Abstract
Although the role of α-synuclein aggregation on Parkinson’s disease is relatively well known, the physiological role and the regulatory mechanism governing the expression of α-synuclein are unclear yet. We recently reported that α-synuclein is expressed and secreted from cultured astrocytes. In this study, we investigated the effect of valproic acid (VPA), which has been suggested to provide neuroprotection by increasing α-synuclein in neuron, on α-synuclein expression in rat primary astrocytes. VPA concentrationdependently increased the protein expression level of α-synuclein in cultured rat primary astrocytes with concomitant increase in mRNA expression level. Likewise, the level of secreted α-synuclein was also increased by VPA. VPA increased the phosphorylation of Erk1/2 and JNK and pretreatment of a JNK inhibitor SP600125 prevented the VPA-induced increase in α-synuclein. Whether the increased α-synuclein in astrocytes is involved in the reported neuroprotective effects of VPA awaits further investigation.
Keywords: α-synuclein, Valproic acid, Stability, JNK, Neuroprotection, Acetylation
INTRODUCTION
Cytoplasmic accumulation of α-synuclein (Spillantini et al., 1997) is commonly observed in patients with sporadic or familial cases of Parkinson’s disease (PD). When α-synuclein is incubated in an aqueous environment, α-synuclein loses its disordered structure and is transformed from monomer to mature fibril suggesting the neurotoxic role of α-synuclein. And also, α-synuclein forms oligomers and its aggregates have a pathological function. Mutations in three loci (A53T, A30P, and E46K) of α-synuclein facilitate α-synuclein aggregate formation and have been linked to the heritable form of PD (Polymeropoulos et al., 1997; Kruger et al., 1998). Recent study reported that soluble oligomers have a toxic effect on neuron. Artificial proline mutations on the α-synuclein protein did not form mature fibrils, however oligomer formation was observed. These mutants were more toxic than wild-type α-synuclein in various cells and in vivo models (Karpinar et al., 2009).
Albeit the general belief that α-synuclein plays neurotoxic role in PD pathogenesis, growing interests also focus on the neuroprotective role of α-synuclein in several brain insult conditions (Alves Da Costa et al., 2002; Seo et al., 2002; Jensen et al., 2003), which might suggest that normal physiological function of α-synuclein may include neuroprotection as well as the regulation of neurotransmitter release. Adding to the above mentioned reports, recent publication from Leng and Chuang suggests that up-regulation of endogenous expression of α-synuclein through modulation of histone deacetylation provides protection of cerebellar granule neurons against excitotoxic injury both in vitro and in vivo (Leng and Chuang, 2006). Interestingly, chronic in vivo administration of a histone deacetylase inhibitor (HDACi), valproic acid (VPA) provided neuroprotection against rotenone-induced neurotoxicity, possibly via increase in α-synuclein level concomitant with the decreased monoubiquitination of α-synuclein (Monti et al., 2010). VPA is a mainstream drug used in the treatment of bipolar disorder as well as epileptic seizure, which possesses HDACi activity along with many other cellular signaling modulator activities with relatively strong neuroprotective effects in numerous pathological conditions (Monti et al., 2009).
Although the predominant cells expressing α-synuclein in brain are neuron, we recently reported that primary astrocytes in culture also express and regulate α-synuclein trafficking as well as physiological responses including regulation of proteolytic enzymes (Lee et al., 2005; Joo et al., 2010). Considering the fact that astrocytes provide neuroprotective role in several brain insults condition (Marchetti, 1997; L’Episcopo et al., 2010; Sofroniew and Vinters, 2010), we reasoned that VPA may modulate the level of α-synuclein in astrocyte. In this study, we investigated the role of VPA on α-synuclein level in cultured rat primary astrocytes as well as the cellular regulatory mechanism mediating the effects.
MATERIALS AND METHODS
Materials
Sprague-Dawley (SD) rats were obtained from Orient Bio (Seoul, Korea) and the Dulbecco’s Modified Eagle Medium (DMEM)/F12, fetal bovine serum (FBS), penicillin/streptomycin and trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA, USA). U0126, SP600125, LY294002 and hydrogen peroxide (H2O2) were purchased from Merck (Darmstadt, Germany). Sodium valproate, sodium butyrate and Trichostatin A (TSA) were purchased from Sigma Chemical Co. (St Louis MO, USA).
Cell culture
Rat primary astrocytes were cultured from the frontal cortices of 2 day-old SD rat pups as described (Shin et al., 2001; Park et al., 2008). In brief, cortices were dissected out and digested with trypsin to isolate single cells. The cells were cultured in DMEM/F12 media containing 10% FBS for 7 days and then were re-plated after trypsin-EDTA digestion. The purity of astrocyte culture was more than 95% as determined by immunostaining with antibodies against astrocyte specific marker GFAP as we reported previously (Kim et al., 2010). The day before experiments, cells were deprived of serum by washing three times with serum free DMEM-F12. Stock solution of VPA was prepared in distilled water and astrocytes were treated with varying concentration of VPA for 24 hr.
Western blot analysis
The protein level of α-synuclein in whole cell lysates as well as in secreted compartment was determined by Western blot using specific antibody against α-synuclein (Syn-1, BD Biosciences, San Diego, CA). Whole cell lysates were prepared using RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl (pH 7.4), 20 mM NaF, 20 mM EGTA, 1 mM DTT, 1 mM Na3VO4 and protease inhibitors) and culture supernatants were concentrated 10 fold by acetone precipitation before electrophoresis. After dissolution with PBS, samples were mixed with 4X SDS sample buffer and then electrophoresed using 12% SDS-PAGE gels. After electrophoresis, the protein bands were electrically transferred to nitrocellulose (NC) membrane and blocked with 5% nonfat dried milk in PBST. The NC membrane was incubated overnight at 4℃ with monoclonal antibody against α-synuclein (1:1,000), phosphor-ERK, phosphor-JNK, phosphor-p38, ERK, JNK, p38 and polyclonal antibody against Ac-H3 and H3 diluted 1:1,000 in PBST. Western blot using a monoclonal antibody against β-actin (Sigma, St. Louis) at a dilution of 1:30,000 was used as a loading control. After washing, the membrane was incubated for 2 hr at RT with HRP-conjugated goat anti-mouse IgG that was diluted 1:5,000 in PBST. Protein bands were visualized using enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ, USA) and quantified with Image J program (NIH, USA).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
For the determination of mRNAs level encoding α-synuclein and GAPDH, semi-quantitative RT-PCR analysis was performed as described previously using cDNA obtained from 0.5 μg total RNA as a template (Joo et al., 2010). Total RNA was extracted from the harvested cells using the Trizol® (Invitrogen, Carlsbad, CA, USA) reagent and total RNA was converted to cDNA using Superscipt II at 42℃ for 90 min using first-strand cDNA synthesis kit. The PCR amplification consisted of 26 cycles (94℃, 1 min; 60℃, 1 min; 72℃, 1 min) with the oligonucleotide primers for α-synuclein and GAPDH. The sequence of the primers used in this study was as follows: α-synuclein (413 bp); 5’-GGA GTG ACA ACA GTG GCT GA-3’ (sense), 5’-CGA TCA CTG CTG ATG GAA GA-3’ (antisense): GAPDH (446 bp); 5’-CAA AAG GGT CAT CAT CTC TG-3’ (sense), 5’-CCT GCT TCA CCA CCT TCT TG-3’(antisense). Final PCR products were resolved by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
Adenoviral infection for α-synuclein overexpression
To examine the effect of VPA on the α-synuclein protein itself, cells were infected with adenoviral vector encoding either wild-type α-synuclein at a multiplicity of infection (m.o.i.) of 20 (Lee et al., 2002). Because the adenoviral vector was devoid of endogenous regulatory promoter sequence for α-synuclein gene, we can chase the changes in α-synuclein protein itself but not the transcriptional regulation of α-synuclein expression. The infected cells were treated with VPA and then the level of α-synuclein was checked by Western blot using a monoclonal antibody against α-synuclein (Syn-1, BD Biosciences, San Diego, CA).
Immunoprecipitation of acetylated α-synuclein
For the identification of acetylation on α-synuclein protein, whole cell extracts were prepared by homogenizing in 200 μl IP buffer containing protease inhibitors (20 mM Tris-HCl at pH 7.4, 150 mM sodium chloride, 10% glycerol, 1% NP-40, 1 mM sodium vanadate, 50 mM sodium fluoride, 50 mM sodiumglycerophosphate, 10 mM sodium butyrate, 10 mM 2-mercaptoethanol, 1mM PMSF and 1 μg/ml aprotinin). Protein concentration of cell extracts was measured by BCA protein assay and equal amounts of protein (500 μg) were incubated overnight at 4℃ with anti-acetyl-lysine (Millipore, CA, USA) or anti-Flag (Sigma) antibody (IP antibody) to immunoprecipitate acetylated protein or flag tagged α-synuclein, respectively. The antibody-antigen complex was then incubated with 20 μl protein A agarose bead (Pierce, Thermo scientific, USA) for 2 hr at 4℃. After washing three times with lysis buffer, the immunoprecipitated material was resolved on a 10% polyacrylamide gel (BioRad, Hercules, CA, USA) and probed with antiacetyl- lysine and anti-Flag antibodies by Western blotting to detect the presence of acetylated α-synuclein.
Statistical analysis
Results were expressed as the mean ± S.E.M. The data were analyzed for statistical significance using one-way ANOVA followed by Tukey’s post hoc test using SPSS software and a value of p<0.05 was considered as significant.
RESULTS
Valproic acid induces the expression of α-synuclein in rat primary astrocyte
First, we examined whether VPA induces the expression of α-synuclein in rat primary astrocyte. In Western blot, α-synuclein was appeared as a band with molecular weight of 19 Kd. VPA concentration dependently increased the level of α-synuclein and as low as 100 μM VPA showed statistically significant increase in α-synuclein level (Fig. 1A). The increased α-synuclein in cellular compartment is accompanied with the increased α-synuclein level in secreted compartment, which appears as doublet bands, suggesting that VPA may increase the secretion of α-synuclein in rat primary astrocytes (Fig. 1B). Consistent with these results, the same concentrations of VPA increased the mRNA expression level of α-synuclein suggesting the increase in α-synuclein is mediated by the up-regulation of mRNA of α-synuclein (Fig. 2).
Fig. 1. Effects of VPA on the protein level of α-synuclein in primary astrocytes. Rat primary astrocytes in serum-free DMEM/F12 were treated with various concentrations of VPA (0.01~1 mM) at 37℃. (A) After 24 hr, cell extracts were collected and analyzed for α-synuclein protein level by Western blotting with α-synuclein antibody. Graph is the quantification data of α-synuclein and β-actin expression by densitometry. (B) Culture spent media were collected and analyzed for released α-synuclein protein by Western blotting. Values are expressed as the mean ± S.E.M. (n=5). **p<0.01 vs. control.
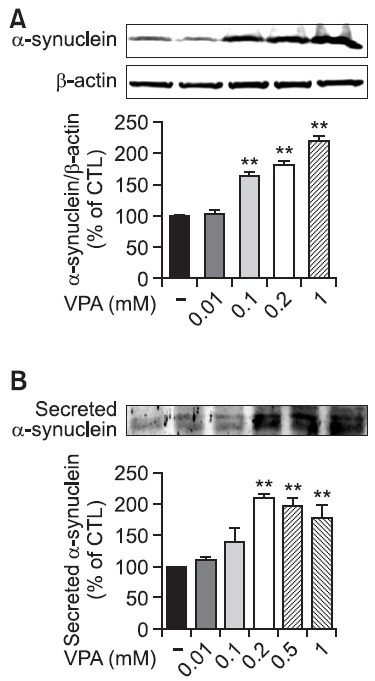
Fig. 2. Effects of VPA on the mRNA expression of α-synuclein in rat primary astrocytes. Rat primary astrocytes were treated with various concentrations of VPA (0.01~1 mM) at 37℃. The expression of α-synuclein mRNA was determined by RT-PCR after the treatment of VPA. Graph is the quantification data of α-synuclein and GAPDH expression level by densitometry. Values are expressed as mean ± S.E.M. of five experiments. *p<0.05, **p<0.01 vs. control.
Inhibition of HDAC activity increases the expression of α-synuclein protein
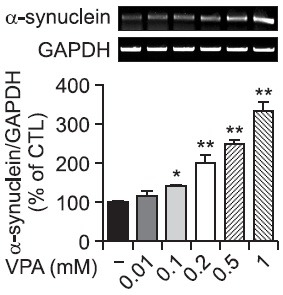
One of the most well-known cellular activities of VPA is the inhibition of HDAC activity. We tested sodium butyrate (SB) and trichostatin A (TSA), two HDAC inhibitors, for their ability to inhibit HDAC activity and to increase α-synuclein. As expected, all three HDAC inhibitors VPA, SB and TSA caused histone H3 hyperacetylation (Fig. 3). SB and TSA induced α-synuclein expression to a similar extent as VPA (Fig. 3). We also tested valpromide (VM), a derivative of valproic acid without HDAC inhibitory activity. In contrast to VPA, VM did not increase α-synuclein expression. These data suggest that the effects of VPA on α-synuclein are related to the direct HDACi activity.
Fig. 3. Effects of HDACis on the expression of α-synuclein in rat primary astrocytes. Rat primary astrocytes in serum-free DMEM/ F12 were treated with HDAC inhibitors, such as VPA (1 mM), sodium butyrate (SB; 1, 5 mM) and trichostatin A (TSA; 0.5, 1 μM) or control compound valpromide (VM; 1 mM). After 24 hr, cultured cells were harvested for Western blot analysis. Immunoblots of lysates from treated primary astrocytes were probed with α-synuclein, acetyl-H3 and H3 antibodies. Values are expressed as the mean ± S.E.M. (n=5). **p<0.01 vs. control.
The effect of VPA is mediated by the JNK signaling pathway in rat primary astrocytes
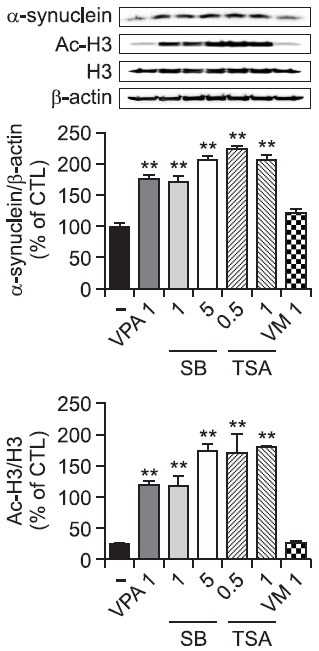
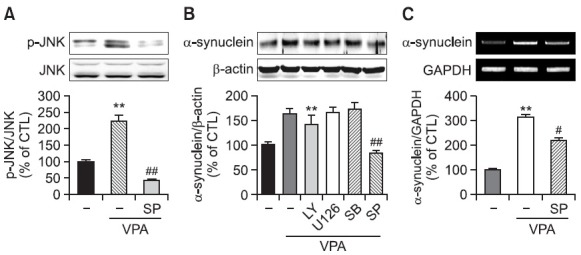
To investigate the signaling pathway mediating VPA-induced increase in α-synuclein expression, we investigated the activation of Akt and MAPK pathways by VPA in rat primary astrocytes. VPA increased the phosphorylation of JNK, Erk1/2 and p38 but not Akt in rat primary astrocytes as early as 6 hr (Fig. 4), which was slightly normalized at 24 hr (data not shown). Interestingly, pretreatment of a JNK inhibitor SP600125 but not other inhibitors such as PI3K-Akt pathway inhibitor LY294002, MEK inhibitor U0126 and p38 inhibitor SB203580, inhibited VPA-induced increase in α-synuclein protein and mRNA (Fig. 5). These data suggest that the activation of JNK pathway mediates VPA-induced increase in the α-synuclein.
Fig. 4. The activation of MAPK pathways by VPA in rat primary astrocytes. Rat primary astrocytes in serum-free DMEM/F12 were treated with VPA (1 mM) at 37℃. Cell extracts were collected for Western blot analysis. Immunoblots of lysates form treated rat primary astrocytes were probed with phospho-ERK, phospho-p38, phospho-JNK, and phosphop-Akt antibodies. As a loading control, total ERK/JNK/p38/Akt levels were also measured. **p<0.01 vs. control.
Fig. 5. Effect of MAPKs inhibitors on VPA-induced α-synuclein expression in rat primary astrocytes. (A) Effects of JNK inhibitor on VPAinduced JNK phosphorylation in rat primary astrocytes. Rat primary astrocytes in serum-free DMEM/F12 were treated with a JNK inhibitor (SP; SP600125, 2 μM) 1 hr before 1 mM VPA treatment. Treated cells were harvested for Western blot. Immunoblots of lysates form treated rat primary astrocytes were probed with phospho-JNK and JNK. Graph is the quantification data of phospho-JNK and JNK expression level by densitometry. Values are expressed as mean ± S.E.M. **p<0.01 vs. control; ##p<0.01 vs. VPA. (B) Rat primary astrocytes in serumfree DMEM/F12 were treated with a PI3K inhibitor (LY; LY294002, 10 μM), a MEK inhibitor (U; U0126, 10 μM), a p38 MAPK inhibitor (SB; SB203580, 20 μM), and a JNK inhibitor (SP; SP600125, 2 μM) 1 hr before 1 mM VPA treatment. After 24 hr, cultured cells were harvested for Western blot analysis. Immunoblots of lysates from treated rat primary astrocytes were probed with α-synuclein antibody. Graph is the quantification data of α-synuclein and β-actin expression by densitometry. Values are expressed as the mean ± S.E.M. (n=5). **p<0.01 vs. control; ##p<0.01 vs. VPA. (C) Effects of JNK inhibitor on α-synuclein mRNA in rat primary astrocytes. Rat primary astrocytes in serum-free DMEM/F12 were treated with a JNK inhibitor (SP; SP600125, 2 μM) 1 hr before 1 mM VPA treatment. After 24 hr, cultured cells were harvested for RT-PCR. Graph is the quantification data of α-synuclein and GAPDH expression level by densitometry. Values are expressed as mean ± S.E.M. **p<0.01 vs. control; #p<0.05 vs. VPA.
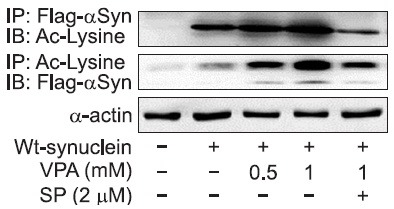
VPA increases the acetylation of α-synuclein in rat primary astrocytes
We investigated the effects of VPA on the acetylation of α-synuclein protein, which might be related to the modulation of stability of α-synuclein (Glozak et al., 2005; Li et al., 2010; Geng et al., 2012). Cultured cells were infected with adenoviral vector encoding flag tagged α-synuclein and after 24 hr, cell extracts were immunoprecipitated using anti-acetyl lysine or anti-flag antibody. As shown in Fig. 6, VPA increased acetylation of α-synuclein protein in rat primary astrocytes and an inhibitor of JNK, SP600125 prevented the acetylation of α-synuclein protein. These findings suggest that VPA induced the acetylation of α-synuclein protein through JNK pathway.
Fig. 6. Effects of VPA on lysine acetylation of α-synuclein in rat primary astrocytes. Viral particles encoding wild type α-synuclein were infected for 24 hr and cells were treated with VPA and SP600125 (2 μM). After 24 hr, cultured cells were harvested for Western blot analysis. Immunoblots of lysates from treated rat primary astrocytes were immunoprecipitated with acetyl lysine or flag antibody as described. Immunoblots were probed with acetyl lysine or flag antibody. The data are representative of two independent experiments.
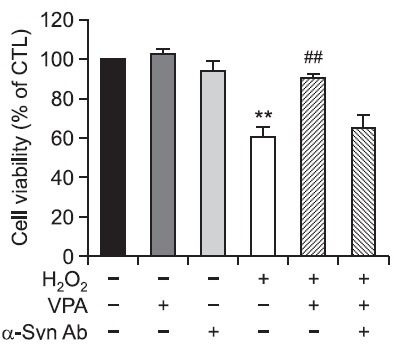
VPA prevents neuronal cell death by increasing the α-synuclein expression
Finally, we examined the effects of α-synuclein inhibition on VPA-induced neuroprotection. Cultured neuronal cells were treated with VPA and an antibody against α-synuclein 1 hr before H2O2 treatment. After 24 hr, cell viability was measured by MTT assay. VPA inhibited H2O2-induced neuronal cell death, and α-synuclein Ab inhibited the neuroprotective effect by VPA (Fig. 7). These results suggest that VPA prevents neuronal cell death, at least in part, by the increased expression of α-synuclein.
Fig. 7. Effects of α-synuclein inhibition on VPA-induced neuroprotection in primary cortical neurons. A. Primary cortical neurons in serum-free MEM were treated with VPA (1 mM) and α-synuclein Ab (10 μg) 1 hr before H2O2 treatment for 24 hr at 37℃. Cell viability was measured by MTT reduction assay. Values are expressed as the mean ± S.E.M. **p<0.01 vs. control; ##p<0.01 vs. H2O2.
DISCUSSION
In this study, we demonstrated that VPA induced the upregulation of α-synuclein in JNK dependent manner in rat primary astrocytes. α-synuclein is a presynaptic protein and controls neurotransmitter release, which is linked to PD and other synucleinopathies. Therefore, the overall- regulation of α-synuclein level is critical for its toxicity and synaptic function. Soluble form of α-synuclein oligomer rather than monomeric α-synuclein appear to present a toxic conformations of α-synuclein in PD brains. In this study, we observed the increase in monomeric α-synuclein by VPA in both cellular and secreted compartment (Fig. 1) suggesting increased secretion of α-synuclein in rat primary astrocytes. α-synuclein can be secreted and affect neuronal and glial function (Vekrellis and Stefanis, 2012). It was reported that soluble α-synuclein is cleared by the proteasome (Bennett et al., 1999) and autophagy (Webb et al., 2003) suggesting not only the transcriptional control but also the proteolytic degradation of α-synuclein may play important role in the regulation of overall α-synuclein level.
Recently, acetylation of key cellular proteins has been suggested as a major post-translational modification that regulates protein stability and transcriptional activity (Glozak et al., 2005; Singh et al., 2010). For example, in a series of biochemical and molecular biological experiments, it has been suggested that p300-dependent acetylation and HDAC1- mediated deacetylation process plays important role on the regulation of the protein stability of Nurr77. Overexpression of p300 or inhibition of HDAC activity using TSA increased the acetylation of Nurr77, which leads to the transient increase in protein level (Kang et al., 2010). Similar stabilizing effects of HDAC inhibition has been reported for ER (Kim et al., 2010) and WRN (Li et al., 2010). Interestingly, Li et al. reported that WRN stability is regulated by the ubiquitination pathway and WRN acetylation by CBP significantly reduces its ubiquitination (Li et al., 2010). Whether VPA-induced acetylation of α-synuclein protein is involved in the decreased ubiquitination and increased protein stability should be investigated further in the future study.
Recently, it has been reported that HDAC6 deficiency inhibits the clearance of α-synuclein resulting in the accumulation of α-synuclein in nucleus. HDAC6 recruits and binds polyubiquitinated proteins and dynein motors (Kawaguchi et al., 2003), and has been implicated in the regulation of formation of aggresome and degradation of misfolded proteins through autophagy pathway. Interestingly, the specific inhibitor of HDAC6, tubacin increased the level as well as the nuclear translocation of α-synuclein in A53T expressing PC12 cells (Su et al., 2011). Although VPA is rather selective against class I HDAC, it is still possible that VPA affects class IIB HDACs or other HDACs in a similar way to affect the ubiquitin-dependent degradation of α-synuclein. For example, at least one study suggested that VPA inhibits HDAC6 activity leading to the hyperacetylation of tubulin, which plays a role in the increased paclitaxel-induced cell death in a thyroid cancer cell line (Catalano et al., 2007). In silico prediction of possible acetylation on α-synuclein predicted lysine residues at position 6, 34 and 96 on α-synuclein are strong candidate for acetylation (supplementary Table 1). The actual site of acetylation modulated by VPA should be experimentally determined in the future study.
Supplementary table 1.
MDVFMKGLSKAKEGVVAAAEKTKQGVAEAAGKTKEGVLYVGSKTKEGVVHGVTTVAEKTKEQVTNVGGAVVTGVTAVAQKTVEGAGNIAAATGFVKKDQMGKGEEGYPQEGILEDMPVDPSSEAYEMPSEEGYQDYEPEA
| Sequence name | Position | Score |
|---|---|---|
|
| ||
| gi|9507125|ref|NP_062042.1| | K6 | 0.994041 |
| gi|9507125|ref|NP_062042.1| | K12 | 0.838264 |
| gi|9507125|ref|NP_062042.1| | K23 | 0.757646 |
| gi|9507125|ref|NP_062042.1| | K32 | 0.803234 |
| gi|9507125|ref|NP_062042.1| | K34 | 0.976814 |
| gi|9507125|ref|NP_062042.1| | K43 | 0.82382 |
| gi|9507125|ref|NP_062042.1| | K96 | 0.989674 |
| gi|9507125|ref|NP_062042.1| | K97 | 0.87061 |
The increase in α-synuclein level by VPA was also reported in cerebellar granule cells (Leng and Chuang, 2006). In this case, the increase in α-synuclein level following VPA treatment is accompanied by the increase in mRNA expression and is dependent on the HDACi activity of VPA. Our results also suggested that α-synuclein protein and mRNA induction in rat primary astrocytes by VPA is mediated through the HDACi activity of VPA. This assumption is based on the results that VPA caused histone H3 hyperacetylation and that two other HDAC inhibitors, such as sodium butyrate and TSA, upregulated α-synuclein protein like VPA.
Mood-stabilizing agents such as VPA affect multiple signaling pathways including ERK (Einat et al., 2003) and JNK (Yamauchi et al., 2010). In this study, we showed that the effect of VPA on the acetylation of α-synuclein is mediated by the JNK signaling pathway. This conclusion is supported by the findings that a JNK inhibitor SP600125 inhibits the accumulation of α-synuclein by VPA and VPA induces the phosphorylation of JNK. S0600125 is a reversible ATP-competitive inhibitor for JNK1, JNK2 and JNK3 with IC50 of 40 nM, 40 nM and 90 nM, respectively, and inhibits the phosphorylation of c-Jun. Recently, it was reported that SP600125 also inhibits aryl hydrocarbon receptor (AhR, ~10 μM), other kinases including Aurora kinase A, FLT3 and MELK with IC50 of 60 nM, 90 nM and 70 nM (Colombo et al., 2010). Therefore, it is also possible that SP600125 regulates the expression of synuclein by modulating the activity of kinases other than JNK. Molecular biological experiments including siRNA knockdown of JNK may provide more direct answer regarding the role of JNK in the regulation of synuclein expression. However, JNK plays an important role in the regulation of cell survival, death and growth, especially, in polarized cells such as neurons, which make it extremely difficult to knock down JNK in neuron without cellular toxicity (Tönges et al., 2011). Further study might be required to unequivocally demonstrate the role of JNK on α-synuclein expression. VPA upregulates Gadd45a, which activates the downstream of JNK cascade to induce neurite extension in N1E-115 neuroblastoma cells (Yamauch et al., 2010). It is reported that JNK pathway is involved not only in the transcriptional up-regulation of target proteins but also in the regulation of protein degradation. JNK signaling pathway is linked to cAMP-dependent proteolysis (Ushijima and Maeda, 2012) and is a target for proteasomal degradation of c-Jun dimerization protein 2 (Weidenfeld-Baranboim et al., 2011). And also, the acetylation of p53 protein by SIRT1 activator is increased through JNK signal in cultured skin keratinocytes, which provides the protection (Cao et al., 2009). It is reported that JNK determines the stability of F-actin protein and increase filopodium numbers (Björkblom et al., 2012).
Although majority of studies suggest that increased α-synuclein may underlie pathological changes in PD, especially when it associated with the increased aggregation, it is not clear whether increased α-synuclein by VPA in astrocytes leads to the increased aggregation of the protein. Considering the expression level of α-synuclein in astrocytes is relatively low compared with neuron, it is doubtful that the increased α-synuclein level leads to the immediate toxicity to neuron or astrocytes. Several researchers have suggested the protective role of VPA against neurotoxic insults such as excitotoxic injury and rotenone-induced PD model in rats (Kanai et al., 2004; Monti et al., 2010). In an experiment using siRNA against α-synuclein, the increased α-synuclein level by VPA has been associated with the regulation of both pro and anti-apoptotic proteins (Leng and Chuang, 2006). In addition to the neuroprotective role, normal physiological function of α-synuclein has also been assigned to the regulation of synaptic differentiation as well as transmission including dopaminergic synapses (Sidhu et al., 2004), although even the modest increase in α-synuclein expression has been suggested to interfere with the normal synaptic recycling process (Nemani et al., 2010).
In conclusion, VPA induced α-synuclein expression by transcriptional control and increased acetylation, possibly through the regulation of JNK activity in rat primary astrocytes. The search for physiological and pathological significance of the present findings may provide insights into the understanding of the exact role of α-synuclein in brain as well as the identification of new targets for the regulation of α-synuclein level in PD.
Supplementary table legend
Supplementary table 1. Predicted acetylation sites on α-synuclein were analyzed using a web-based prediction algorithm (http://www.aporc.org/EnsemblePail/index.html). Lysine residue at position 6, 34 and 96 on α-synuclein showed high probability of putative acetylation (Hubinger et al.). Residues with mid to low probability of acetylation were also shown in the table. Above is the peptide sequence of human α-synuclein.
Acknowledgments
This work was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120029).
References
- 1.Alves Da Costa C., Paitel E., Vincent B., Checler F. Alpha- synuclein lowers p53-dependent apoptotic response of neuronal cells. Abolishment by 6-hydroxydopamine and implication for Parkinson's disease. J. Biol. Chem. (2002);277:50980–50984. doi: 10.1074/jbc.M207825200. [DOI] [PubMed] [Google Scholar]
- 2.Bennett M. C., Bishop J. F., Leng Y., Chock P. B., Chase T. N., Mouradian M. M. Degradation of alpha-synuclein by proteasome. J. Biol. Chem. (1999);274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 3.Björkblom B., Padzik A., Mohammad H., Westerlund N., Komulainen E., Hollos P., Parviainen L., Papageorgiou A. C., Iljin K., Kallioniemi O., Kallajoki M., Courtney M. J., Mågård M., James P., Coffey E.T. c-Jun N-terminal kinase phosphorylation of MARCKSL1 determines actin stability and migration in neurons and in cancer cells. Mol. Cell. Biol. (2012);32:3513–3526. doi: 10.1128/MCB.00713-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao C., Lu S., Kivlin R., Wallin B., Card E., Bagdasarian A., Tamakloe T., Wang W. J., Song X., Chu W. M., Kouttab N., Xu A., Wan Y. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. (2009);13:3632–3643. doi: 10.1111/j.1582-4934.2008.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalano M. G., Poli R., Pugliese M., Fortunati N., Boccuzzi G. Valproic acid enhances tubulin acetylation and apoptotic activity of paclitaxel on anaplastic thyroid cancer cell lines. Endocr. Relat. Cancer. (2007);14:839–845. doi: 10.1677/ERC-07-0096. [DOI] [PubMed] [Google Scholar]
- 6.Colombo R., Caldarelli M., Mennecozzi M., Giorgini M. L., Sola F., Cappella P., Perrera C., Depaolini S. R., Rusconi L., Cucchi U., Avanzi N., Bertrand J. A., Bossi R. T., Pesenti E., Galvani A., Isacchi A., Colotta F., Donati D., Moll J. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. (2010);70:10255–10264. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
- 7.Einat H., Yuan P., Gould T. D., Li J., Du J., Zhang L., Manji H. K., Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J. Neurosci. . (2003);23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng H., Liu Q., Xue C., David L., Beer T. M., Thomas G. V., Dai M. S., Qian D. Z. HIF1α protein stability is increased by acetylation at lysine-K709. J. Biol. Chem. (2012);287:35496–35505. doi: 10.1074/jbc.M112.400697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glozak M. A., Sengupta N., Zhang X., Seto E. Acetylation and deacetylation of non-histone proteins. Gene . (2005);363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Jensen P. J., Alter B. J., O'Malley K. L. Alpha-synuclein protects naive but not dbcAMP-treated dopaminergic cell types from 1-methyl-4-phenylpyridinium toxicity. J. Neurochem. (2003);86:196–209. doi: 10.1046/j.1471-4159.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 11.Joo S. H., Kwon K. J., Kim J. W., Hasan M. R., Lee H. J., Han S. H., Shin C. Y. Regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by alpha-synuclein in rat primary glial cells. Neurosci. Lett. (2010);469:352–356. doi: 10.1016/j.neulet.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Kanai H., Sawa A., Chen R. W., Leeds P., Chuang D. M. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. (2004);4:336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- 13.Kang S. A., Na H., Kang H. J., Kim S. H., Lee M. H., Lee M. O. Regulation of Nur77 protein turnover through acetylation and deacetylation induced by p300 and HDAC1. Biochem. Pharmacol. (2010);80:867–873. doi: 10.1016/j.bcp.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Karpinar D. P., Balija M. B., Kügler S., Opazo F., Rezaei-Ghaleh N., Wender N., Kim H. Y., Taschenberger G., Falkenburger B. H., Heise H., Kumar A., Riedel D., Fichtner L., Voigt A., Braus G. H., Giller K., Becker S., Herzig A., Baldus M., Jäckle H., Eimer S., Schulz J. B., Griesinger C., Zweckstetter M. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. (2009);28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y., Kovacs J. J., McLaurin A., Vance J. M., Ito A., Yao T. P. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell . (2003);115:727–738. doi: 10.1016/S0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim S. H., Kang H. J., Na H., Lee M. O. Trichostatin A enhances acetylation as well as protein stability of ERalpha through induction of p300 protein. Breast Cancer Res. (2010);12:R22. doi: 10.1186/bcr2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruger R., Kuhn W., Muller T., Woitalla D., Graeber M., Kosel S., Przuntek H., Epplen J. T., Schols L., Riess O. Ala-30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. (1998);18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 18.Lee H. J., Patel S., Lee S. J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J. Neurosci. (2005);25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H. J., Shin S. Y., Choi C., Lee Y. H., Lee S. J. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Biol. Chem. . (2002);277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- 20.Leng Y., Chuang D. M. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J. Neurosci. (2006);26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.L'Episcopo F., Tirolo C., Testa N., Caniglia S., Morale M. C., Marchetti B. Glia as a turning point in the therapeutic strategy of Parkinson's disease. CNS Neurol. Disord. Drug Targets. (2010);9:349–372. doi: 10.2174/187152710791292639. [DOI] [PubMed] [Google Scholar]
- 22.Li K., Wang R., Lozada E., Fan W., Orren D. K., Luo J. Acetylation of WRN protein regulates its stability by inhibiting ubiquitination. PloS One. (2010);5:e10341. doi: 10.1371/journal.pone.0010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchetti B. Cross-talk signals in the CNS: Role of neurotrophic and hormonal factors, adhesion molecules and intercellular signaling agents in luteinizing hormone-releasing hormone (LHRH) neuron-astroglia interactive network. Front. Biosci. (1997);2:d88–125. doi: 10.2741/a177. [DOI] [PubMed] [Google Scholar]
- 24.Monti B., Gatta V., Piretti F., Raffaelli S. S., Virgili M., Contestabile A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson's disease: involvement of alpha-synuclein. Neurotox. Res. (2010);17:130–141. doi: 10.1007/s12640-009-9090-5. [DOI] [PubMed] [Google Scholar]
- 25.Monti B., Polazzi E., Contestabile A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. (2009);2:95–109. doi: 10.2174/1874467210902010095. [DOI] [PubMed] [Google Scholar]
- 26.Nemani V. M., Lu W., Berge V., Nakamura K., Onoa B., Lee M. K., Chaudhry F. A., Nicoll R. A., Edwards R. H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. (2010);65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J. Y., Paik S. R., Jou I., Park S. M. Microglial phagocytosis is enhanced by monomeric alpha-synuclein, not aggregated alpha-synuclein: implications for Parkinson's disease. Glia . (2008);56:1215–1223. doi: 10.1002/glia.20691. [DOI] [PubMed] [Google Scholar]
- 28.Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di I. G., Golbe L. I., Nussbaum R. L. Mutation in the alphasynuclein gene identified in families with Parkinson's disease. Science. (1997);276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 29.Seo J. H., Rah J. C., Choi S. H., Shin J. K., Min K., Kim H. S., Park C. H., Kim S., Kim E. M., Lee S. H., Lee S., Suh S. W., Suh Y. H. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. (2002);16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- 30.Shin C. Y., Choi J. W., Jang E. S., Ryu J. H., Kim W. K., Kim H. C., Ko K. H. Glucocorticoids exacerbate peroxynitrite mediated potentiation of glucose deprivation-induced death of rat primary astrocytes. Brain Res. (2001);923:163–171. doi: 10.1016/S0006-8993(01)03212-7. [DOI] [PubMed] [Google Scholar]
- 31.Sidhu A., Wersinger C., Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. (2004);18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- 32.Singh B. N., Zhang G., Hwa Y. L., Li J., Dowdy S. C., Jiang S. W. Nonhistone protein acetylation as cancer therapy targets. Expert Rev. Anticancer Ther. . (2010);10:935–954. doi: 10.1586/era.10.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sofroniew M., Vinters H. B. Astrocytes: biology and pathology. Acta Neuropathol. . (2010);119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. (1997);388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 35.Su M., Shi J. J., Yang Y. P., Li J., Zhang Y. L., Chen J., Hu L. F., Liu C. F. HDAC6 regulates aggresome-autophagy degradation pathway of alpha-synuclein in response to MPP+-induced stress. J. Neurochem. . (2011);117:112–120. doi: 10.1111/j.1471-4159.2011.07180.x. [DOI] [PubMed] [Google Scholar]
- 36.Tönges L., Planchamp V., Koch J. C., Herdegen T., Bähr M., Lingor P. JNK isoforms differentially regulate neurite growth and regeneration in dopaminergic neurons in vitro. J. Mol. Neurosci. (2011);45:284–293. doi: 10.1007/s12031-011-9519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ushijima H., Maeda M. cAMP-dependent proteolysis of GATA-6 is linked to JNK-signaling pathway. Biochem. Biophys. Res. Commun. . (2012);423:679–683. doi: 10.1016/j.bbrc.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Vekrellis K., Stefanis L. Targeting intracellular and extracellular alpha-synuclein as a therapeutic strategy in Parkinson's disease and other synucleinopathies. Expert Opin. Ther. Targets. (2012);16:421–432. doi: 10.1517/14728222.2012.674111. [DOI] [PubMed] [Google Scholar]
- 39.Webb J. L., Ravikumar B., Atkins J., Skepper J. N., Rubinsztein D. C. Alpha-Synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. (2003);278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 40.Weidenfeld-Baranboim K., Koren L., Aronheim A. Phosphorylation of JDP2 on threonine-148 by the c-Jun N-terminal kinase targets it for proteosomal degradation. Biochem. J. (2011);436:661–669. doi: 10.1042/BJ20101031. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi J., Torii T., Kusakawa S., Sanbe A., Nakamura K., Takashima S., Hamasaki H., Kawaguchi S., Miyamoto Y., Tanoue A. The mood stabilizer valproic acid improves defective neurite formation caused by Charcot-Marie-Tooth diseaseassociated mutant Rab7 through the JNK signaling pathway. J. Neurosci. Res. (2010);88:3189–3197. doi: 10.1002/jnr.22460. [DOI] [PubMed] [Google Scholar]


