and lasts for a period of time. In FD patients, impaired gastric accommodation prevalence was reported up to about 40%, and it was reported that impaired accommodation was related to early satiety and weight decrease (Bisschops and Tack, 2007).
Currently there are limited available animal models for evaluating the fundus relaxing effect. In humans, the barostat method is the gold standard to measure gastric tone and accommodation (Mundt et al., 2002; Tomita et al., 2013). Most of the well-known studies on fundus relaxation are using barostat in the canine (Azpiroz and Malagelada, 1985; De Ponti et al., 2003; Lei et al., 2005; Yin et al., 2007), but rat (Graca et al., 2002; Monroe et al., 2004; Romer et al., 2005; Zhao et al., 2005), ferret (Blackshaw et al., 1987) and feline (Mayrand et al., 1994; Janssen et al., 2004) are also known in vivo models. There are 2 kinds of end points evaluating fundus relaxation in the canine barostat model: accommodation and compliance (Azpiroz and Malagelada, 1985; De Ponti et al., 2003). The physiological gastric accommodation response enables relaxation of the proximal stomach providing space to receive foods without an increase in gastric pressure (Azpiroz and Malagelada, 1986). Especially meal-induced gastric accommodation is thought to be the most important motor index that can be studied in this model, since it is impaired in FD patients and it is a physiological test (Tack, 2009). Gastric compliance, which is tested in the fasting state, measures gastric tone in the resting state, and seems to be related to pain and discomfort perception threshold (De Ponti et al., 2003; Kuiken et al., 2005). Sumatriptan, known as a migraine treatment, was reported to enhance gastric accommodation in both humans and canines. Additionally, the triptan class of drugs was reported to have enhancing effects on gastric accommodation in canine (Moro et al., 2004). However, currently there is no established fundic relaxant and some agents are in development (Tack and Janssen, 2011b).
It was evaluated that DA-9701 can relax the proximal stomach in dog. The fundus relaxing effect was first established using the gastric compliance model, and it was shown that the pressure volume curve was shifted left and up in the DA-9701 treated group (Lee et al., 2008). Also, in the meal-induced gastric accommodation model, DA-9701 increased the intragastric volume more than in the vehicle-treated group, and this lasted for more than the normal accommodation response in dog barostat studies (approximately 1 h) (Kim et al., 2012). DA-9701 may be effective to treat stress-related disorders because one of its active ingredients (AI), tetrahydroberberine (THB), alleviates impaired gastric compliance in the poststress rat (Lee et al., 2011), and it prevented stress induced feeding inhibition (Kim et al., 2010). The common mechanism between stress-induced feeding inhibition and impaired gastric relaxation is not known. However, it is known that THB relaxes the proximal stomach via 5-HT1A agonism (Lee et al., 2011) and that DA-9701 significantly inhibits feeding inhibition induced by restraint stress via 5-HT1A activation in the rat (Kim et al., 2010). DA-9701 also has 5-HT4 and adrenergic α2 agonistic properties but it is not fully established that activation of these receptors effect proximal stomach relaxation. However, there are reports that a 5-HT4 agonist and an adrenergic α2 agonist have a fundus relaxation effect (Tack et al., 1998a; Tack et al., 2004).
Visceral analgesic effect
In the past decade, treatment development for functional gastrointestinal diseases (FGIDs) has focused on gastrointestinal motility. Particularly for IBS, several agents were developed to correct bowel movement, but the overall effect was not sufficient. Thus, visceral hypersensitivity was targeted as an alternative approach for FGID treatment development (Bradesi et al., 2008). Visceral hypersensitivity is the lowered perception and pain threshold to visceral stimuli that normal subjects do not perceive, and this is related to the visceral pain experienced by FGID patients (Mayer and Gebhart, 1994). Visceral hypersensitivity was identified as a major therapeutic target of many gastrointestinal disorders, including irritable bowel syndrome, functional dyspepsia, gastroesophageal reflux disease, and gastroparesis. Currently, there is no established agent for correcting visceral hypersensitivity, but several agents are being developed. Psychotropic agents and antidepressants are often used to correct visceral hypersensitivity in FD but the true benefit was not yet been fully proven (Tack and Janssen, 2011b).
There is no other method to evaluate analgesia in animals except through the use of surrogate markers, for example abdominal contraction or change of blood pressure (Mayer et al., 2008). Various stimulations inducing pain and discomfort can be used: distension of the lumen of the intestine, chemical irritant contact with the intestine, and parasitic infection. Experimentally, visceral hypersensitivity can also be induced by neonatal maternal separation and neonatal experiences of pain (Al-Chaer et al., 2000; Lidow, 2002; Lin and Al-Chaer, 2003; Chung et al., 2007a; Chung et al., 2007b).
In gastric distention induced nociception model, DA-9701 showed an analgesic effect: the perception of intragastric distension and the perception threshold was increased dose dependently and significantly in canine (unpublished). In colorectal distension induced model also, DA-9701 showed analgesic effect (unpublished). Furthermore corydaline and tetrahydropalmatine, the AI of DA-9701, was known to have antinociceptive effects on visceral and somatic nociception in rats (Wang et al., 2010; Cao et al., 2011). The pain signal that arises from the peripheral region transfers to the spinal cord (dorsal root ganglion) and there the information is processed for transfer to the central nervous system (CNS). It is certain that DA-9701 has an effect at the pain signal transduction level and is related with downregulation of phosphorylated extracellular-signal-regulated kinase (p-ERK) (unpublished).
DA-9701 has a high affinity to multiple receptors related to gastrointestinal function. Among them, it was suggested that 5-HT1A and adrenergic α2 might be involved in increased perception and pain threshold. The affinity of DA-9701 to 5-HT1A and adrenergic α2 receptors are 6.87 and 4.81 μg/ml respectively. 5-HT1A agonism was known to relax smooth muscle (Coulie et al., 1999; De Ponti et al., 2003), a possible mechanism of increased perception (Kuiken et al., 2005) and pain threshold related to tension-sensitive mechanoreceptor inactivation (Blackshaw et al., 1987). Moreover, an adrenergic α2 agonist was known to have antinociceptive effect to visceral, somatic and noxious stimuli (Wang and Mo, 1989; Danzebrink and Gebhart, 1990; Harada et al., 1995).
Prokinetic effect
The prokinetic effect was the traditional therapeutic target of FGIDs for the treatment of impaired gastrointestinal motility and the fundamental feature of gastrointestinal prokinetics. Prokinetics was traditionally regarded as the first step of PDS (postprandial distress syndrome), with an assumption that delayed gastric emptying was a major pathophysiological mechanism and that most symptoms arise from it (Tack, 2008). However, delayed gastric emptying did not well corre-late with other symptoms suggesting other causes of the symptoms might be present (Tack et al., 1998b; Tack et al., 2001).
There are numerous animal models to evaluate the effect of enhanced gastric emptying. In rat, the method of administering a meal and measuring the amount of remaining meal after a period of time is widely used to evaluate gastric emptying. The meals used for experimental purposes vary according to the purpose of the experiment, and include semisolid meals, solid meals, and liquid meals. A pathophysiological model of delayed gastric emptying includes delayed gastric emptying by dopamine, cisplatin, and opioids (Ramsbottom and Hunt, 1970; Tanila et al., 1993; Hirokawa et al., 1998). Intestinal transit is also widely used for evaluating intestinal motility. A pathophysiological model includes delayed gastrointestinal transit by opioids or postoperative ileus (Galligan and Burks, 1983; Tanila et al., 1993). Marker materials used for transit studies include charcoal and FITC-conjugated, amongst others (Miller et al., 1981; Williams et al., 1992).
The ability for DA-9701 to promote gastric emptying and gastrointestinal transit was evaluated in these models, and showed comparable prokinetic effects with other prokinetics (Lee et al., 2008). DA-9701 enhances gastric emptying and gastrointestinal transit via dopamine D2 antagonism and 5-HT4 agonism (Lim et al., 2012). The receptor affinities of DA-9701 to D2 receptor and 5-HT4 receptor are 0.381 and 13.2 μg/ml, respectively (Eurofins Panlabs). There can be some argue on coexistence of relaxation effect and contraction effect on stomach but, it can be explained by one report about serotonergic receptors’ distribution in stomach in which contractile receptors were dominant in antrum region and relaxing receptors were dominant in fundus region.
SAFETY OF DA-9701
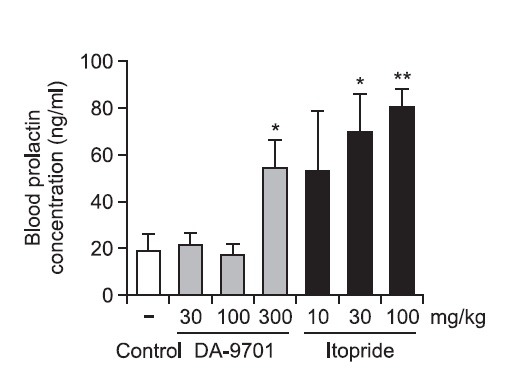
The LD50 of DA-9701 was found to be 2,000 mg/kg as a single treatment, and the no observed adverse effect level (NOAEL) was identified as 150 mg/kg in a pivotal repeated treatment study (26 weeks) in rats. NOAEL was 100 mg/kg in both 1-week and 13-week repeated-treatment studies in dogs. DA-9701 had no genotoxicity. The hyperprolactinemia due to D2 antagonism, which is one of major mechanisms of action of DA-9701, was one of the safety concerns. The prolactin ED200 of DA-9701 was about 70-fold lower than itopride (3.78 vs 270.1 mg/kg) in rats (Fig. 2). The CNS distribution of corydaline, one of the marker compounds of DA-9701, was studied because D2 antagonists such as metoclopramide had a direct effect on the CNS across the blood brain barrier. The pharmacologically effective concentration of corydaline is greater than 15 μM (Adsersen et al., 2007; Ma et al., 2008). The distribution of corydaline was not as much enough to have a pharmacological effect based on the blood concentration of 12.42~17.64 nM using the human dose. Chlorogenic acid, another marker compound of DA-9701, does not cross the blood brain barrier. 5-HT4 agonism is involved in DA-9701 pharmacology, and 5-HT4 agonist such as cisapride and tegaserod were withdrawn from the market due to adverse cardiac effects. Thus, a single-treatment study in beagle dog and rat was performed using telemetry to assess cardiac safety. No abnormal event was observed up to 250 mg/kg in dog and 100 mg/kg in rat. This is 67- and 167-fold higher than the human dose, respectively.
Fig. 2. The comparative blood prolactin elevation effect of DA-9701 versus itopride in SD rat (In briefly, blood was recovered in 30 min after drug administration in 8 weeks old SD rats and the prolactn level was analyzed by Rat PRL Elisa kit (AER011) within 6 hours from recovery. 5 animals were used in each group. Open bar; control, dark gray bar; motilitone, Black bar; Itopride *p<0.05, **p<0.01 vs. control; student’s t-test, value of result; Mean ± SD).
SUMMARY
FD is a highly prevalent chronic gastrointestinal disorder that is of considerable burden to both the patient and society. First of all, the pathophysiology should be understood completely for better treatment but, it may be a long way. Multiple action mechanisms may be a good solution for multifactorial and chronic disease. DA-9701 is a leading phytomedicine which implicated the possibility of multiple drug target synergism versus single molecular target against functional dyspepsia which has complex pathophysiology.
DA-9701 showed a beneficial effect on gastric accommodation, visceral hypersensitivity, gastrointestinal motility and possibly stress-induced disorder in vivo. These effects counteract major pathophysiological causes of FD, and good clinical outcome is anticipated. Moreover, in FD there are some overlapping features with the other FGIDs and thus the effect of Motilitone may not be limited to FD.
Acknowledgments
This work was supported by the Global Leading Technology Program of the Office of Strategic R&D Planning (OSP), funded by the Ministry of Knowledge Economy, Republic of Korea (10039321).
References
- 1.Adsersen A., Kjolbye A, Dall O., Jager A. K. Acetylcholinesterase and butyrylcholinesterase inhibitory compounds from Corydalis cava Schweigg. & Kort. J. Ethnopharmacol. (2007);113:179–182. doi: 10.1016/j.jep.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Agreus L., Svardsudd K., Nyren O., Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology . (1995);109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 3.Al-Chaer E. D., Kawasaki M., Pasricha P. J. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology . (2000);119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 4.Azpiroz F., Malagelada J. R. Physiological variations in canine gastric tone measured by an electronic barostat. Am. J. Physiol. . (1985);248:G229–237. doi: 10.1152/ajpgi.1985.248.2.G229. [DOI] [PubMed] [Google Scholar]
- 5.Azpiroz F., Malagelada J. R. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am. J. Physiol. (1986);251:G727–735. doi: 10.1152/ajpgi.1986.251.6.G727. [DOI] [PubMed] [Google Scholar]
- 6.Banani S. J., Lankarani K. B., Taghavi A, Bagheri M. H, Sefidbakht S., Geramizadeh B. Comparison of metoclopramide oral tablets and solution in treatment of dysmotility-like dyspepsia. Am. J. Health Syst. Pharm. (2008);65:1057–1061. doi: 10.2146/ajhp070381. [DOI] [PubMed] [Google Scholar]
- 7.Bisschops R., Tack J. Dysaccommodation of the stomach: therapeutic nirvana? Neurogastroenterol. Motil. (2007);19:85–93. doi: 10.1111/j.1365-2982.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- 8.Blackshaw L. A, Grundy D., Scratcherd T. Vagal afferent discharge from gastric mechanoreceptors during contraction and relaxation of the ferret corpus. J. Auton. Nerv. Syst. (1987);18:19–24. doi: 10.1016/0165-1838(87)90130-5. [DOI] [PubMed] [Google Scholar]
- 9.Boeckxstaens G. E., Tytgat G. N., Wajs E., Van Nueten L., De Ridder F., Meulemans A., Tack J. The influence of the novel 5-HT1A agonist R137696 on the proximal stomach function in healthy volunteers. Neurogastroenterol. Motil. (2006);18:919–926. doi: 10.1111/j.1365-2982.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 10.Bradesi S, Herman J., Mayer E. A. Visceral analgesics: drugs with a great potential in functional disorders? Curr. Opin. Pharmacol. (2008);8:697–703. doi: 10.1016/j.coph.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun R., Kuo B. Functional dyspepsia. Therap. Adv. Gastroenterol. (2010);3:145–164. doi: 10.1177/1756283X10362639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camilleri M., Bueno L., de Ponti F., Fioramonti J., Lydiard R. B., Tack J. Pharmacological and pharmacokinetic aspects of functional gastrointestinal disorders. Gastroenterology . (2006);130:1421–1434. doi: 10.1053/j.gastro.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Cannon W. B., Lieb C. W. The receptive relaxation of the stomach. Am. J. Physiol. (1911);29:267–273. [Google Scholar]
- 14.Cao F. L., Shang G. W., Wang Y., Yang F., Li C. L., Chen J. Antinociceptive effects of intragastric dl-tetrahydropalmatine on visceral and somatic persistent nociception and pain hypersensitivity in rats. Pharmacol. Biochem. Behav. . (2011);100:199–204. doi: 10.1016/j.pbb.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Choi S., Choi J. J., Jun J. Y., Koh J. W., Kim S. H., Kim D. H., Pyo M. Y., Son J. P., Lee I., Son M., Jin M. Induction of pacemaker currents by DA-9701, a prokinetic agent, in interstitial cells of Cajal from murine small intestine. Mol. Cells . (2009);27:307–312. doi: 10.1007/s10059-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 16.Choung R. S., Talley N. J., Peterson J., Camilleri M., Burt D., Harmsen W. S., Zinsmeister A. R. A double-blind, randomized, placebo-controlled trial of itopride (100 and 200 mg three times daily) on gastric motor and sensory function in healthy volunteers. Neurogastroenteol. Motil. (2007);19:180–187. doi: 10.1111/j.1365-2982.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 17.Chung E. K., Zhang X., Li Z., Zhang H., Xu H., Bian Z. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain. Res. (2007a);1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Chung E. K., Zhang X. J., Xu H. X., Sung J. J., Bian Z. X. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience . (2007b);149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Coffin B., Bouhassira D, Chollet R, Fraitag B, De Meynard C, Geneve J, Lemann M, Willer J. C., Jian R. Effect of the kappa agonist fedotozine on perception of gastric distension in healthy humans. Aliment. Pharmacol. Ther. . (1996);10:919–925. doi: 10.1046/j.1365-2036.1996.109280000.x. [DOI] [PubMed] [Google Scholar]
- 20.Coulie B., Tack J., Sifrim D., Andrioli A., Janssens J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor- mediated relaxation of gastric fundus. Am. J. Physiol. (1999);276:G373–377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- 21.Danzebrink R. M., Gebhart G. F. Antinociceptive effects of intrathecal adrenoceptor agonists in a rat model of visceral nociception. J. Pharmacol. Exp. Ther. (1990);253:698–705. [PubMed] [Google Scholar]
- 22.De Ponti F., Crema F., Moro E., Nardelli G., Frigo G., Crema A. Role of 5-HT1B/D receptors in canine gastric accommodation: effect of sumatriptan and 5-HT1B/D receptor antagonists. Am. J. Physiol. Gastrointest. Liver Physiol. (2003);285:G96–104. doi: 10.1152/ajpgi.00280.2002. [DOI] [PubMed] [Google Scholar]
- 23.Degen L., Matzinger D., Merz M., Appel-Dingemanse S., Osborne S., Luchinger S., Bertold R., Maecke H., Beglinger C. Tegaserod, a 5-HT4 receptor partial agonist, accelerates gastric emptying and gastrointestinal transit in healthy male subjects. Aliment. Pharmacol. Ther. . (2001);15:1745–1751. doi: 10.1046/j.1365-2036.2001.01103.x. [DOI] [PubMed] [Google Scholar]
- 24.Degryse H, De Schepper A., Verlinden M. A double-blind fluoroscopic study of cisapride on gastrointestinal motility in patients with functional dyspepsia. Scand. J. Gastroenterol. Suppl. . (1993);195:1–4. doi: 10.3109/00365529309098321. [DOI] [PubMed] [Google Scholar]
- 25.Delvaux M., Louvel D., Mamet J. P., Campos-Oriola R., Frexinos J. Effect of alosetron on responses to colonic distension in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. (1998);12:849–855. doi: 10.1046/j.1365-2036.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 26.Ding B., Zhou T., Fan G., Hong Z., Wu Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/MS and LCDAD. J. Pharm. Biomed. Anal. (2007);45:219–226. doi: 10.1016/j.jpba.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Drossman D. A. The functional gastrointestinal disorders and the Rome II process. Gut . (1999);45(Suppl. 2):II1–5. doi: 10.1136/gut.45.2008.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dukes G. E., Talley N. J., van Zanten S. V., Heath M., Sorrells S.C., Perschy T. B., Kleoudis C., Mangel A. W. A doseranging, placebo-controlled, randomized clinical trial of alosetron, a 5-HT3 receptor antagonist, in the treatment of functional (nonulcer) dyspepsia. Am. J. Gastroenterol. . (2000);95:2451. doi: 10.1111/j.1572-0241.2000.02498.x. [DOI] [Google Scholar]
- 29.Dumitrascu D. L., Ungureanu O., Verzea D., Pascu O. The effect of metoclopramide on antral emptying of a semisolid meal in patients with functional dyspepsia. A randomized placebo controlled sonographic study. Rom. J. Intern. Med. . (1998);36:97–104. [PubMed] [Google Scholar]
- 30.Dumuis A., Sebben M., Bockaert J. The gastrointestinal prokinetic benzamide derivatives are agonists at the non-classical 5-HT receptor (5-HT4) positively coupled to adenylate cyclase in neurons. Naunyn Schmiedebergs Arch. Pharmacol. . (1989);340:403–403. doi: 10.1007/BF00167041. [DOI] [PubMed] [Google Scholar]
- 31.Ejskjaer N., Vestergaard E. T., Hellstrom P. M., Gormsen L. C., Madsbad S., Madsen J. L., Jensen T. A., Pezzullo J. C., Christiansen J. S., Shaughnessy L., Kosutic G. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment. Pharmacol. Ther. (2009);29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 32.Galligan J. J., Burks T. F. Centrally mediated inhibition of small intestinal transit and motility by morphine in the rat. J. Pharmacol. Exp. Ther. (1983);226:356–361. [PubMed] [Google Scholar]
- 33.Gilja O. H., Hausken T., Bang C. J., Berstad A. Effect of glyceryl trinitrate on gastric accommodation and symptoms in functional dyspepsia. Dig. Dis. Sci. (1997);42:2124–2131. doi: 10.1023/A:1018839122354. [DOI] [PubMed] [Google Scholar]
- 34.Graca J. R., Leal P. R., Gondim F. A., Rola F. H., Santos A.A. Variations in gastric compliance induced by acute blood volume changes in anesthetized rats. Braz. J. Med. Biol. Res. . (2002);35:405–410. doi: 10.1590/S0100-879X2002000300018. [DOI] [PubMed] [Google Scholar]
- 35.Halder S. L., Talley N. J. Functional Dyspepsia: A New Rome III Paradigm. Curr. Treat. Options Gastroenterol. . (2007);10:259–272. doi: 10.1007/s11938-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 36.Hallerback B. I., Bommelaer G., Bredberg E., Campbell M., Hellblom M., Lauritsen K., Wienbeck M., Holmgren L. L. Dose finding study of mosapride in functional dyspepsia: a placebo- controlled, randomized study. Aliment. Pharmacol. Ther. (2002);16:959–967. doi: 10.1046/j.1365-2036.2002.01236.x. [DOI] [PubMed] [Google Scholar]
- 37.Hancock B. D., Bowen-Jones E., Dixon R., Dymock I. W., Cowley D. J. The effect of metoclopramide on gastric emptying of solid meals. Gut . (1974);15:462–467. doi: 10.1136/gut.15.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada Y., Nishioka K., Kitahata L. M., Kishikawa K., Collins J.G. Visceral antinociceptive effects of spinal clonidine combined with morphine, [D-Pen2, D-Pen5] enkephalin, or U50,488H. Anesthesiology . (1995);83:344–352. doi: 10.1097/00000542-199508000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Herman M. A., Niedringhaus M., Alayan A., Verbalis J. G., Sahibzada N., Gillis R. A. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in refl ex control of fundus tone. Am. J. Physiol. Regul. Integr. Comp.Physiol. (2008);294:R720–729. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- 40.Hirokawa Y., Yamazaki H., Yoshida N., Kato S. A novel series of 6-methoxy-1H-benzotriazole-5-carboxamide derivatives with dual antiemetic and gastroprokinetic activities. Bioorg. Med. Chem. Lett. (1998);8:1973–1978. doi: 10.1016/S0960-894X(98)00341-2. [DOI] [PubMed] [Google Scholar]
- 41.Houghton L. A., Cremonini F., Camilleri M., Busciglio I., Fell C., Cox V., Alpers D. H., Dewit O. E., Dukes G. E., Gray E., Lea R., Zinsmeister A. R., Whorwell P. J. Effect of the NK(3) receptor antagonist, talnetant, on rectal sensory function and compliance in healthy humans. Neurogastroenterol. Motil. (2007);19:732–743. doi: 10.1111/j.1365-2982.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 42.Jahnberg T., Martinson J., Hulten L., Fasth S. Dynamic gastric response to expansion before and after vagotomy. Scand. J. Gastroenterol. (1975);10:593–598. [PubMed] [Google Scholar]
- 43.Janssen P., Tack J., Sifrim D., Meulemans A. L., Lefebvre R. A. Influence of 5-HT1 receptor agonists on feline stomach relaxation. Eur. J. Pharmacol. (2004);492:259–267. doi: 10.1016/j.ejphar.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 44.Jia W., Wang X., Xu D., Zhao A., Zhang Y. Common traditional Chinese medicinal herbs for dysmenorrhea. Phytother. Res. (2006);20:819–824. doi: 10.1002/ptr.1905. [DOI] [PubMed] [Google Scholar]
- 45.Jung Y. S., Kim M. Y., Lee H. S., Park S. L., Lee K. J. Effect of DA-9701, a novel prokinetic agent, on stress-induced delayed gastric emptying and hormonal changes in rats. Neurogastroenterol. Motil. (2013);25:254–259. doi: 10.1111/nmo.12053. [DOI] [PubMed] [Google Scholar]
- 46.Kamigauchi M., Iwasa K. Corydalis spp.: in vitro culture and the biotransformation of protoberberines. Biotechnol. Agric. For. . (1994);26:93–105. [Google Scholar]
- 47.Kanaizumi T., Nakano H., Matsui Y., Ishikawa H., Shimizu R., Park S., Kuriya N. Prokinetic effect of AS-4370 on gastric emptying in healthy adults. Eur. J. Clin. Pharmacol. (1991);41:335–337. doi: 10.1007/BF00314963. [DOI] [PubMed] [Google Scholar]
- 48.Kessler R. M., Ansari M. S, de Paulis T., Schmidt D. E., Clanton J. A., Smith H. E., Manning R. G., Gillespie D., Ebert M.H. High affinity dopamine D2 receptor radioligands. 1. Regional rat brain distribution of iodinated benzamides. J. Nucl. Med. (1991);32:1593–1600. [PubMed] [Google Scholar]
- 49.Kim E. R., Min B. H., Lee S. O., Lee T. H., Son M., Rhee P.L. Effects of DA-9701, a novel prokinetic agent, on gastric accommodation in conscious dogs. J. Gastroenterol. Hepatol. (2012);27:766–772. doi: 10.1111/j.1440-1746.2011.06924.x. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y. S., Lee M. Y., Choi E. s., Oh J. T., Sohn Y. W., Oh Y. L., Ryu H. S., Seo G. S., Choi S. C. W1406 DA-9701, a new prokinetic agent, improves feeding inhibition by restraint stress in rats-comparative study with in vivo micro-animal. Gastroenterology. (2010);138:S–717. doi: 10.1053/j.gastro.2009.09.061. [DOI] [Google Scholar]
- 51.Kuiken S. D., Tytgat G. N., Boeckxstaens G. E. Review article: drugs interfering with visceral sensitivity for the treatment of functional gastrointestinal disorders--the clinical evidence. Aliment. Pharmacol. Ther. . (2005);21:633–651. doi: 10.1111/j.1365-2036.2005.02392.x. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S., Kumar D, Singh J, Narender R., Kaushik D. Anti-inflammatory, Analgesic and Antioxidant Activities of Ipomoea hederaceae Linn. Planta Med. (2009);75:P–100. doi: 10.1055/s-2009-1216538. [DOI] [Google Scholar]
- 53.Lee T. H., Choi J. J., Kim D. H., Choi S., Lee K. R., Son M., Jin M. Gastroprokinetic effects of DA-9701, a new prokinetic agent formulated with Pharbitis Semen and Corydalis Tuber. Phytomedicine. (2008);15:836–843. doi: 10.1016/j.phymed.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 54.Lee T. H., Kim K. H., Lee S. O., Lee K. R., Son M., Jin M. Tetrahydroberberine, an isoquinoline alkaloid isolated from corydalis tuber, enhances gastrointestinal motor function. J. Pharmacol. Exp. Ther. . (2011);338:917–924. doi: 10.1124/jpet.111.182048. [DOI] [PubMed] [Google Scholar]
- 55.Lei Y, Zhu H, Xing J., Chen J. D. Rectal distension modulates canine gastric tone and accommodation. Dig. Dis. Sci. . (2005);50:2134–2140. doi: 10.1007/s10620-005-3020-z. [DOI] [PubMed] [Google Scholar]
- 56.Lidow M. S. Long-term effects of neonatal pain on nociceptive systems. Pain. (2002);99:377–383. doi: 10.1016/S0304-3959(02)00258-0. [DOI] [PubMed] [Google Scholar]
- 57.Lim H. C., Park H., Lee S. I., Jahng J., Lee Y. J. Tu1985 effects of DA-9701, a novel prokinetic agent on gastric motor function in guinea pig. Gastroenterology . (2012);142:S893–S894. [Google Scholar]
- 58.Lin C., Al-Chaer E. D. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. (2003);971:73–82. doi: 10.1016/S0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 59.Ma Z. Z., Xu W., Jensen N. H., Roth B. L., Liu-Chen L. Y., Lee D. Y. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affi nities at the dopamine D1 receptor. Molecules . (2008);13:2303–2312. doi: 10.3390/molecules13092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackie A. D., Ferrington C., Cowan S., Merrick M. V., Baird J. D., Palmer K. R. The effects of renzapride, a novel prokinetic agent, in diabetic gastroparesis. Aliment. Pharmacol. Ther. (1991);5:135–142. doi: 10.1111/j.1365-2036.1991.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 61.Madsen J. L., Fuglsang S. A randomized, placebo-controlled, crossover, double-blind trial of the NK1 receptor antagonist aprepitant on gastrointestinal motor function in healthy humans. Aliment. Pharmacol. Ther. (2008);27:609–615. doi: 10.1111/j.1365-2036.2008.03618.x. [DOI] [PubMed] [Google Scholar]
- 62.Mansi C., Borro P., Giacomini M., Biagini R., Mele M. R., Pandolfo N., Savarino V. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol. Ther. (2000);14:561–569. doi: 10.1046/j.1365-2036.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 63.Matsueda K., Hongo M., Tack J., Saito Y., Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut . (2012);61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayer E. A., Bradesi S., Chang L., Spiegel B. M., Bueller J. A., Naliboff B. D. Functional GI disorders: from animal models to drug development. Gut . (2008);57:384–404. doi: 10.1136/gut.2006.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayer E. A., Gebhart G. F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology . (1994);107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 66.Mayrand S., Tremblay L., Diamant N. In vivo measurement of feline esophageal tone. Am. J. Physiol. . (1994);267:G914–921. doi: 10.1152/ajpgi.1994.267.5.G914. [DOI] [PubMed] [Google Scholar]
- 67.McCallum R. W., Cynshi O. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment. Pharmacol. Ther. (2007a);26:1121–1130. doi: 10.1111/j.1365-2036.2007.03461.x. [DOI] [PubMed] [Google Scholar]
- 68.McCallum R. W., Cynshi O. Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment. Pharmacol. Ther. . (2007b);26:107–116. doi: 10.1111/j.1365-2036.2007.03346.x. [DOI] [PubMed] [Google Scholar]
- 69.Miller M. S., Galligan J. J., Burks T. F. Accurate measurement of intestinal transit in the rat. J. Pharmacol. Methods. (1981);6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 70.Monroe M. J., Hornby P. J., Partosoedarso E. R. Central vagal stimulation evokes gastric volume changes in mice: a novel technique using a miniaturized barostat. Neurogastroenterol. Motil. (2004);16:5–11. doi: 10.1046/j.1365-2982.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 71.Moro E., Crema F., De Ponti F., Frigo G. Triptans and gastric accommodation: pharmacological and therapeutic aspects. Dig. Liver Dis. . (2004);36:85–92. doi: 10.1016/j.dld.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Mundt M. W., Hausken T., Samsom M. Effect of intragastric barostat bag on proximal and distal gastric accommodation in response to liquid meal. Am. J. Physiol. Gastrointest. Liver Physiol. (2002);283:G681–686. doi: 10.1152/ajpgi.00499.2001. [DOI] [PubMed] [Google Scholar]
- 73.Nagahata Y., Urakawa T., Kurod H., Tomonaga K., Idei H., Kawakita N., Yoshizumi K., Saitoh Y. The effect of dopamine on rat gastric motility. Gastroenterol. Jpn. (1992);27:482–487. doi: 10.1007/BF02777783. [DOI] [PubMed] [Google Scholar]
- 74.Noh Y. W., Jung H. K., Kim S. E., Jung S. A. Overlap of erosive and non-erosive refl ux diseases with functional gastrointestinal disorders according to Rome III criteria. J. Neurogastroenterol. Motil. (2010);16:148–156. doi: 10.5056/jnm.2010.16.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piessevaux H., De Winter B., Louis E., Muls V., De Looze D., Pelckmans P., Deltenre M., Urbain D., Tack J. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol. Motil. (2009);21:378–388. doi: 10.1111/j.1365-2982.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 76.Ramsbottom N., Hunt J. N. Studies of the effect of metoclopramide and apomorphine on gastric emptying and secretion in man. Gut . (1970);11:989–993. doi: 10.1136/gut.11.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Read N. W., Abitbol J. L., Bardhan K. D., Whorwell P. J., Fraitag B. Efficacy and safety of the peripheral kappa agonist fedotozine versus placebo in the treatment of functional dyspepsia. Gut. (1997);41:664–668. doi: 10.1136/gut.41.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rhie S. Y., Choi C. H., Lee H. W., Do M. Y., Lee S. H., Han S. P., et al. The frequency of functional dyspepsia subtypes and its related factors for health check up subjects. Korean. J. Neurogastroenterol. Motil. (2007);13:31–37. [Google Scholar]
- 79.Romer M., Painsipp E., Schwetz I., Holzer P. Facilitation of gastric compliance and cardiovascular reaction by repeated isobaric distension of the rat stomach. Neurogastroenterol. Motil. (2005);17:399–409. doi: 10.1111/j.1365-2982.2005.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosch W., Liebregts T., Gundermann K. J., Vinson B., Holtmann G. Phytotherapy for functional dyspepsia: a review of the clinical evidence for the herbal preparation STW 5. Phytomedicine. (2006);13(Suppl 5):114–121. doi: 10.1016/j.phymed.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 81.Sarnelli G., Sifrim D., Janssens J., Tack J. Influence of sildenafil on gastric sensorimotor function in humans. Am. J. Physiol Gastrointest. Liver Physiol. (2004);287:G988–992. doi: 10.1152/ajpgi.00419.2003. [DOI] [PubMed] [Google Scholar]
- 82.Schemann M., Michel K., Zeller F., Hohenester B., Ruhl A. Region-specific effects of STW 5 (Iberogast) and its components in gastric fundus, corpus and antrum. Phytomedicine. (2006);13(Suppl 5):90–99. doi: 10.1016/j.phymed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 83.Sekino Y., Yamada E., Sakai E., Ohkubo H., Higurashi T., Iida H., Endo H., Takahashi H., Koide T., Sakamoto Y., Nonaka T., Gotoh E., Maeda S., Nakajima A., Inamori M. Influence of sumatriptan on gastric accommodation and on antral contraction in healthy subjects assessed by ultrasonography. Nuerogastroenterol Motil. (2012);24:1083–e1564. doi: 10.1111/j.1365-2982.2012.01984.x. [DOI] [PubMed] [Google Scholar]
- 84.Soji Y., Kadokawa T., Masuda Y., Kawashima K., Nakamura K. Effects of Corydalis alkaloid upon inhibition of gastric juice secretion and prevention of gastric ulcer in experimental animals. Nihon Yakurigaku Zasshi. (1969);65:196–209. doi: 10.1254/fpj.65.196. [DOI] [PubMed] [Google Scholar]
- 85.Stanghellini V., De Giorgio R., Barbara G., Cogliandro R., Tosetti C., De Ponti F., Corinaldesi R. Delayed gastric emptying in functional dyspepsia. Curr. Treat. Options Gastroenterol. (2004);7:259–264. doi: 10.1007/s11938-004-0011-7. [DOI] [PubMed] [Google Scholar]
- 86.Stanghellini V., De Ponti F., De Giorgio R., Barbara G., Tosetti C., Corinaldesi R. New developments in the treatment of functional dyspepsia. Drugs . (2003);63:869–892. doi: 10.2165/00003495-200363090-00003. [DOI] [PubMed] [Google Scholar]
- 87.Stevens J. E., Russo A., Maddox A. F., Rayner C. K., Phillips L., Talley N. J., Giguere M., Horowitz M., Jones K. L. Effect of itopride on gastric emptying in longstanding diabetes mellitus. Neurogastroenterol. Motil. (2008);20:456–463. doi: 10.1111/j.1365-2982.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 88.Suzuki H., Nishizawa T., Hibi T. Therapeutic strategies for functional dyspepsia and the introduction of the Rome III classification. J. Gastroenterol. (2006);41:513–523. doi: 10.1007/s00535-006-1847-5. [DOI] [PubMed] [Google Scholar]
- 89.Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Curr. Opin. Pharmacol. (2008);8:690–696. doi: 10.1016/j.coph.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Tack J. Mandatory and optional function tests in gastroduodenal disorders. Best Pract. Res. Clin. Gastroenterol. . (2009);23:387–393. doi: 10.1016/j.bpg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Tack J., Broeckaert D., Coulie B., Janssens J. The influence of cisapride on gastric tone and the perception of gastric distension. Aliment. Pharmacol. Ther. (1998a);12:761–766. doi: 10.1046/j.1365-2036.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 92.Tack J., Broekaert D., Coulie B., Fischler B., Janssens J. Influence of the selective serotonin re-uptake inhibitor, paroxetine, on gastric sensorimotor function in humans. Aliment. Pharmacol. Ther. (2003a);17:603–608. doi: 10.1046/j.1365-2036.2003.01469.x. [DOI] [PubMed] [Google Scholar]
- 93.Tack J., Caenepeel P., Corsetti M., Janssens J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology . (2004);127:1058–1066. doi: 10.1053/j.gastro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Tack J., Caenepeel P., Fischler B., Piessevaux H., Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. (2001);121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 95.Tack J., Janssen P. Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin. Investig. Drugs . (2011a);20:701–712. doi: 10.1517/13543784.2011.562890. [DOI] [PubMed] [Google Scholar]
- 96.Tack J., Janssen P. Emerging drugs for functional dyspepsia. Expert Opin. Emerg. Drugs. (2011b);16:283–292. doi: 10.1517/14728214.2011.558502. [DOI] [PubMed] [Google Scholar]
- 97.Tack J., Masclee A., Heading R., Berstad A., Piessevaux H., Popiela T., Vandenberghe A., Kato H. A dose-ranging, placebo-controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterol. Motil. (2009a);21:272–280. doi: 10.1111/j.1365-2982.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 98.Tack J., Piessevaux H., Coulie B., Caenepeel P., Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. (1998b);115:1346–1352. doi: 10.1016/S0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 99.Tack J., Talley N. J., Camilleri M., Holtmann G., Hu P., Malagelada J. R., Stanghellini V. Functional gastroduodenal disorders. Gastroenterology . (2006);130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 100.Tack J., Van Den Elzen B., Tytgat G., Wajs E., Van Nueten L., De Ridder F., Boeckxstaens G. A placebo-controlled trial of the 5-HT1A agonist R-137696 on symptoms, visceral hypersensitivity and on impaired accommodation in functional dyspepsia. Neurogastroenterol. Motil. (2009b);21:619–626. doi: 10.1111/j.1365-2982.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- 101.Tack J., Vos R., Janssens J., Salter J., Jauffret S., Vandeplassche G. Influence of tegaserod on proximal gastric tone and on the perception of gastric distension. Aliment. Pharmacol.Ther. (2003b);18:1031–1037. doi: 10.1046/j.1365-2036.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- 102.Talley N. J., Camilleri M., Burton D., Thomforde G., Koch K., Rucker M. J., Peterson J., Zinsmeister A. R., Earnest D. L. Double-blind, randomized, placebo-controlled study to evaluate the effects of tegaserod on gastric motor, sensory and myoelectric function in healthy volunteers. Aliment. Pharmacol. Ther. (2006);24:859–867. doi: 10.1111/j.1365-2036.2006.03049.x. [DOI] [PubMed] [Google Scholar]
- 103.Talley N. J., Locke G. R., 3rd, Herrick L. M., Silvernail V. M., Prather C. M., Lacy B. E., DiBaise J. K., Howden C. W., Brenner D. M., Bouras E. P., El-Serag H. B., Abraham B. P., Moayyedi P., Zinsmeister A. R. Functional Dyspepsia Treatment Trial (FDTT): a double-blind, randomized, placebo-controlled trial of antidepressants in functional dyspepsia, evaluating symptoms, psychopathology, pathophysiology and pharmacogenetics. Contemp. Clin. Trials. (2012);33:523–533. doi: 10.1016/j.cct.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Talley N. J., Tack J., Ptak T., Gupta R., Giguere M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut . (2008);57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 105.Talley N. J., Zinsmeister A. R., Schleck C. D., Melton L. J. 3rd. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. (1992);102:1259–1268. [PubMed] [Google Scholar]
- 106.Tanila H., Kauppila T., Taira T. Inhibition of intestinal motility and reversal of postlaparotomy ileus by selective alpha 2-adrenergic drugs in the rat. Gastroenterology. (1993);104:819–824. doi: 10.1016/0016-5085(93)91018-d. [DOI] [PubMed] [Google Scholar]
- 107.Taniyama K., Nakayama S., Takeda K., Matsuyama S., Shirakawa J., Sano I., Tanaka C. Cisapride stimulates motility of the intestine via the 5-hydroxytryptamine receptors. J. Pharmacol. Exp. Ther. . (1991);258:1098–1104. [PubMed] [Google Scholar]
- 108.Thumshirn M., Camilleri M., Choi M. G., Zinsmeister A. R. Modulation of gastric sensory and motor functions by nitrergic and alpha2-adrenergic agents in humans. Gastroenterology. (1999);116:573–585. doi: 10.1016/S0016-5085(99)70179-4. [DOI] [PubMed] [Google Scholar]
- 109.Tomita T., Okugawa T., Yamasaki T., Kondo T, Toyoshima F., Sakurai J., Oshima T., Fukui H., Daimon T., Watari J., Kashiwagi T., Matsumoto T., Miwa H. Use of scintigraphy to evaluate gastric accommodation and emptying: comparison with barostat. J. Gastroenterol. Hepatol. (2013);28:106–111. doi: 10.1111/j.1440-1746.2012.07261.x. [DOI] [PubMed] [Google Scholar]
- 110.Van Oudenhove L., Kindt S., Vos R., Coulie B., Tack J. Influence of buspirone on gastric sensorimotor function in man. Aliment. Pharmacol. Ther. . (2008);28:1326–1333. doi: 10.1111/j.1365-2036.2008.03849.x. [DOI] [PubMed] [Google Scholar]
- 111.Veldhuyzen van Zanten S. J., Jones M. J., Verlinden M., Talley N. J. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am. J. Gastroenterol . (2001);96:689–696. doi: 10.1016/S0002-9270(00)02314-5. [DOI] [PubMed] [Google Scholar]
- 112.Wang C., Wang S., Fan G., Zou H. Screening of antinociceptive components in Corydalis yanhusuo W.T. Wang by comprehensive two-dimensional liquid chromatography/tandem mass spectrometry. Anal. Bioanal. Chem. (2010);396:1731–1740. doi: 10.1007/s00216-009-3409-1. [DOI] [PubMed] [Google Scholar]
- 113.Wang S., Mo W. Y. Effect of intraseptal noradrenaline on somatic and visceral pain threshold of rabbits. Sheng Li Xue Bao. (1989);41:128–135. [PubMed] [Google Scholar]
- 114.Williams P. D., Colbert W. E., Shetler T. J., Turk J. A. Comparative pharmacological profile of muscarinic agonists in the isolated ileum, the pithed rat, and the mouse charcoal meal transit test. Gen. Pharmacol. . (1992);23:177–185. doi: 10.1016/0306-3623(92)90006-6. [DOI] [PubMed] [Google Scholar]
- 115.Yin J., Ouyang H., Chen J. D. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring) (2007);15:1133–1138. doi: 10.1038/oby.2007.615. [DOI] [PubMed] [Google Scholar]
- 116.Zhao J., Liao D., Gregersen H. Tension and stress in the rat and rabbit stomach are location- and direction-dependent. Neurogastroenterol. Motil. . (2005);17:388–398. doi: 10.1111/j.1365-2982.2004.00635.x. [DOI] [PubMed] [Google Scholar]


