Abstract
Osteoprotegerin (OPG) is a secreted glycoprotein and a member of the tumor necrosis factor receptor superfamily. It usually functions in bone remodeling, by inhibiting osteoclastogenesis through interaction with a receptor activator of the nuclear factor κB (RANKL). Transglutaminases-2 (Tgase-2) is a group of multifunctional enzymes that plays a role in cancer cell metastasis and bone formation. However, relationship between OPG and Tgase-2 is not studied. Therefore, we investigated the involvement of 12-O-Tetradecanoylphorbol 13-acetate in the expression of OPG in MG-63 osteosarcoma cells. Interleukin-1β time-dependently induced OPG and Tgase-2 expression in cell lysates and media of the MG-63 cells by a Western blot. Additional 110 kda band was found in the media of MG-63 cells. 12-O-Tetradecanoylphorbol 13-acetate also induced OPG and Tgase-2 expression. However, an 110 kda band was not found in TPA-treated media of MG-63 cells. Cystamine, a Tgase-2 inhibitor, dose-dependently suppressed the expression of OPG in MG-63 cells. Gene silencing of Tgase-2 also signifi cantly suppressed the expression of OPG in MG-63 cells. Next, we examined whether a band of 110 kda of OPG contains an isopeptide bond, an indication of Tgase-2 action, by monoclonal antibody specifi c for the isopeptide bond. However, we could not fi nd the isopeptide bond at 110 kda but 77 kda, which is believed to be the band position of Tgase-2. This suggested that 110 kda is not the direct product of Tgase-2’s action. All together, OPG and Tgase-2 is induced by IL-1β or TPA in MG-63 cells and Tgase-2 is involved in OPG expression in MG-63 cells.
Keywords: Osteoprotegerin, Transglutaminase-2, MG-63 cell, Cystamine, IL-1β, TPA
INTRODUCTION
Osteoprotegerin (OPG) is a secreted glycoprotein belonging to the tumour necrosis factor receptor (TNFR) superfamily (Simonet et al., 1997; Tsuda et al., 1997). OPG is central in the regulation of bone turnover through the inhibition of osteoclastogenesis (Tsuda et al., 1997). OPG is absent of a transmembrane domain, making this a decoy receptor with the ability to bind a number of different ligands, and is produced as a monomer (55-62 kDa). It is secreted as a disulfidelinked homo dimeric glycoprotein with four or five potential glycosylation sites, generating a mature form (110-120 kDa). OPG functions both in bone remodeling, by inhibiting osteoclastogenesis through its interaction with receptor activator of the nuclear factor κB, and in survival, by acting as a decoy receptor for TNF-related apoptosis-inducing ligand, preventing its interaction with the functional death receptors; thus, allowing cells to escape cell death (Emery et al., 1998; Yasuda et al., 1999). Furthermore, recently, OPG is also reported as a marker for several diseases, including marker of atherosclerosis in diabetic patients (Zauli et al., 2009; Augoulea et al., 2013). Recently, a high level of OPG was an independent risk marker of all-cause mortality in a high-risk population of hemodialysis patients with previously documented cardiovascular disease and OPG is also regarded as one of biochemical markers of vascular calcification (Osorio et al., 2013; Winther et al., 2013).
Transglutaminase-2 (Tgase-2) is a multifunctional protein with both intracellular and extracellular functions (Lee et al., 2012). In addition to catalyzing Ca2+-dependent transamidation reactions, Tgase-2 can bind and hydrolyze GTP/GDP with a similar affinity and catalytic rate to the α subunit of large heterotrimeric G proteins and small Ras-type G proteins (Lorand and Graham, 2003; Mhaouty-Kodja, 2004; Lee et al., 2012). Tgase-2 activates NF-κB via polymerization of I-κB (Lee et al., 2012). Recently, we also showed that Tgase-2 is involved in JNK activation and PP2A downregulation (Park et al., 2011; Park et al., 2012). Transamidation activity of Tgase is in-creased in osteoarthritis (OA) joint cartilage (Rosenthal et al., 1997). Tgase-2 is also expressed hypertrophic chondrocytes (Nurminskaya et al., 2003). Tgase-2 is also an essential mediator of Interleukin-1β (IL-1β)-induced calcification, as well as hypertrophic differentiation and calcification in articular chondrocytes in vivo and in vitro (Johnson and Terkeltaub, 2005). Tgase-2 is described as a biomarker of OA severity in Hartley guinea pig knees (Johnson et al., 2004) Tgase-2 is central to induction of the arterial calcification (Johnson et al., 2008).
Several inflammatory mediators, including IL-1β, TNF-α and TGF-β, induce OPG expression (Brandstrom et al., 1998; Vidal et al., 1998; Thirunavukkarasu et al., 2001). Both p38 and ERK signaling pathways are involved in OPG expression and involvement of NF-κB is still controversial (Kobayashi- Sakamoto et al., 2004; Lambert et al., 2007; McCarthy et al., 2009). Therefore, details of signaling pathways of OPG are still unclear. Moreover, both of Tgase-2 and OPG are involved in calcification, relationship between OPG and Tgase-2 is not studied. Especially involvement of Tgase-2 in OPG expression is not reported.
In this report, we showed that Tgase-2 is induced in IL-1β or TPA-treated MG-63 cells with concomitant induction of OPG, and that Tgase-2 is involved in OPG expression.
MATERIALS AND METHODS
Materials
Recombinant human IL-1β was purchased from the R&D systems, Inc. (St. Louis, MO, USA). A 12-O-Tetradecanoylphorbol 13-acetate (TPA) and cystamine (CTM) were purchased from Sigma Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and DMEM were obtained from WelGENE Inc. (Daegu, South Korea). All other chemicals were of standard analytical grade. Antibody against OPG was obtained from the R&D systems, Inc. (St. Louis, MO, USA), antibody to Tgase-2 was from NeoMarkers (Fremont, CA, USA) and antibody to β-actin was from Cell Signaling Technology (Beverly, MA, USA).
Cell culture
Human osteosarcoma MG-63 cells were purchased from ATCC (Rockville, MD, USA). The cells cultured in DMEM supplemented with 10% (v/v) heat-activated fetal bovine serum, streptomycin (100 μg/ml), and penicillin (100 U/ml) at 37℃ in a 5% CO2 atmosphere.
Protein extracts from culture media or cells
Cell culture supernatants were collected and centrifuged at 1,000 rpm, 5 min. The cell free supernatant was incubated with 80% cold acetone at -20℃ for 60 min. Subsequently, the samples were centrifuged at 15,000 rpm at 4℃ for 30 minutes. The supernatants were carefully aspirated and the pellets were allowed to air dry at 23℃.
For whole-cell lysate, the cells were washed twice with cold PBS. The cells were lysed in a lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% triton X-100, 2 mM EDTA, 1% DOC (Deoxycholic acid), 0.1% SDS, 1 mM NaVO3, 10 mM NaF, 1 mM DTT] and centrifuged to yield whole-cell lysates. Protein concentration was measured using the Bradford method.
Western blot analysis
Aliquots of the lysates (20-30 μg of protein) were separated on a 4-12% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) with a glycine transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8), 10% MeOH (v/v)]. After blocking the nonspecific site with 5% non-fat dry milk, the membrane was then incubated with specific primary antibody in 3% BSA at 4℃ overnight. The membrane was further incubated for 60 min with a peroxidase-conjugated secondary antibody (1:2,000, Santa Cruz, CA, USA) at room temperature. Immunoactive proteins were detected using the WEST-ZOL (plus) Western Blot Detection System (iNtRON, Gyeonggi, Korea).
Cell proliferation assay
Cell proliferation was measured using the EZ-Cytox Cell viability assay kit (Daeillab service, Seoul, Korea). Briefly, 100 μl of cell suspension (3,000 cells per well) was added into each well of a 96-well plate. After the required incubation with the stimulants for indicated time, 10 μl of EZ-Cytox solution was added to each well of the plate and incubated at 37℃ for 2 h. The absorbance was measured by a spectrophotometer (Multscan, Thermo, USA) at 450 nm. The cell proliferation (%) was calculated using the formula: [As/Ac]×%. As: the absorbance of well containing cell, culture medium, EZ-Cytox solution and stimulants; Ac: the absorbance of well containing cell, culture medium and EZ-Cytox solution.
Tgase 2 gene silencing by small interfering RNA
A small interfering RNA (siRNA) duplex targeting human Tgase 2,5'-AAGAGCGAGAUGAUCUGGAAC-3' (Invitrogen) was introduced into the cells, using Lipofectamine 2,000 (Invitrogen), according to the manufacturer's instruction. Fortyeight hours after transfection, the cells were harvested, and a cytosolic faction was prepared in order to analyze the level of Tgase 2 and OPG by Western blotting. Cells incubated with Lipofectamine 2,000 and Stealth Negative control (Invitrogen) were employed as the negative control.
RESULTS
IL-1β increased Tgase-2 and OPG expression in MG-63 oste - osarcoma cells
OPG is induced by several cytokines, such as IL-1β and TNF-α (Vidal et al., 1998; Thirunavukkarasu et al., 2001). We chose IL-1β as an inducer. In order to investigate the expression of OPG in IL-1β-induced osteosarcoma cell line, MG-63 cells were treated with IL-1β for the indicated time. IL-1β time-dependently induced OPG and Tgase-2 expression in cell lysates and media of MG-63 cells as shown by a Western blot analysis (Fig. 1). Additional 110 kda band was found in the media of MG-63 cells (Fig. 1B).
Fig. 1. IL-1β increased Tgase-2 and OPG expression in MG-63 osteosarcoma cells. (A) IL-1β time-dependently induced OPG and Tgase-2 expression in cell lysates. MG-63 cells were treated with control media or IL-1β (5 ng/ml) for indicated time. Whole-cell lysates were subjected to 4-12% SDS-PAGE, and expression of Tgase-2 and OPG were determined by Western blotting. β-actin was used here as an internal control. (B) IL-1β time-dependently induced 110 kDa OPG expressions in the cell media. Culture media were subjected to 4-12% SDS-PAGE, and the expression of OPG was determined by Western blotting.
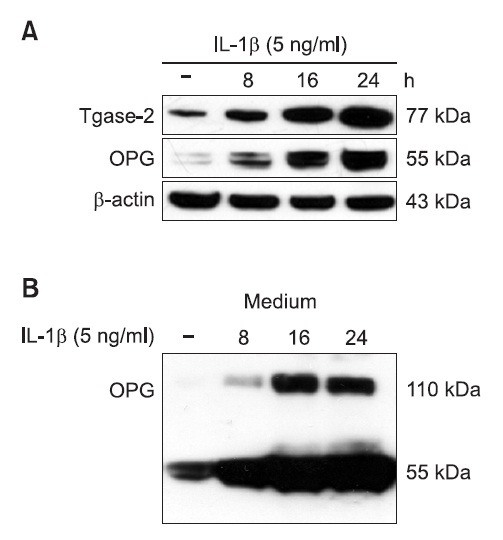
TPA increased Tgase-2 and OPG expression in MG-63 osteosarcoma cells
OPG is reported to be induced by TPA (Kondo et al., 2002). Therefore, we also examined the effect of TPA on the expression of OPG and Tgase-2 in MG-63 cells. TPA also time-dependently induced OPG and Tgase-2 expression (Fig. 2A). In contrast to media of IL-1β treated MG-63 cells, a 110 kda band was not found in TPA-treated media of MG-63 cells (Fig. 2B). The expression was not strong as seen with IL-1β.
Fig. 2. TPA increased Tgase-2 and OPG expression in MG-63 osteosarcoma cells. (A) TPA induced OPG and Tgase-2 expression in cell lysates. MG-63 cells were treated with control media or TPA (10 nM) for the indicated time. Whole-cell lysates were subjected to 4-12% SDS-PAGE, and expression of Tgase-2 and OPG were determined by Western blotting. β-actin was used here as an internal control. (B) TPA induced OPG expressions in the cell media. Culture mediums were subjected to 4-12% SDS-PAGE, and expression of OPG was determined by Western blotting.
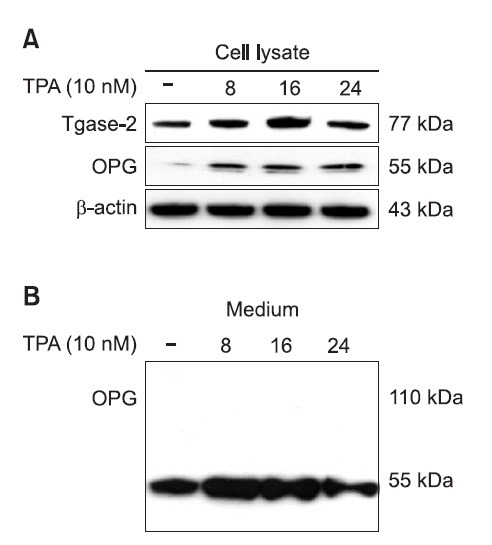
CTM and gene silencing of Tgase-2 suppressed IL-1β-induced OPG expression in MG-63 osteosarcoma cells
To investigate the role of Tgase-2 in OPG expression, we examined the effects of CTM, well known inhibitor, on the OPG expression in MG-63 cells. CTM dose-dependently suppressed the IL-1β-induced OPG expression of MG-63 cells (Fig. 3A). CTM also dose-dependently suppressed the TPA-induced OPG expression of MG-63 cells (Fig. 3B). To confirm the involvement of Tgase-2, we also examined the involvement of Tgase-2 in OPG expression by gene silencing of Tgase-2. To be consistent with the effects of CTM, gene silencing of Tgase-2 also significantly suppressed the expression of Tgase-2 in MG-63 cells (Fig. 3C).
Fig. 3. Effects of CTM, and gene silencing of Tgase-2 on the OPG expression in activated-MG-63 osteosarcoma cells. (A) CTM, dose-dependently suppressed the IL-1β-induced OPG expression of MG-63 cells. MG-63 cells were treated with CTM (0.1, 0.5, 1 mM) for 1 hr. Then, cells were treated with IL-1β for 24 h. Whole-cell lysates were prepared and the protein level was subjected to 4-12% SDS-PAGE, and expression of Tgase-2 and OPG were determined by Western blotting. (B) CTM, dose-dependently suppressed the TPA -induced OPG expression of MG-63 cells. MG-63 cells were treated with CTM (0.1, 0.5, 1 mM) for 1 hr. Then, cells were treated with TPA for 16 h. (C) Involvement of Tgase-2 in activated-MG-63 cells. MG-63 cells were transfected with siRNA of Tgase-2 (50 nM) for 48 hr. The cells were then treated with IL-1β (5 ng/ml) for 24 h. Whole-cell lysates were prepared and the protein level was subjected to 4-12% SDS-PAGE, and expression of Tgase-2 and OPG were determined by Western blotting.
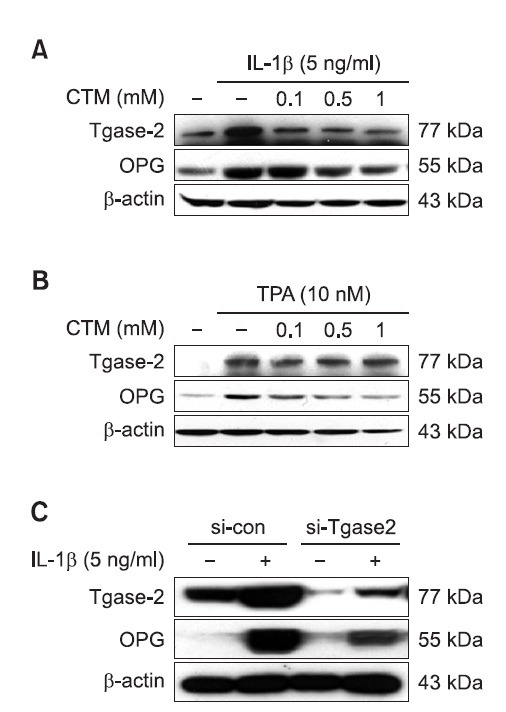
110 kDa protein is not detected by isopeptide specific monoclonal antibody
Tgase-2 is known to selectively cross-link osteopontin by isopeptide bonding (Higashikawa et al., 2007). Therefore, we examined whether the IL-1β-induced Tgase-2 crosslink the OPG protein using monoclonal antibody specific for isopeptide bond. However, we could not find the isopeptide bond at 110 kda but detect 77 kda, which is believed to be the band position of Tgase-2 (Fig. 4).
Fig. 4. Expression of N-epsilon gamma glutamyl lysine in IL-1β stimulated MG-63 osteosarcoma. MG-63 cells were treated with the control media or IL-1β (5 ng/ml) for the indicated time. Conditional media were prepared and the protein level was subjected to 4-12% SDS-PAGE, and expression of N-epsilon gamma glutamyl lysine was determined by Western blotting.
DISCUSSION
OPG is a soluble member of the tumor necrosis factor receptor superfamily, which potently inhibits RANKL-mediated osteoclastogenesis (Zauli et al., 2009). The administration of OPG-Fc counteracted bone loss in several preclinical models of cancers. In addition, several in vitro studies reported that full-length OPG also antagonizes the death-inducing ligand TRAIL (Emery et al., 1998; Truneh et al., 2000; Schneeweis et al., 2005). Furthermore, full-length OPG possesses RANKL-and TRAIL-independent biological properties, mainly related to the promotion of endothelial cell survival as well as angiogenesis and metastasis of breast cancer, ameloblastomas, and multiple myeloma (Holen et al., 2002; Shipman and Croucher, 2003; Zauli et al., 2009). Therefore, OPG expression in tumor cells seems to be important in aspects of hallmark of cancer, such as metastasis. However, the details of OPG expression in cancer cells are still unclear.
OPG expression was induced by IL-1β or TPA in MG-63 cells, which is consistent with the report by Mori et al. (2007). Several papers reported the expression of OPG in MG-63 cells via ELISA. In this paper, we examined the expression of OPG by Western blotting. In Fig. 1,2, we also examined the OPG expression in the media from IL-1β- or TPA-treated treated MG-63 cells by Western blotting. A 110 kda band, which corresponds to the double molecular weight of 55 kda OPG, were observed in the media of IL-1β treated MG-63 cells by Western blotting (Fig. 1B). Induction of OPG by IL-1β is stronger than that by TPA.
Tgase-2 was also highly expressed in MG-63 cells by IL- 1β or TPA. To our knowledge, IL-1β- or TPA-induced Tgase-2 expression was firstly observed in MG-63 cells (Fig. 1,2), although, the expression of Tgase-2 in MG-63 cells was also reported by Heath et al. (2001).
MG-63 cells treatment with IL-1β resulted in the phosphorylation of c-Jun NH2-terminal kinase (JNK), p38, and extracellular signal-regulated kinase (ERK). Both p38 and ERK, but not JNK, were needed for IL-1β induced OPG production. In contrast, NF-κB was not essential for IL-1β induction of OPG (Lambert et al., 2007), although it was reported that P. ginivalis upregulated the expression of OPG via a NF-κB dependent pathway (Kobayashi-Sakamoto et al., 2004). As such, we questioned the role of Tgase-2 in OPG expression since Tgase-2 is also induced by several inducers of OPG, such as IL-1β or TPA.
CTM, a Tgase inhibitor, suppressed the expression of OPG and Tgase-2 in IL-1β or TPA treated MG-63 cells (Fig. 2). It seemed that the inhibitory effects of CTM is strong in TPAtreated MG-63 cells since the expression of OPG by IL-1β is stronger that that by TPA (Fig. 2B).
We examined the gene silencing of Tgase-2 on the IL- 1β-induced OPG expression since CTM is not a specific Tgase-2 inhibitor. Gene silencing of Tgase-2 suppressed the expression of OPG (Fig. 3C). However, the detailed effects of Tgase-2 on OPG expression are still unknown. Tgase-2 induced the upregulation and polymerization of osteopontin, which is a mineral-binding protein abundant in most mineralized tissues and pathologically calcifying tissues, including blood vessels (Speer et al., 2005; Kaartinen et al., 2007). Polymerized osteopontin showed enhanced biological activity, such as cell adhesion, spreading, focal contact formation, and migration (Higashikawa et al., 2007). Therefore, OPG also might be a substrate and polymerized by Tgase-2. Hence, we speculated that a 110 kda band might be the result of Tgase-2’s action on OPG. Thus, we tested the existence of the isopeptide bond in a 110 kda band since isopeptide bond is formed by Tgase-2. However, we did not observe the isopeptide bond in 110 kda position (Fig. 4). Therefore, a 110 kda band might be a homodimer form of OPG and disulfide linkage might be involved.
Recently, the roles of OPG in cancer are reported in several groups. For example, OPG overexpression by breast cancer cells enhanced tumor growth, following orthotopic inoculation (Fisher et al., 2006). Investigation of various human cancers demonstrated endothelial OPG expression in 59% of malignant tumors (n=512), but in contrast, OPG was absent in endothelial cells associated with benign tumors and normal tissues (n=178) (Cross et al., 2006). OPG functions as a paracrine survival factor for human myeloma cells (Shipman and Croucher, 2003).
Osteosarcoma is the most common skeletal sarcoma, which appears more commonly in the second to third decades of life. Although the outcome of osteosarcoma treatment has been improved by the chemotherapy-based combination therapy, progress has been painfully slow for the past 20 years (De Toni et al., 2008). Therefore, Tgase-2 expression and involvement in OPG expression in MG-63 cells might be a clue for understanding the role of OPG in osteosarcoma cancer (Fig. 1,2).
All together, we showed that Tgase-2 is involved in OPG expression and Tgase-2 might be a new way of controlling the expression of OPG in cancer cells.
Acknowledgments
This work was supported by the GRRC program of Gyeonggi province ([GRRC-DONGGUK2012-B01] Development of new health supplements/therapeutics for neurodegenerative diseases).
References
- 1.Augoulea A., Vrachnis N., Lambrinoudaki I., Dafopoulos K., Iliodromiti Z., Daniilidis A., Varras M., Alexandrou A., Deligeoroglou E., Creatsas G. Osteoprotegerin as a marker of ath-erosclerosis in diabetic patients. Int. J. Endocrinol. (2013);2013:182060. doi: 10.1155/2013/182060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandstrom H., Jonsson K. B., Vidal O., Ljunghall S., Ohlsson C., Ljunggren O. Tumor necrosis factor-alpha and -beta upregulate the levels of osteoprotegerin mRNA in human osteosarcoma MG-63 cells. Biochem. Biophys. Res. Commun. (1998);248:454–457. doi: 10.1006/bbrc.1998.8993. [DOI] [PubMed] [Google Scholar]
- 3.Cross S. S., Yang Z., Brown N. J., Balasubramanian S. P., Evans C. A., Woodward J. K., Neville-Webbe H. L., Lippitt J. M., Reed M. W., Coleman R. E., Holen I. Osteoprotegerin (OPG)--a potential new role in the regulation of endothelial cell phenotype and tumour angiogenesis? Int. J. Cancer . (2006);118:1901–1908. doi: 10.1002/ijc.21606. [DOI] [PubMed] [Google Scholar]
- 4.De Toni E. N., Thieme S. E., Herbst A., Behrens A., Stieber P., Jung A., Blum H., Goke B., Kolligs F. T. OPG is regulated by beta-catenin and mediates resistance to TRAIL-induced apoptosis in colon cancer. Clin. Cancer Res. (2008);14:4713–4718. doi: 10.1158/1078-0432.CCR-07-5019. [DOI] [PubMed] [Google Scholar]
- 5.Emery J. G., McDonnell P., Burke M. B., Deen K. C., Lyn S., Silverman C., Dul E., Appelbaum E. R., Eichman C., DiPrinzio R., Dodds R. A., James I. E., Rosenberg M., Lee J. C., Young P. R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. (1998);273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J. L., Thomas-Mudge R. J., Elliott J., Hards D. K., Sims N. A., Slavin J., Martin T. J., Gillespie M. T. Osteoprotegerin overexpression by breast cancer cells enhances orthotopic and osseous tumor growth and contrasts with that delivered therapeutically. Cancer Res. (2006);66:3620–3628. doi: 10.1158/0008-5472.CAN-05-3119. [DOI] [PubMed] [Google Scholar]
- 7.Heath D. J., Downes S., Verderio E., Griffin M. Characterization of tissue transglutaminase in human osteoblast-like cells. J. Bone Miner. Res. . (2001);16:1477–1485. doi: 10.1359/jbmr.2001.16.8.1477. [DOI] [PubMed] [Google Scholar]
- 8.Higashikawa F., Eboshida A., Yokosaki Y. Enhanced biological activity of polymeric osteopontin. FEBS Lett. . (2007);581:2697–2701. doi: 10.1016/j.febslet.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Holen I., Croucher P. I., Hamdy F. C., Eaton C. L. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. (2002);62:1619–1623. [PubMed] [Google Scholar]
- 10.Johnson K., Svensson C. I., Etten D. V., Ghosh S. S., Murphy A. N., Powell H. C., Terkeltaub R. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. (2004);50:1216–1225. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- 11.Johnson K. A., Polewski M., Terkeltaub R. A. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ. Res. (2008);102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson K. A., Terkeltaub R. A. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J. Biol. Chem. (2005);280:15004–15012. doi: 10.1074/jbc.M500962200. [DOI] [PubMed] [Google Scholar]
- 13.Kaartinen M. T., Murshed M., Karsenty G., McKee M. D. Osteopontin upregulation and polymerization by transglutaminase 2 in calcifi ed arteries of Matrix Gla protein-deficient mice. J. Histochem. Cytochem. (2007);55:375–386. doi: 10.1369/jhc.6A7087.2006. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi-Sakamoto M., Hirose K., Isogai E., Chiba I. NF-kappaB-dependent induction of osteoprotegerin by Porphyromonas gingivalis in endothelial cells. Biochem. Biophys. Res. Commun. (2004);315:107–112. doi: 10.1016/j.bbrc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 15.Kondo H., Guo J., Bringhurst F. R. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/ parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J. Bone Miner. Res. (2002);17:1667–1679. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- 16.Lambert C., Oury C., Dejardin E., Chariot A., Piette J., Malaise M., Merville M. P., Franchimont N. Further insights in the mechanisms of interleukin-1beta stimulation of osteoprotegerin in osteoblast-like cells. J. Bone Miner. Res. (2007);22:1350–1361. doi: 10.1359/jbmr.070508. [DOI] [PubMed] [Google Scholar]
- 17.Lee H. L., Park M. K., Bae H. C., Yoon H. J., Kim S. Y., Lee C. H., Lee C. H. Transglutaminase-2 is involved in all-trans retinoic acid-induced invasion and matrix metalloproteinases expression of SH-SY5Y neuroblastoma cells via NF-κB pathway. Biomol.Ther. (2012);20:286–292. doi: 10.4062/biomolther.2012.20.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorand L., Graham R. M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. (2003);4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy H. S., Williams J. H., Davie M. W., Marshall M. J. Platelet-derived growth factor stimulates osteoprotegerin production in osteoblastic cells. J. Cell. Physiol. (2009);218:350–354. doi: 10.1002/jcp.21600. [DOI] [PubMed] [Google Scholar]
- 20.Mhaouty-Kodja S. Ghalpha/tissue transglutaminase 2: an emer ging G protein in signal transduction. Biol. Cell . (2004);96:363–367. doi: 10.1016/j.biolcel.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Mori K., Le Goff B., Berreur M., Riet A., Moreau A., Blanchard F., Chevalier C., Guisle-Marsollier I., Leger J., Guicheux J., Masson M., Gouin F., Redini F., Heymann D. Human osteosarcoma cells express functional receptor activator of nuclear factorkappa B. J. Pathol. (2007);211:555–562. doi: 10.1002/path.2140. [DOI] [PubMed] [Google Scholar]
- 22.Nurminskaya M., Magee C., Faverman L., Linsenmayer T. F. Chondrocyte-derived transglutaminase promotes maturation of preosteoblasts in periosteal bone. Dev. Biol. (2003);263:139–152. doi: 10.1016/s0012-1606(03)00445-7. [DOI] [PubMed] [Google Scholar]
- 23.Osorio A., Ortega E., Torres J. M., Sanchez P., Ruiz-Requena E. Biochemical markers of vascular calcifi cation in elderly hemodialysis patients. Mol. Cell. Biochem. (2013);374:21–27. doi: 10.1007/s11010-012-1500-y. [DOI] [PubMed] [Google Scholar]
- 24.Park M. K., Lee H. J., Shin J., Noh M., Kim S. Y., Lee C. H. Novel participation of transglutaminase-2 through c-Jun Nterminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim. Biophys. Acta. (2011);1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Park M. K., Park Y., Shim J., Lee H. J., Kim S., Lee C. H. Novel involvement of leukotriene B(4) receptor 2 through ERK activation by PP2A down-regulation in leukotriene B(4)-induced keratin phosphorylation and reorganization of pancreatic cancer cells. Biochim. Biophys. Acta. (2012);1823:2120–2129. doi: 10.1016/j.bbamcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal A. K., Derfus B. A., Henry L. A. Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum. (1997);40:966–970. doi: 10.1002/art.1780400526. [DOI] [PubMed] [Google Scholar]
- 27.Schneeweis L. A., Willard D., Milla M. E. Functional dissection of osteoprotegerin and its interaction with receptor activator of NF-kappaB ligand. J. Biol. Chem. . (2005);280:41155–41164. doi: 10.1074/jbc.M506366200. [DOI] [PubMed] [Google Scholar]
- 28.Shipman C. M., Croucher P. I. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. (2003);63:912–916. [PubMed] [Google Scholar]
- 29.Simonet W. S., Lacey D. L., Dunstan C. R., Kelley M., Chang M. S., Luthy R., Nguyen H. Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H. L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T. M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W. J. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell . (1997);89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 30.Speer M. Y., Chien Y. C., Quan M., Yang H. Y., Vali H., McKee M. D., Giachelli C. M. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc. Res. . (2005);66:324–333. doi: 10.1016/j.cardiores.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Thirunavukkarasu K., Miles R. R., Halladay D. L., Yang X., Galvin R. J., Chandrasekhar S., Martin T. J., Onyia J. E. Stimulation of osteoprotegerin (OPG) gene expression by transforming growth factor-beta (TGF-beta) J. Biol. Chem. (2001);276:36241–36250. doi: 10.1074/jbc.M104319200. [DOI] [PubMed] [Google Scholar]
- 32.Truneh A., Sharma S., Silverman C., Khandekar S., Reddy M. P., Deen K. C., McLaughlin M. M., Srinivasula S. M., Livi G. P., Marshall L. A., Alnemri E. S., Williams W. V., Doyle M. L. Temperature-sensitive differential affinity of TRAIL for its receptors. J. Biol. Chem. (2000);275:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 33.Tsuda E., Goto M., Mochizuki S., Yano K., Kobayashi F., Morinaga T., Higashio K. Isolation of a novel cytokine from human fibroblasts that specifi cally inhibits osteoclastogenesis. Biochem. Biophys. Res. Commun. (1997);234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- 34.Vidal O. N., Sjogren K., Eriksson B. I., Ljunggren O., Ohlsson C. Osteoprotegerin mRNA is increased by interleukin-1 alpha in the human osteosarcoma cell line MG-63 and in human osteoblast-like cells. Biochem. Biophys. Res. Commun. (1998);248:696–700. doi: 10.1006/bbrc.1998.9035. [DOI] [PubMed] [Google Scholar]
- 35.Winther S., Christensen J. H., Flyvbjerg A., Schmidt E. B., Jorgensen K. A., Skou-Jorgensen H., Svensson M. Osteoprotegerin and mortality in hemodialysis patients with cardiovascular disease. Osteoprotegerin; (2013). [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Goto M., Mochizuki S. I., Tsuda E., Morinaga T., Udagawa N., Takahashi N., Suda T., Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone . (1999);25:109–113. doi: 10.1016/s8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 37.Zauli G., Melloni E., Capitani S., Secchiero P. Role of full-length osteoprotegerin in tumor cell biology. Cell. Mol. Life Sci. (2009);66:841–851. doi: 10.1007/s00018-008-8536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


