Abstract
Aromadendrin, a flavonol, has been reported to possess a variety of pharmacological activities such as anti-inflammatory, antioxidant, and anti-diabetic properties. However, the underlying mechanism by which aromadendrin exerts its biological activity has not been extensively demonstrated. The objective of this study is to elucidate the anti-inflammatory mechanism of aromadedrin in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. Aromadendrin significantly suppressed LPS-induced excessive production of pro-inflammatory mediators such as nitric oxide (NO) and PGE2. In accordance, aromadendrin attenuated LPSinduced overexpression iNOS and COX-2. In addition, aromadendrin significantly suppressed LPS-induced degradation of IκB, which sequesters NF-κB in cytoplasm, consequently inhibiting the nuclear translocation of pro-inflammatory transcription factor NF- κB. To elucidate the underlying signaling mechanism of anti-inflammatory activity of aromadendrin, MAPK signaling pathway was examined. Aromadendrin significantly attenuated LPS-induced activation of JNK, but not ERK and p38, in a concentration-dependent manner. Taken together, the present study clearly demonstrates that aromadendrin exhibits anti-inflammatory activity through the suppression of nuclear translocation of NF-κB and phosphorylation of JNK in LPS-stimulated RAW 264.7 macrophage cells.
Keywords: Aromadendrin, COX-2, iNOS, JNK, Lipopolysaccharide, NF-κB, RAW 264.7 cells
INTRODUCTION
Macrophages play essential roles in host defense against bacterial infection (Rehman et al., 2012). Macrophages orchestrate the innate immunity response to infection by expressing various inflammatory cytokines such as TNF-α, IL-1, NO, and PGE2 (Kim et al., 2012). The most common cause of macrophage activation is an exposure to LPS, the principal component of the outer membrane Gram-negative bacteria (Rietschel and Brade, 1992). However, aberrantly over-activated macrophages have also been attributed to detrimental responses in inflammatory diseases including sepsis, septic shock, or systemic inflammatory response syndrome (Rackow and Astiz, 1991; Shapiro et al., 2010). Therefore, suppression of aberrant activation of macrophage may have valuable therapeutic potential for the treatment of inflammatory diseases such as sepsis.
Aromadendrin is a flavonol, which is a type of flavonoids. Flavonoids have been widely reported to exhibit various pharmacological activities including anti-inflammatory, anti-oxidant, anti-tumor, and neuroprotective properties (Havsteen, 1983; Dixon and Steele, 1999). Aromadendrin has been reported to possess a variety of biological properties such as anti-inflammatory activity (Zhang et al., 2006), radical scavenging activity (Lee et al., 2009), and anti-tumor activity (Kwak et al., 2009). Recently, it has been reported that aromadendrin possesses anti-diabetic activity by stimulating glucose uptake and ameliorating insulin resistance (Zhang et al., 2011). In addition, it has been also reported that aromadendrin derivatives exhibit various biological activities such as anti-proliferative (Duh et al., 2012) and osteogenic actions (Swarnkar et al., 2011). However, the underlying mechanism by which aromadendrin exerts its pharmacological activity, especially anti-inflammatory, has not been clearly demonstrated. Aromadendrin has been reported to be present in various medicinal herbs including Chionanthus retusus (Kwak et al., 2009), Cudrania tricuspidata (Lee et al., 2009), Populus davidiana (Zhang et al., 2006), and Gelditsia sinensis (Zhang et al., 2011). Aromadendrin, used in the present study, was isolated from the heartwood of Hemiptelea davidii (Chang et al., 2004).
In the present study, to provide a novel pharmacological agent that could suppress aberrantly activated macrophages, the anti-inflammatory activity of aromadendrin and its underlying mechanism were elucidated in LPS-stimulated RAW 264.7 macrophage cells.
MATERIALS AND METHODS
Reagents and cell culture
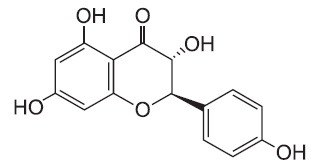
Bacterial lipopolysaccharide (LPS) from Escherichia coli serotype 055:B5 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Aromadendrin was isolated and identified from Hemiptelea davidii (Fig. 1) (Chang et al., 2004). Aromadendrin was dissolved in dimethyl sulfoxide (DMSO) and added to the cell culture at the desired concentrations. The macrophage RAW 264.7 cell line was maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 5% heat-inactivated fetal bovine serum and penicillin-streptomycin (Gibco BRL) at 37℃, 5% CO2. In all experiments, cells were incubated in the presence of the indicated concentration of Aromadendrin before the addition of LPS (200 ng/ml).
Fig. 1. Chemical structure of aromadendrin.
Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW 264.7 macrophage cells were seeded at 5×105 cells per well and incubated with aromadendrin at various concentrations for 24 hr at 37℃. After incubation, MTT (0.5 mg/ml in PBS) was added to each well, and the cells were incubated for 3 hr at 37℃ and 5% CO2. The resulting formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance was determined at 540 nm. The results were expressed as a percentage of surviving cells over control cells.
Nitrite quantification assay
The production of NO was estimated by measuring the amount of nitrite, a stable metabolite of NO, using the Griess reagent as described (Lee et al., 2012). After aromadendrinpretreated RAW 264.7 macrophage cells were stimulated with LPS in 12-well plates for 24 hr, 100 μl of the cell supernatant was mixed with an equal volume of Griess reagent. Light absorbance was read at 540 nm. The results were expressed as a percentage of released NO from LPS-stimulated RAW 264.7 cells. To prepare a standard curve, sodium nitrite was used to prepare a standard curve.
Isolation of nuclear extract
The isolation of the nuclear fraction was performed according to the protocol of NF-κB activation assay kits (FIVE photon).
Western blot analysis
The RAW 264.7 macrophage cells were pretreated with aromadendrin for 1 hr and stimulated with LPS. Cells were washed with PBS and lysed in PRO-PREP lysis buffer (iNtRON Biotechnology, Seongnam, Korea). Equal amounts of protein were separated on 10% SDS-polyacrylamide gel. Proteins were transferred to Hypond PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA) and blocked in 5% skim milk in TBST for 1 hr at room temperature. Specific antibodies against inducible NO synthase (iNOS), COX-2, extracellular signal-regulated kinase (ERK), phosphorylated (p)-ERK, p38, p-p38, c-Jun N-terminal kinase (1:1,000; Cell signaling Technology), IκB-α (1:1,000; Santa Cruz Biotechnology Inc), and β-actin (1:2,500; Sigma) were diluted in 5% skim milk. After thoroughly washing with TBST, horseradish peroxidase-conjugated secondary antibodies were applied. The blots were developed by the enhanced chemiluminescence detection (Amersham Biosciences).
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
The RAW 264.7 macrophage cells were treated with aromadendrin in the absence or presence of LPS (200 ng/ml) for 6 hr. Total RNA was isolated using the Total RNA Extraction Kit (iNtRON Biotechnology, Inc, USA) according to the manufacturer's instruction. The total RNA (2 μg) obtained from cells was reverse-transcribed using oligo-(dT) 15 primers (Promega, Madison, WI, USA). PCR amplification conditions using primer sets specific for iNOS, COX-2 and β-actin were optimized for each pair of primers. PCR primers were as follows: mouse iNOS forward, 5’-TCCTACACCACACCAAAC-3’; iNOS reverse, 5’-CTCCAATCTCTGCCTATCC-3’; COX-2 forward, 5’-CTTCACGCATCAGTTTTTCAAG-3’; COX-2 reverse, 5’-TCACCGTAAATATGATTTAAGTCCAC-3’; GAPDH forward, 5’-ACCCAGAAGACTGTGGAT-3’; GAPDH reverse, 5’-TCGTTGAGGGCAATGCCA- 3’. Parallel PCR analysis was run for the house keeping gene GAPDH to normalize data for differences in mRNA quantity and integrity. PCR products were separated on agarose gel.
Statistical analysis
All values shown in the figures are expressed as the mean ± SD obtained from at least three independent experiments. Statistical significance was analyzed by two-tailed Student’s t-test. Data with values of p<0.05 were considered as statistically significant. Single (* and #) and double (** and ##) marks represent statistical significance in p<0.05 and p<0.01, respectively.
RESULTS
Aromadendrin inhibits NO and PGE2 production in LPSstimulated RAW 264.7 macrophage cells
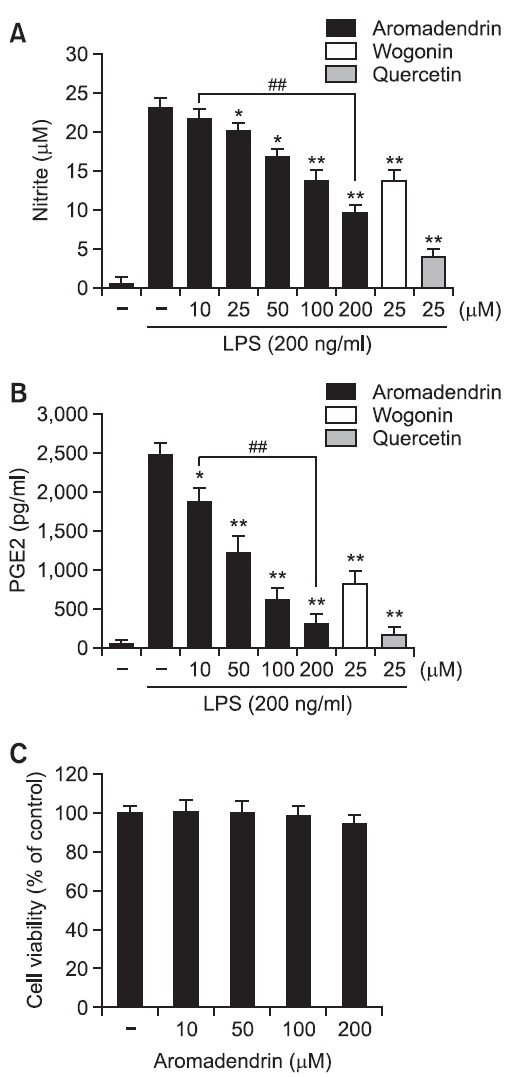
Given the previous reports that pro-inflammatory mediators such as NO and PGE2 play key roles in the progression of in- flammation (Ock et al., 2009; Lee et al., 2012), the inhibitory effects of aromadendrin on NO and PGE2 release in LPS-stimulated RAW 264.7 macrophage cells. Cells were incubated with aromadendrin (10, 50, 100, or 200 μM) for 1 hr prior to LPS treatment (200 ng/ml) LPS. LPS markedly increased NO and PGE2 production in RAW 264.7 cells. However, aromadendrin significantly inhibited NO and PGE2 release in LPS-stimulated RAW 264.7 cells in concentration dependent manners (Fig. 2 A and B). Furthermore, to examine the anti-inflammatory magnitude of aromadedrin, inhibitory potency of aromadendrin on LPS-induced NO production was compared to prototype flavonoids, such as wogonin and quercetin (Fig. 2A). Although aromadendrin significantly attenuated LPS-induced NO production in a concentration-dependent manner, the inhibitory effect of aromadendrin was less potent than those of wogonin and quercetin. In addition, aromadendrin showed no significant cytotoxicity in concentration ranges used in the study (Fig. 2C).
Fig. 2. Effects of aromadendrin on LPS-induced overproduction of NO (A) and PGE2 (B) in RAW 264.7 macrophage cells. RAW 264.7 cells were pretreated with various concentrations of aromadendrin for 1 hr before incubation with LPS (200 ng/ml) for 24 hr. The level of nitrite and PGE2 were measured using Griess reagent and ELISA assay, respectively. Aromadendrin significantly suppressed LPS-induced NO and PGE2 production in concentration-dependent manners. (C) Effect of aromadendrin on the viability of RAW 264.7 cells. No noticeable cell death was observed with aromadendrin concentrations used in the present study. The data are expressed as mean ± S.D. (n=3), and representative of three independent experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. ##p<0.01 indicates statistically significant differences between indicated groups.
Aromadendrin inhibits LPS-induced mRNA and protein expressions of iNOS and COX-2
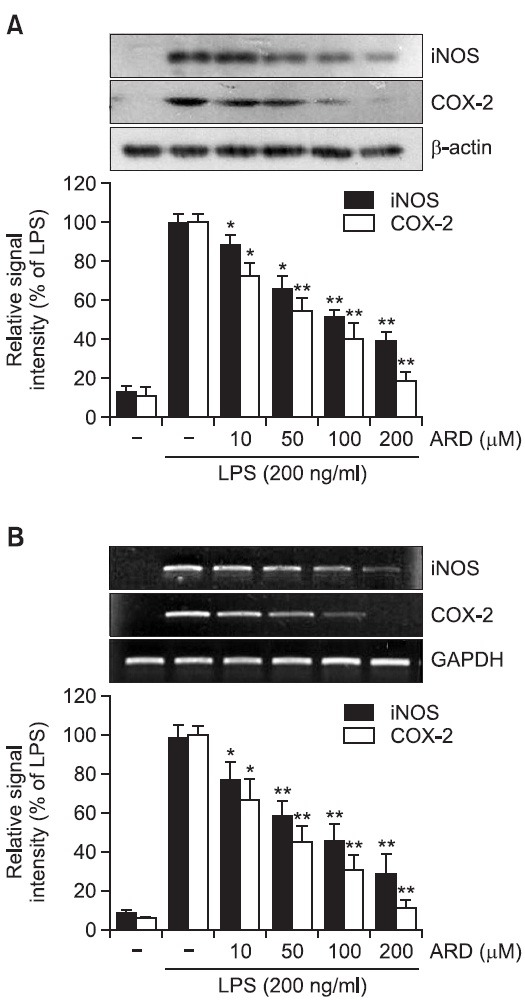
As aromadendrin inhibited LPS-induced NO and PGE2 production in RAW 264.7 cells (Fig. 2), whether the suppression of NO and PGE2 were attributable to downregulation of iNOS and COX-2 expression was examined in the presence or absence of aromadendrin. LPS stimulation of the RAW 264.7 cells strongly upregulated iNOS and COX-2 protein levels. Pretreatment of aromadendrin resulted in a significant decrease in LPS-induced iNOS and COX-2 overexpression levels in a concentration-dependent manner (Fig. 3A). Consistent with the decrease of protein levels, LPS-induced up-regulation of iNOS and COX-2 mRNA expression was significantly attenuated with aromadendrin (Fig. 3B), indicating that attenuated release of NO and PGE2 is due to decreased expression of their responsible genes by aromadendrin.
Fig. 3. Effects of aromadendrin on LPS-induced iNOS and COX-2 protein (A) and mRNA (B) expressions in RAW 264.7 cells. (A) The cell lysates were subjected to SDS-PAGE, and then protein levels of iNOS and COX-2 were determined by Western blot analysis. Aromadendrin significantly attenuated LPS-induced overexpression of iNOS and COX-2. (B) After LPS treatment for 6 hr, total mRNA levels of iNOS and COX-2 were examined by RT-PCR. GAPDH was used as an internal control. Aromadendrin significantly suppressed LPS-induced iNOS and COX-2 expression in concentration-dependent manners. Images are representative of three independent experiments that shows reproducible results. ARD stands for aromadendrin.
Aromadendrin inhibits IκB degradation and NF-κB translocation in in LPS-stimulated RAW 264.7 cells
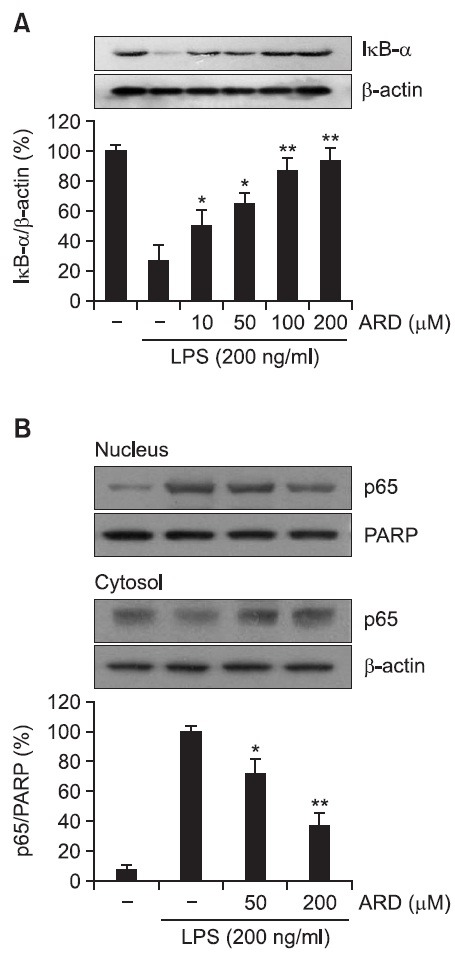
Activation of the transcription factor NF-κB has been reported to result in the increased transcription of pro-inflammatory mediators including iNOS and COX-2 (Baldwin, 1996; Moynagh, 2005). IκB has been demonstrated to inhibit nuclear translocation of NF-κB by retaining it in cytoplasm (Kim et al., 2012; Rehman et al., 2012). It has been also reported that LPS causes activation of IκB kinases, which phosphorylate IκB and then lead to proteasomal degradation of IκB (Baeuerle and Baichwal, 1997). Therefore, in the present study, whether aromadendrin could suppress LPS-induced IκB degradation was examined. LPS treatment resulted in obvious degradation of IκB. However, aromadendrin significantly attenuated LPS-induced degradation of IκB in a concentration-dependent manner (Fig. 4A). Furthermore, whether aromadendrin could prevent nuclear translocation of the p65 subunit of NF-κB was examined. LPS treatment caused a significantly increased nuclear translocation of NF-κB. However, aromadendrin resulted in a significant reduction of LPS-induced nuclear translocation of NF-κB in a concentration-dependent manner (Fig. 4B).
Fig. 4. Effect of aromadendrin on IκB-α degration (A) and p65 translocation (B) to the nucleus in LPS-stimulated RAW 264.7 macrophage cells. Total cell lysates obtained 15 and 60 min after the LPS stimulation were subjected to Western blotting to measure the levels of IκB-α degradation (A) and p65 translocation (B). Data from triplicate determination are shown (mean ± S.D.). *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. ARD stands for aromadendrin.
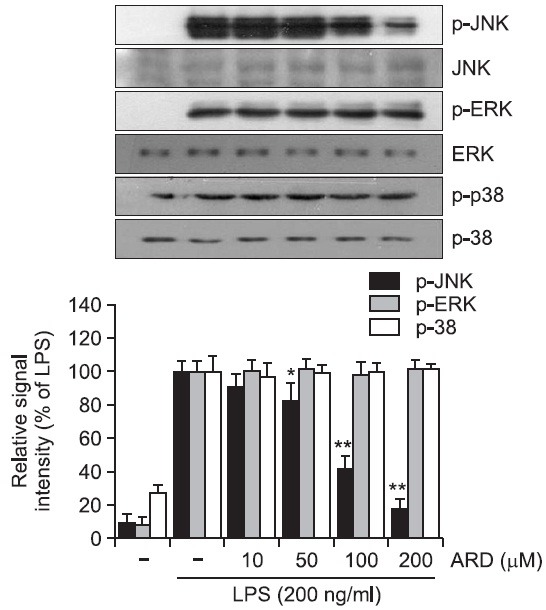
Aromadendrin inhibits the phosphorylation of JNK in LPS-stimulated RAW 264.7 cells
It has been previously reported that MAP kinase signaling pathway is implicated in the LPS-induced inflammatory responses (Guha and Mackman, 2001; Rushworth et al., 2005). In the present study, MAP kinase pathway was examined to understand the underlying signaling mechanism by which aromadendrin exhibits its anti-inflammatory activity. The effect of aromadendrin on LPS-stimulated phosphorylation of JNK, ERK, and p38 kinase in RAW 264.7 macrophage cells was determined. Cells were pretreated with aromadendrin at indicated concentrations (10, 50, 100, and 200 μM), and then were treated with LPS (200 ng/ml) for 30 min. Aromadendrin significantly suppressed the LPS-induced phosphorylation of JNK in a concentration-dependent manner in RAW 264.7 cells (Fig. 5). However, no significant attenuation of ERK and p38 phosphorylation was observed with aromadendrin. The data strongly suggest that JNK signaling plays a key role in LPSinduced inflammatory response in RAW 264.7 cells.
Fig. 5. Effect of aromadendrin on LPS-induced MAPK signaling pathway in RAW 264.7 macrophage cells. RAW 264.7 cells were stimulated with 200 ng/ml LPS in the absence or presence of aromadendrin. Western blot analysis was then performed to evaluate the activation of MAP kinase signaling pathway. LPSinduced phosphorylation of JNK was significantly attenuated with aromadendrin treatment, but phosphorylation of ERK and p38 was not decreased with aromadendrin treatment, suggesting that JNK signaling might play a key role in the LPS-induced overactivation of RAW 264.7 cells. β-Actin was used as an internal control. Images are representative of three independent experiments that shows reproducible results. ARD stands for aromadendrin.
DISCUSSION
The present study clearly demonstrated that aromadendrin suppresses inflammatory responses through the suppression of nuclear translocation of NF-κB and phosphorylation of JNK in LPS-stimulated RAW 264.7 macrophage cells. Aromadendrin significantly inhibited LPS-stimulated excessive production of NO and PGE2 with the suppression of their responsible proteins, iNOS and COX-2, respectively. Aromadendrin also attenuated LPS-induced IκB degradation, nuclear NF-κB translocation, and JNK phosphorylation.
Aromadendrin, [(2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)- 2,3-dihydrochromen-4-one], has been reported to possess pharmacological properties such as anti-inflammatory (Zhang et al., 2006) and anti-oxidant (Lee et al., 2009) actions. However, the underlying mechanism by which aromadendrin exerts its anti-inflammatory activity has not been extensively elucidated. The present study demonstrated that aromadenrin exerts its anti-inflammatory properties in LPS-induced RAW 264.7 macrophage cells through the suppression of LPS-induced IκB degradation, nuclear NF-κB translocation, and JNK activation.
Although macrophages play essential roles in innate immunity for the host defense against infections (Rehman et al., 2012), macrophages also play a key role in sepsis by producing pro-inflammatory mediators, expression of adhesion molecules and coagulation factors, phagocytosis, and cytoskeletal rearrangement (Sweet and Hume, 1996). It has been reported that aberrantly activated macrophages are implicated in the pathogenesis of many infection-associated disorders including sepsis and septic shock (Rackow and Astiz, 1991; Shapiro et al., 2010). Therefore, regulation of aberrant macrophage activation may have valuable therapeutic potential for the treatment of inflammatory diseases such as sepsis. It has been reported that LPS activates gene transcription by binding to its membrane receptor in macrophages, TLR4, which induces signal transcription pathways leading to the phosphorylation of kinases such as IκB kinases, protein kinase C, PI3K, and MAPKs. Subsequently, these kinases activate various transcription factors such as NF-κB and AP-1 families (O'Connell et al., 1998; Guha and Mackman, 2001). In accordance, the present study showed that aromadendrin resulted in the significant attenuation of LPS-induced degradation of IκB and nuclear NF-κB translocation. However, effects of aromadendrin on other pro-inflammatory transcription factors have not been studied. Therefore, further studies are necessary to clearly understand the role of aromadendrin on various inflammation-related transcription factors.
It has been reported that LPS is a potent activator of all three MAPK pathways in human monocytic cells (Guha and Mackman, 2001). Many studies have shown that LPS activates ERK1/2, JNK, and p38 in macrophages (Sweet and Hume, 1996) and that many of the downstream targets of MAPK pathways are transcription factors, which regulate various genes encoding inflammatory mediators (Zhang et al., 2006; Lee et al., 2009; Zhang et al., 2011). In the present study, LPS treatment exhibited increased phosphorylation of all three MAPKs. However, aromadendrin selectively attenuated LPS-induced JNK activation in a concentration-dependent manner. No noticeable changes were observed in phosphorylation of ERK and p38 with aromadendrin. Selective suppression of JNK activation appeared to be sufficient to exert anti-inflammatory effects of aromadendrin in RAW 264.7 cells, suggesting that JNK pathway rather than ERK and p38 plays a key role in pro-inflammatory responses in RAW 264.7 cells.
NF-κB is a major transcription factor for the expression of pro-inflammatory mediators such as iNOS, IL-1β, and TNF-α (Siebenlist et al., 1994; Kuprash et al., 1995) and aberrant activation of NF-κB and its downstream genes have been implicated in various pathological conditions including sepsis and autoimmune diseases (Karin et al., 2001; Li and Verma, 2002). LPS has been reported to cause the nuclear translocation of NF-κB through IκB degradation (Moon et al., 2007; Zheng et al., 2008). Accordingly, the present study showed that aromadendrin significantly attenuated LPS-induced IκB degradation and nuclear translocation of NF-κB in LPS-induced RAW 264.7 macrophages.
In conclusion, the present data demonstrate that aromadendrin possesses anti-inflammatory activity such as suppression of NO and PGE2 production, and inhibition of nuclear transclocation of NF-κB and phosphorylation of JNK in LPS-stimulated RAW 264.7 macrophage cells. The present study strongly suggests that aromadendrin might be a valuable therapeutic agent in the treatment of inflammation-related pathologies including sepsis. However, further studies are necessary to clearly elucidate the exact mechanism by which aromadendrin inhibits LPS-induced activation of JNK signaling pathway and the possible crosstalk between NF-κB activation and JNK signaling pathway.
References
- 1.Baeuerle P. A., Baichwal V. R. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. . (1997);65:111–137. [PubMed] [Google Scholar]
- 2.Baldwin A. S. Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. (1996);14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Chang B. S., Kwon Y. S., Kim C. M. The chemical structures and their antioxidant activity of the components isolated from the heartwood of Hemiptelea davidii. Korean J. Pharmacogn. (2004);35:80–87. [Google Scholar]
- 4.Dixon R. A., Steele C. L. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends Plant Sci. . (1999);4:394–400. doi: 10.1016/S1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- 5.Duh P. D., Chen Z. T., Lee S. W., Lin T. P., Wang Y. T., Yen W. J., Kuo L. F., Chu H. L. Antiproliferative activity and apoptosis induction of Eucalyptus Citriodora resin and its major bioactive compound in melanoma B16F10 cells. J. Agric. Food Chem. (2012);60:7866–7872. doi: 10.1021/jf301068z. [DOI] [PubMed] [Google Scholar]
- 6.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signal. . (2001);13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 7.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. (1983);32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 8.Karin M., Takahashi T., Kapahi P., Delhase M., Chen Y., Makris C., Rothwarf D., Baud V., Natoli G., Guido F., Li N. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors . (2001);15:87–89. doi: 10.1002/biof.5520150207. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y. J., Shin Y., Lee K. H., Kim T. J. Anethum graveloens flower extracts inhibited a lipopolysaccharide-induced inflammatory response by blocking iNOS expression and NF-kappaB activity in macrophages. Biosci. Biotechnol. Biochem. (2012);76:1122–1127. doi: 10.1271/bbb.110950. [DOI] [PubMed] [Google Scholar]
- 10.Kuprash D. V., Udalova I. A., Turetskaya R. L., Rice N. R., Nedospasov S. A. Conserved kappa B element located downstream of the tumor necrosis factor alpha gene: distinct NF-kappa B binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene. (1995);11:97–106. [PubMed] [Google Scholar]
- 11.Kwak J. H., Kang M. W., Roh J. H., Choi S. U., Zee O. P. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. (2009);32:1681–1687. doi: 10.1007/s12272-009-2203-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee J. W., Bae C. J., Choi Y. J., Kim S. I., Kim N. H., Lee H. J., Kim S. S., Kwon Y. S., Chun W. 3,4,5-Trihydroxycinnamic acid inhibits lps-induced iNOS expression by suppressing NF-kappaB activation in BV2 microglial cells. Korean J. Physiol. Pharmacol. (2012);16:107–112. doi: 10.4196/kjpp.2012.16.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y. J., Kim S., Lee S. J., Ham I., Whang W. K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. (2009);32:195–200. doi: 10.1007/s12272-009-1135-z. [DOI] [PubMed] [Google Scholar]
- 14.Li Q., Verma I. M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. (2002);2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 15.Moon D. O., Park S. Y., Lee K. J., Heo M. S., Kim K. C., Kim M. O., Lee J. D., Choi Y. H., Kim G. Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharidestimulated BV2 microglia. Int. Immunopharmacol. (2007);7:1092–1101. doi: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Moynagh P. N. The NF-kappaB pathway. J. Cell Sci. (2005);118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- 17.Ock J., Kim S., Suk K. Anti-inflammatory effects of a fluorovinyloxyacetamide compound KT-15087 in microglia cells. Pharmacol. Res. (2009);59:414–422. doi: 10.1016/j.phrs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell M. A., Bennett B. L., Mercurio F., Manning A. M., Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J. Biol. Chem. (1998);273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 19.Rackow E. C., Astiz M. E. Pathophysiology and treatment of septic shock. JAMA . (1991);266:548–554. doi: 10.1001/jama.1991.03470040112032. [DOI] [PubMed] [Google Scholar]
- 20.Rehman M. U., Yoshihisa Y., Miyamoto Y., Shimizu T. The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages. Inflamm. Res. (2012);61:1177–1185. doi: 10.1007/s00011-012-0512-0. [DOI] [PubMed] [Google Scholar]
- 21.Rietschel E. T., Brade H. Bacterial endotoxins. Sci. Am. (1992);267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 22.Rushworth S. A., Chen X. L., Mackman N., Ogborne R. M., O'Connell M. A. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. (2005);175:4408–4415. doi: 10.4049/jimmunol.175.7.4408. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro N. I., Khankin E. V., Van Meurs M., Shih S. C., Lu S., Yano M., Castro P. R., Maratos-Flier E., Parikh S. M., Karumanchi S. A., Yano K. Leptin exacerbates sepsis-mediated morbidity and mortality. J. Immunol. (2010);185:517–524. doi: 10.4049/jimmunol.0903975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. (1994);10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 25.Swarnkar G., Sharan K., Siddiqui J. A., Chakravarti B., Rawat P., Kumar M., Arya K. R., Maurya R., Chattopadhyay N. A novel flavonoid isolated from the steam-bark of Ulmus wallichiana planchon stimulates osteoblast function and inhibits osteoclast and adipocyte differentiation. Eur. J. Pharmacol. (2011);658:65–73. doi: 10.1016/j.ejphar.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Sweet M. J., Hume D. A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. (1996);60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W. Y., Lee J. J., Kim I. S., Kim Y., Myung C. S. Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin. Pharmacology. (2011);88:266–274. doi: 10.1159/000331862. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Hung T. M., Phuong P. T., Ngoc T. M., Min B. S., Song K. S., Seong Y. H., Bae K. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. (2006);29:1102–1108. doi: 10.1007/BF02969299. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L. T., Ock J., Kwon B. M., Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int. Immunopharmacol. . (2008);8:484–494. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]


