Abstract
Ionizing radiation can induce cellular oxidative stress through the generation of reactive oxygen species, resulting in cell damage and cell death. The aim of this study was to determine whether the antioxidant effects of the flavonoid fisetin (3,7,3',4'-tetrahydroxyflavone) included the radioprotection of cells exposed to γ-irradiation. Fisetin reduced the levels of intracellular reactive oxygen species generated by γ-irradiation and thereby protected cells against γ-irradiation-induced membrane lipid peroxidation, DNA damage, and protein carbonylation. In addition, fisetin maintained the viability of irradiated cells by partially inhibiting γ-irradiation-induced apoptosis and restoring mitochondrial membrane potential. These effects suggest that the cellular protective effects of fisetin against γ-irradiation are mainly due to its inhibition of reactive oxygen species generation.
Keywords: Apoptosis, Cell damage, Fisetin, γ-irradiation, Reactive oxygen species
INTRODUCTION
Ionizing radiation induces high cellular levels of reactive oxygen species (ROS), including superoxide anions, hydroxyl radicals, and hydrogen peroxide (Dubner et al., 1995), resulting in oxidative stress and cellular injury (Sies, 1983). The damage to irradiated cells in part reflects the macromolecular targets of ROS, primarily DNA, protein, and lipid. In addition, ROS-mediated disruption of the mitochondrial membrane is a trigger for apoptosis (programmed cell death), in which apoptotic factors are released from the damaged mitochondria into the cytosol (Simon et al., 2000).
Flavonoids are a group of low-molecular-weight polyphenolic compounds synthesized by members of the plant kingdom. Their wide-ranging biological activities include antioxidant, anti-inflammatory, and anti-tumor effects (Middleton and Kandaswami, 1992; Middleton et al., 2000; Beecher, 2003). The flavonoid fisetin (3,7,3',4'-tetrahydroxyflavone; Fig. 1A) is a natural component in many fruits and vegetables, such as strawberry, apple, persimmon, grape, onion, and cucumber (Arai et al., 2000), and it has been demonstrated to have anti-cancer (Sung et al., 2007), anti-angiogenic (Fotsis et al., 1998), neuroprotective (Zbarsky et al., 2005), and neurotrophic (Maher et al., 2006) effects. While the antioxidant activity of fisetin has been reported in human retinal pigment epithelial cells (Hanneken et al., 2006) and macrophages (Wang et al., 2006), whether this activity also mediates protection against oxidative stress and cell damage induced by γ-irradiation has not yet been determined. Thus, this study investigated the ability of fisetin to inhibit cell damage in Chinese hamster lung fibroblasts exposed to γ-irradiation, and the underlying mechanisms conferring protection.
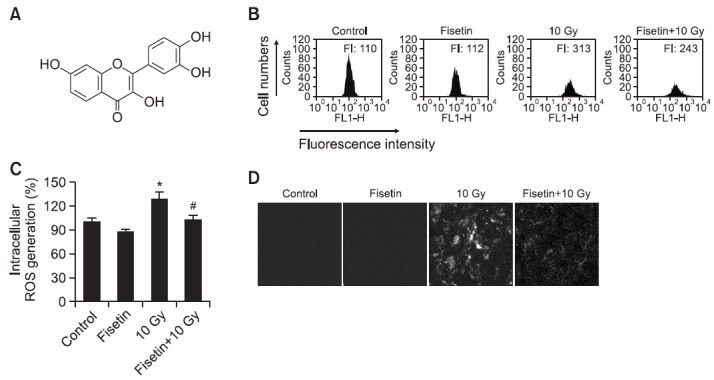
Fig. 1. Effect of fisetin on the levels of intracellular ROS generated following γ-irradiation (A) Chemical structure of fisetin (3,7,3',4'-tetrahydroxyflavone) (B-D) ROS-scavenging effects of fisetin in cells exposed to γ-irradiation. Cells were treated with 2.5 μg/ml fisetin and 1 h later were exposed to 10 Gy of γ-irradiation. After incubation for 24 h, cells were stained with DCF-DA and intracellular ROS were detected using (B) flow cytometry, (C) fluorescence spectrophotometry, and (D) confocal microscopy. *Significantly different from non-irradiated control cells (p<0.05) and #significantly different from 10 Gy-irradiated cells (p<0.05).
MATERIALS AND METHODS
Reagents
Fisetin was purchased from Fluka (Buchs, Switzerland). 2′,7′-Dichlorodihydrofluorescein diacetate (DCF-DA) and Hoechst 33342 were purchased from Sigma (St. Louis, MO, USA). 5,5′, 6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1), and diphenyl-1-pyrenylphosphine (DPPP) were purchased from Invitrogen (Carlsbad, CA, USA, and Poole, Dorset, UK, respectively). Antibodies against Bcl-2, Bax, caspase- 9, and caspase-3 were from Santa Cruz (Santa Cruz, CA, USA).
Cell culture and irradiation
Chinese hamster lung fibroblasts (V79-4) from the American Type Culture Collection (Rockville, MD, USA) were grown in Dulbecco's modified Eagle's medium containing 10% heatinactivated fetal calf serum, streptomycin (100 μg/ml), and penicillin (100 units/ml). The cultures were maintained in a 37℃ incubator in a humidified atmosphere of 5% CO2. Irradiated cells were exposed to 10 Gy of γ-irradiation at 1.5 Gy/min using a 60Co γ-ray source (MDS Nordion C-188 standard source, Jeju National University, Jeju, Republic of Korea). Control cells were cultured and treated as described but were not irradiated.
Intracellular ROS
Cells were pre-treated (or not) with 2.5 μg/ml fisetin for 1 h and then irradiated as described above. After further incubation for 24 h at 37℃, DCF-DA was added to a final concentration of 25 μM and the fluorescence of 2′,7′-dichlorofluorescein was measured with a flow cytometer (Becton Dickinson, Mountain View, CA, USA) and a Perkin Elmer LS-5B spectrofluorometer (Rosenkranz et al., 1992), respectively. In addition, the generation of intracellular ROS was imaged. First, 2×105 cells/well were seeded into a cover-slip-loaded 6-well plate. Sixteen hours after plating, cells were treated with fisetin for 1 h and then irradiated with 10 Gy of γ-rays. Twenty-four hours later, DCF-DA was added to each well to a final concentration of 100 μM and cells were incubated for an additional 30 min at 37℃. After washing with phosphate-buffered saline (PBS), the stained cells were mounted in mounting medium (DAKO, Carpinteria, CA, USA) on a microscope slide and were then observed by confocal microscopy, and images were collected using the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss, Jena, Germany).
Lipid peroxidation assay
Lipid peroxidation was estimated using the fluorescent probe DPPP as described by Okimoto et al. (2000). Image analysis was carried out as follows: 2×105 cells/well were seeded into a cover-slip-loaded 4-well glass slide and then treated (or not) with fisetin for 1 h, after which they were irradiated as described above. Forty-eight hours later, DPPP was added to each well to a final concentration of 5 μM and the plate was incubated for an additional 15 min in the dark. The cells were then processed and imaged as described in the previous section. Lipid peroxidation was also assayed by the thiobarbituric acid (TBA) reaction (Ohkawa et al., 1979). Cells were treated with fisetin for 1 h and then subjected to 10 Gy of γ-irradiation. After incubation for 48 h at 37℃, cells were washed with cold PBS, scraped, and then homogenized in ice-cold 1.15% KCl. A 100 μl aliquot of the resulting lysate was mixed with 0.2 ml of 8.1% sodium dodecylsulfate, 1.5 ml of 20% acetic acid (pH 3.5), and 1.5 ml of 0.8% TBA. The mixture was made up to a final volume of 4 ml with distilled water, heated to 95℃ for 2 h, and then cooled to room temperature. Five ml of a mixture of n-butanol and pyridine (15:1, v/v) was added to each sample with shaking. The sample was centrifuged at 1,000×g for 10 min, and the supernatant was isolated and its absorbance at 532 nm was measured with a spectrophotometer. The amount of TBA-reactive substance (TBARS) was determined using a standard curve prepared with 1,1,3,3-tetrahydroxypropane.
Single-cell gel electrophoresis (comet assay)
The degree of oxidative DNA damage was determined by an alkaline comet assay (Singh, 2000; Rajagopalan et al., 2003). Cells were pre-treated (or not) with fisetin and subjected to 10 Gy of γ-irradiation, and then harvested after 48 h. The resulting cell suspensions were mixed with 75 μl of 0.5% low melting agarose at 39℃ and spread on fully-frosted microscope slides pre-coated with 200 μl of 1% normal melting agarose (NMA). After solidification of the agarose, the slides were covered with another 75 μl of 0.5% low melting agarose, immersed in a lysis solution (2.5 M NaCl, 100 mM Na-EDTA, 10 mM Tris, 1% Triton X-100, and 10% DMSO, pH 10) for 1 h at 4℃, and then subjected to electrophoresis in 300 mM NaOH and 10 mM Na-EDTA (pH 13) for 40 min to allow for DNA unwinding and expression of alkali-labile damage. Next, an electrical field was applied (300 mA, 25 V) for 20 min at 4℃ to draw the negatively charged DNA toward the anode. After electrophoresis, the slides were washed three times for 5 min at 4℃ in a neutralizing buffer (0.4 M Tris, pH 7.5), stained with 75 μl of propidium iodide (20 μg/ml), and observed with a fluorescence microscope and image analyzer (Kinetic Imaging, Komet 5.5, UK). The percentage of total fluorescence in the tail and the tail length were determined in 50 cells per slide.
Protein carbonyl formation
Cells were plated and treated (or not) with fisetin, and 1 h later cells were subjected to 10 Gy of γ-irradiation, followed by incubation at 37℃ for 48 h. The amount of protein carbonyl formation was determined using an Oxiselect protein carbonyl ELISA kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. Cellular protein was isolated using protein lysis buffer (50 mM Tris (pH 7.5), 10 mM EDTA (pH 8), and 1 mM phenylmethanesulfonyl fluoride) and quantified using a spectrophotometer.
Cell viability
The effect of fisetin on cell viability was determined in a [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide (MTT) assay, which is based on the reduction of a tetrazolium salt by mitochondrial dehydrogenase in viable cells (Carmichael et al., 1987). Cells were treated (or not) with fisetin and subjected to γ-irradiation as described above. Forty eight hours later, 50 μl of MTT stock solution (2 mg/ml) was added to each well, to generate a total reaction volume of 200 μl. After incubation for 4 h, the plate was centrifuged at 800×g for 5 min, and the supernatants were discarded. The formazan crystals remaining in each well were dissolved in 150 μl of DMSO and the absorbance at 540 nm was measured with a scanning multi-well spectrophotometer. Cell viability was also assessed by trypan blue exclusion assay. Briefly, cells were treated (or not) with fisetin and subjected to γ-irradiation and plated onto 24-well plates (2×105 cells/well). Forty eight hours later, cells were trypsinized, pelleted by centrifugation and resupended in serum free media and stained with trypan blue. Dead (stained blue) and live (unstained) cells were counted on Neubauer hemacytometer.
Nuclear staining with Hoechst 33342
Cells were treated with fisetin and then exposed to γ-irradiation as described above. After incubation for 48 h at 37℃, 1.5 μl of the DNA-specific fluorescent dye Hoechst 33342 (stock 10 mg/ml) was added to each well and the plates were incubated for 10 min at 37℃. Stained cells were visualized by fluorescence microscopy and imaged with a CoolSNAPPro color digital camera to determine the degree of nuclear condensation.
Mitochondrial membrane potential (Δψm) analysis
To measure Δψm, cells treated (or not) with fisetin were exposed to 10 Gy of γ-irradiation. After incubation for 48 h at 37℃, cells were harvested, washed, and then incubated with the mitochondrial-membrane-permeable dye JC-1 (10 μg/ml in PBS) for 15 min at 37℃. The stained cells were analyzed by flow cytometry (Troiano et al., 2007). In addition, cells were harvested and processed as described above except that cells were incubated with the dye for 30 min. The stained cells were washed with PBS and the cover-slips were mounted onto microscope slides using mounting medium. The slides were examined using a confocal microscope, and images were collected using the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss, Jena, Germany) (Cossarizza et al., 1993).
Western blot analysis
Cells were treated with fisetin, exposed to 10 Gy of γ-irradiation, and then incubated for a further 48 h at 37℃. Harvested cells were washed with PBS, lysed in lysis buffer (120 mM NaCl, 40 mM Tris (pH 8), and 0.1% NP-40), and centrifuged at 13,000×g for 15 min. Aliquots of the lysates (50 μg of protein) were boiled at 95℃ for 5 min and electrophoresed on SDS-PAGE gels. Proteins in the gels were transferred onto nitrocellulose membranes for blotting and the membranes were subsequently probed with primary antibodies to Bcl- 2, Bax, caspase-9, and caspase-3 and then with secondary immunoglobulin G horseradish peroxidase conjugates. After enhanced chemiluminescence using a Western blotting detection kit (Amersham, Buckinghamshire, UK), the protein bands were visualized with a luminescent image analyzer.
Statistical analysis
All values are expressed as means ± standard error of the mean (S.E.M.) based on triplicate measurements. The data were subjected to an analysis of variance (ANOVA) using Tukey’s test to analyze differences. Significance was defined as p<0.05.
RESULTS
The effects of fisetin on γ-irradiation-induced ROS generation
In our system, the optimal dose of fisetin was 2.5 μg/ml, as higher concentrations resulted in cytotoxicity (data not shown). Accordingly, this dose was used to study the radicalscavenging effects of fisetin on the ROS generated in irradiated Chinese hamster lung fibroblasts. Twenty-four hours after irradiation, flow cytometry showed a peak fluorescence intensity of 243 (arbitrary units) in fisetin-treated irradiated cells compared to 313 in irradiated cells not treated with the flavonoid (Fig. 1B). ROS levels following γ-irradiation, as detected spectrofluorometrically, were lower in cells pre-treated with fisetin than in cells that were not pre-treated (Fig. 1C). These results were confirmed by confocal microscopy, which showed that red fluorescence intensity, indicative of ROS, was lower in fisetin-treated γ-irradiated cells than in non-treated γ-irradiated cells (Fig. 1D).
Effects of fisetin on γ-irradiation-induced lipid peroxidation, DNA damage, and protein oxidation
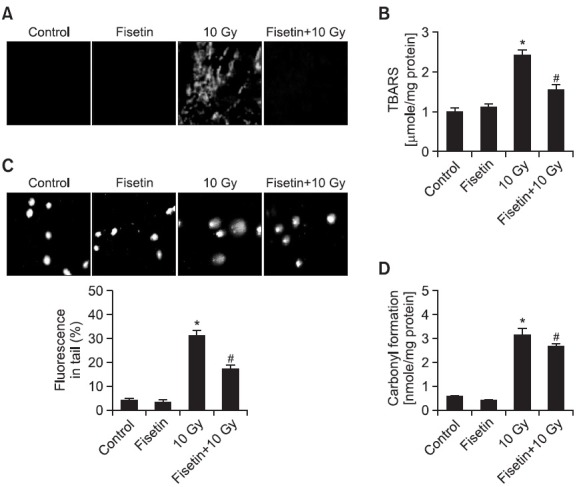
The ability of fisetin to inhibit membrane lipid peroxidation, cellular DNA damage, and protein oxidation in γ-irradiated cells was investigated. Lipid peroxidation in Chinese hamster lung fibroblasts, as visualized microscopically in cells stained with the fluorescent probe DPPP and by the generation of TBARS, was induced by γ-irradiation but this induction was partially inhibited by pre-treatment with fisetin (Fig. 2A and 2B). The damage to cellular DNA induced by γ-irradiation was detected using an alkaline comet assay. In irradiated fisetin-treated cells, 17% of the DNA was in the comet tail compared to 32% in irradiated untreated cells (Fig. 2C). Protein carbonyl formation, which is a biomarker for cellular oxidative damage (Stadtman, 1993), was detected in cells exposed to γ-irradiation but this was partially prevented in fisetin-treated cells (Fig. 2D).
Fig. 2. Inhibitory effects of fisetin on γ-irradiation-induced cellular damage. Cells were treated with 2.5 μg/ml fisetin and 1 h later were exposed to 10 Gy of γ-irradiation. After incubation for 48 h, lipid peroxidation was assayed by (A) DPPP fluorescence and (B) the level of TBARS. *Significantly different from non-irradiated control cells (p<0.05) and #significantly different from 10 Gy-irradiated cells (p<0.05). (C) Representative images showing cellular DNA damage as detected in an alkaline comet assay. *Significantly different from the control (p<0.05) and #significantly different from irradiated cells (p<0.05). (D) Protein oxidation as determined by measuring the level of carbonyl formation. *Significantly different from control cells (p<0.05) and #significantly different from irradiated cells (p<0.05).
Effect of fisetin on γ-irradiation-induced apoptotic cell death
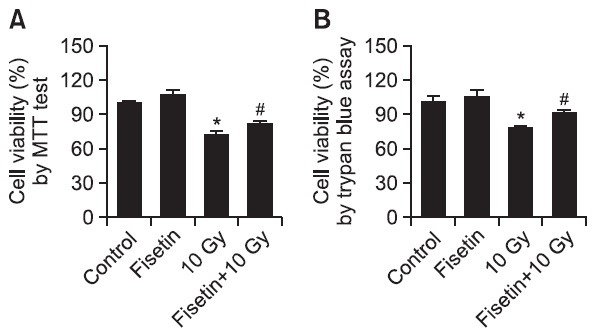
The protective effect of fisetin on cell survival in irradiated cells was also measured by using MTT test and trypan blue exclusion dye assay. MTT results demonstrated that whereas 72% of untreated cells survived after γ-irradiation, this was increased to 82% following fisetin pre-treatment (Fig. 3A). Trypan blue exclusion dye assay results were consistent to the MTT results; 77% of cell viability in γ-irradiated cells and 90% of cell viability in γ-irradiated cells with fisetin treatment (Fig. 3B).
Fig. 3. Inhibitory effect of fisetin on γ-irradiation-induced cell death. Cells were treated with 2.5 μg/ml fisetin and 1 h later were exposed to 10 Gy of γ-irradiation. Cell viability was determined 48 h later using (A) the MTT assay and (B) trypan blue exclusion assay. *Significantly different from control cells (p<0.05) and #significantly different from 10 Gy-irradiated cells (p<0.05).
To determine whether the cytoprotective effect of fisetin involves the inhibition of apoptosis induced by γ-ray exposure, the nuclei of irradiated cells pre-treated or not with fisetin were stained with Hoechst 33342 and assessed by microscopy. Non-irradiated control cells had intact nuclei while there was significant nuclear fragmentation in irradiated cells, indicative of apoptosis (Fig. 4A). However, there was a significant reduction in nuclear fragmentation in irradiated cells pre-treated for 1 h with fisetin. These morphological observations were confirmed by qualitative assessment of mitochondrial membrane potential (Δψm). During apoptosis, mitochondrial membrane pores become leaky (Zamzami et al., 1995), which induces the loss of Δψm (Zamzami et al., 1996; Cai et al., 1998). In irradiated cells stained with JC-1, FL-1 fluorescence intensity was increased indicating a loss of Δψm (Fig. 4B), which was partially prevented in fisetin-treated irradiated cells. These changes in Δψm could be followed microscopically. JC-1 forms oligomeric mitochondrial aggregates that emit red fluorescence in non-apoptotic cells, whereas JC-1 remains a monomer that emits green fluorescence in apoptotic cells (Reers et al., 1991). While non-irradiated control cells exhibited intense red fluorescence localized to the mitochondria, there was a decrease in mitochondrial red fluorescence and an increase in green fluorescence in γ-irradiated cells, consistent with a disruption of Δψm (Fig. 4C). However, the level of red fluorescence was much higher and the level of green fluorescence was lower in cells treated with fisetin for 1 h prior to irradiation than in non-treated irradiated cells.
Fig. 4. Mechanisms by which fisetin protects against γ-irradiation-induced apoptotic cell death. Cells were treated 2.5 μg/ml fisetin and 1 h later were exposed to 10 Gy of γ-irradiation, and then incubated for 48 h. (A) Formation of apoptotic bodies (arrows) was examined in cells stained with Hoechst 33342. (B, C) The mitochondrial membrane potential (Δψm) of JC-1-stained cells was analyzed by flow cytometry and confocal microscopy. (D) Cell lysates were electrophoresed and Bcl-2, Bax, cleaved caspase-9, and cleaved caspase-3 were detected by immunoblot analysis using the corresponding.
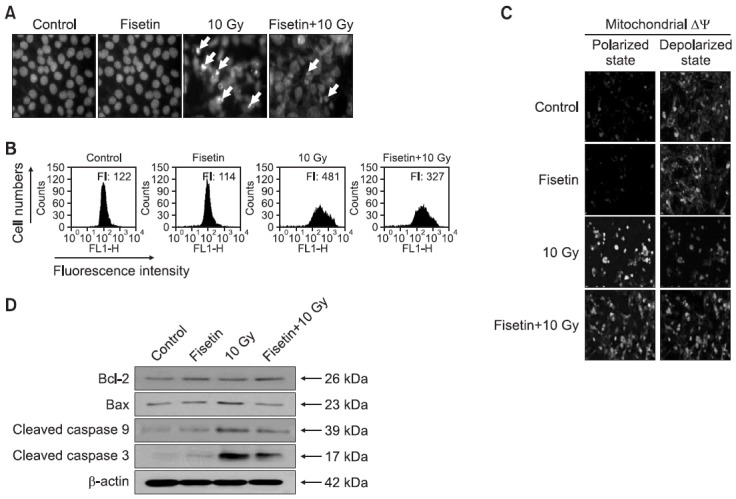
Disruption of Δψm causes the release of cytochrome c and subsequently caspase activation (Regula et al., 2003). During apoptosis, Bcl-2 (a negative regulator of apoptosis) prevents mitochondrial membrane pore formation whereas Bax (a positive regulator of apoptosis) has the opposite effect (Zamzami et al., 1995). Bax expression was higher and Bcl-2 expression was lower in γ-irradiated cells than in control cells (Fig. 4D), suggesting that the γ-irradiation-induced loss of Δψm follows downregulation of Bcl-2 and upregulation of Bax. Mitochondrial membrane disruption by γ-irradiation was followed by activation of caspase-9 (39 kDa) and its target, caspase-3 (17 kDa), both of which were reduced in fisetin-treated irradiated cells (Fig. 4D).
DISCUSSION
Fisetin is a flavonoid with high trolox-equivalent antioxidative activity. As a hydrophobic compound, it readily traverses the cell membrane to accumulate in cells, where it exerts its antioxidant effects (Ishige et al., 2001).
The exposure of cells to ionizing radiation can lead to the increased generation of ROS, including hydroxyl radicals, superoxide anions, singlet oxygen, and hydrogen peroxide, which are major effectors of cellular damage. ROS attack vital cellular structures, in particular the cell membrane, DNA, and protein, and the damage they cause accordingly can be lethal (Riley, 1994; Halliwell and Gutteridge, 1999). ROS-scavenging agents, which include flavonoids, act as radioprotectors (Spitz et al., 2004; Gudkov et al., 2006). In this study, fisetin was found to inhibit DNA damage in cells exposed to γ-irradiation and to protect cell membrane lipids and cellular proteins from radiation-induced peroxidation and carbonylation, respectively. Together, these effects of fisetin increased the viability of irradiated cells. γ-irradiation was previously shown to induce cell death via apoptosis (Kim et al., 2007; Lee et al., 2007), as evidenced by the formation of apoptotic bodies, the loss of Δψm, and changes in apoptosis-related protein expression. During apoptosis, the loss of mitochondrial membrane integrity is due to ROS generation; however, we found that fisetin partially restored cell viability by inhibiting γ-irradiationinduced Δψm disruption.
Thus, while fisetin was previously shown to exert anti-cancer (Sung et al., 2007), anti-angiogenic (Fotsis et al., 1998), neuroprotective (Zbarsky et al., 2005), neurotrophic (Maher et al., 2006), and antioxidant (Hanneken et al., 2006) activities, our data add to this list by demonstrating that fisetin has cytoprotective effects against γ-irradiation-induced apoptosis through its ROS-scavenging abilities.
Acknowledgments
This research was supported by the 2013 scientific promotion program funded by Jeju National University.
References
- 1.Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary intakes of flavonols, flavones and isofl avones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. (2000);130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 2.Beecher G. R. Overview of dietary flavonoids: nomenclature, occurrence and intake. J. Nutr. (2003);133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 3.Cai J., Yang J., Jones D. P. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim. Biophys. Acta. (1998);1366:139–149. doi: 10.1016/S0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. (1987);47:936–942. [PubMed] [Google Scholar]
- 5.Cossarizza A., Baccarani-Contri M., Kalashnikova G., Franceschi C. A new method for the cytofl uorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidaz olcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. (1993);197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- 6.Dubner D., Gisone P., Jaitavich I., Perez M. Free radicals production and estimation of oxidative stress related to gamma irradiation. Biol. Trace Elem. Res. (1995);47:265–270. doi: 10.1007/BF02790126. [DOI] [PubMed] [Google Scholar]
- 7.Fotsis T., Pepper M. S., Montesano R., Aktas E., Breit S., Schweigerer L., Rasku S., Wähälä K., Adlercreutz H. Phytoestrogens and inhibition of angiogenesis. Baillieres Clin. Endocrinol. Metab. (1998);12:649–666. doi: 10.1016/S0950-351X(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 8.Gudkov S. V., Shtarkman I. N., Smirnova V. S., Chernikov A. V., Bruskov V. I. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat. Res. . (2006);165:538–545. doi: 10.1667/RR3552.1. [DOI] [PubMed] [Google Scholar]
- 9.Hanneken A., Lin F. F., Johnson J., Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress induced death. Invest. Ophthalmol. Vis. Sci. (2006);47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B., Gutteridge J. M. C. Oxidative stress: adaptation, damage, repair and death. In Free Radicals in Biology and Medicine (B. Halliwell and J.M.C. Gutteridge, Eds.) 3rd ed. Oxford University; Oxford: (1999). pp. 246–350. [Google Scholar]
- 11.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. (2001);30:433–446. doi: 10.1016/S0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim S. Y., Seo M., Oh J. M., Cho E. A., Juhnn Y. S. Inhibition of gamma ray-induced apoptosis by stimulatory heterotrimeric GTP binding protein involves Bcl-xL downregulation in SH-SY5Y human neuroblastoma cells. Exp. Mol. Med. . (2007);39:583–593. doi: 10.1038/emm.2007.64. [DOI] [PubMed] [Google Scholar]
- 13.Lee J. H., Kim S. Y., Kil I. S., Park J. W. Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. . (2007);282:13385–13394. doi: 10.1074/jbc.M700303200. [DOI] [PubMed] [Google Scholar]
- 14.Maher P., Akaishi T., Abe K. Flavonoid fisetin promotes ERK dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. U.S.A. (2006);103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton E. Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. (1992);43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 16.Middleton E. Jr, Kandaswami C., Theoharides T. C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. (2000);52:673–751. [PubMed] [Google Scholar]
- 17.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. . (1979);95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Okimoto Y., Watanabe A., Niki E., Yamashita T., Noguchi N. A novel fluorescent probe diphenyl-1-pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett. (2000);474:137–140. doi: 10.1016/S0014-5793(00)01587-8. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan R., Ranjan S. K., Nair C. K. Effect of vinblastine sulfate on gamma-radiation-induced DNA single-strand breaks in murine tissues. Mutat. Res. (2003);536:15–25. doi: 10.1016/S1383-5718(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 20.Reers M., Smith T. W., Chen L. B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry . (1991);30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 21.Regula K. M., Ens K., Kirshenbaum L. A. Mitochondriaassisted cell suicide: a license to kill. J. Mol. Cell Cardiol. (2003);35:559–567. doi: 10.1016/S0022-2828(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 22.Riley P. A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. . (1994);65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkranz A. R., Schmaldienst S., Stuhlmeier K. M., Chen W., Knapp W., Zlabinger G. J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofl uorescein-diacetate. J. Immunol. Methods. (1992);156:39–45. doi: 10.1016/0022-1759(92)90008-H. [DOI] [PubMed] [Google Scholar]
- 24.Sies H. In Oxidative Stress (H. Sies, Ed.). Academic Press; New York: (1983). [Google Scholar]
- 25.Simon H. U., Haj-Yehia A., Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. (2000);5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 26.Singh N. P. Microgels for estimation of DNA strand breaks, DNA protein cross links and apoptosis. Mutat. Res. (2000);455:111–127. doi: 10.1016/S0027-5107(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 27.Spitz D. R., Azzam E. I., Li J. J., Gius D. Metabolic oxidation/ reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. (2004);23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- 28.Stadtman E. R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. (1993);62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 29.Sung B., Pandey M. K., Aggarwal B. B. Fisetin, an inhibitor of cyclindependent kinase 6, down-regulates nuclear factorkappaB- regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor interacting protein-regulated IkappaBalpha kinase activation. Mol. Pharmacol. . (2007);71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 30.Troiano L., Ferraresi R., Lugli E., Nemes E., Roat E., Nasi M., Pinti M., Cossarizza A. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat. Protoc. . (2007);2:2719–2727. doi: 10.1038/nprot.2007.405. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Tu Y. C., Lian T. W., Hung J. T., Yen J. H., Wu M. J. Distinctive antioxidant and antiinfl ammatory effects of flavonols. J. Agric. Food Chem. (2006);54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 32.Zamzami N., Marchetti P., Castedo M., Zanin C., Vayssiere J. L., Petit P. X., Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. . (1995);181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zamzami N., Susin S. A., Marchetti P., Hirsch T., Gomez-Monterrey I., Castedo M., Kroemer G. Mitochondrial control of nuclear apoptosis. J. Exp. Med. . (1996);183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zbarsky V., Datla K. P., Parkar S., Rai D. K., Aruoma O. I., Dexter D. T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic. Res. (2005);39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]


