Abstract
This study was aimed at investigating the possible effects of phytoceramide (Pcer) on learning and memory and their underlying mechanisms. Phytoceramide was orally administered to ICR mice for 7 days. Memory performances were assessed using the passive avoidance test and Y-maze task. The expressions of phosphorylated cAMP response element binding protein (pCREB), brain-derived neurotrophic factor (BDNF) were measured with immunoblot. The incorporation of 5-bromo-2-deoxyuridine (BrdU) in hippocampal regions was investigated by using immunohistochemical methods. Treatment of Pcer enhanced cognitive performances in the passive avoidance test and Y-maze task. Immunoblotting studies revealed that the phosphorylated CREB and BDNF were significantly increased on hippocampus in the Pcer-treated mice. Immunohistochemical studies showed that the number of immunopositive cells to BrdU was significantly increased in the hippocampal dentate gyrus regions after Pcer-treatment for 7 days. These results suggest that Pcer contribute to enhancing memory and BDNF expression and it could be secondary to the elevation of neurogenesis.
Keywords: Memory, Neurogenesis, Phytoceramide, Y-maze
INTRODUCTION
It has been emphasized the importance of sphingolipids as bioactive molecules that regulate various cellular processes such as proliferation, growth, differentiation, migration, and apoptosis (Posse de Chaves, 2006). In sphingolipid pathway, the phosphorylation of sphingosine by sphingosine kinase generated sphingosin-1-phosphate (S1P). The fatty acyl-CoA is connected to dihydrosphingosine or phytosphingosine (Pso) by amide bond to generate dihydroceramide or phytoceramide (Pcer) (Garcia et al., 2008). Phytosphingosine is the primary sphingoid base which is produced by hydroxylated dihydroceramide at C4 (Garcia et al., 2008). Phytoceramide generated from acylated Pso by many fatty acids and Pcer has the structural backbone with trans double bond at C4 it can be hydrolyzed to Pso by ceramidase (Mao et al., 2001). The possible physiological role of these complicated molecules for neuronal function has been extensively studied. The S1P concentration increasing mostly promotes cell proliferation and survival (Hait et al., 2006; Hannun and Obeid, 2008). An anti-apoptotic and pro-autophagic effects of S1P could modulate the progress of senescence (Patschan et al., 2008), but correctly how S1P functioned in this system was not clear. A direct connection to the sphingolipid metabolism with the neuronal function has not been established, and it is not understood how this lipid metabolites lead to neuronal function. Phytoceramide showed neuroprotective activity in the glutamate-induced neurotoxicity in cultured cells and recovered the scopolamine-induced reduction of memory in the previous research (Jung et al., 2011). In the present study, we wanted to investigate whether the Pcer demonstrate memory enhancement and modulate the memory-involving signals as well as neurogenesis. To achieve the aim of this study, the memory performance was examined in memory impaired mice by scopolamine treatment using passive avoidance test and Y-maze task. Passive avoidance test is greatly dependent on long-term memory (Ambrogi Lorenzini et al., 1997). And it is well known that learning and memory are closely related to the cholinergic and glutamatergic neurotransmitter systems in brain; (Bartus et al., 1982; Durand et al., 1996). The scopolamine, a muscarinic receptor antagonist, blocked the cholinergic neurotransmitter and damaged learning and memory in mice (Bartus et al., 1982; Renner et al., 2005). The scopolamine-induced amnesic animal models have been used to evaluate for potential drug treatments for cognitive function (Ennaceur and Meliani, 1992). Y-maze task is used for screening working memory, a form of short-term memory (Sarter et al., 1988). In addition, neurogenesis in the hippocampus is also accompanied in learning and memory (Gould et al., 1999; Shors et al., 2001).
Immature neurons in hippocampus are accepted to play a role in the composition of new synaptic connections which are required for memory consolidation (van Praag et al., 2002). And adult hippocampal neurogenesis is involved in memory and affected by many factors which are connected with either memory enhancement or memory formation (Gould et al., 1999; Dupret et al., 2008). In addition, many previous researches demonstrated that neurotrophic factors, including BDNF, are critically demanded for neuronal survival during development and neurogenesis (Goldberg and Barres, 2000). Collectively, it indicates that BDNF signaling and neurogenesis indicate a mechanism of memory enhancement. In this study, we investigated whether Pcer affects hippocampal neurogenesis and hippocampus-dependent learning and memory performance in mice.
MATERIALS AND METHODS
Animals
Male ICR mice (28-30 g) were purchased from Orient Labanimal (Seoul, Korea). Mice allowed access to water and food ad libitum were grouped 5-6 per cage, and maintained at an ambient temperature of 23℃ and a 12 h diurnal light cycle (light on 07:00-19:00). All behavioral experiments were carried out in a room adjacent to that in which the mice were housed under the same conditions of temperature and light cycle. All the experiments were carried out according to the guidelines of the Animal Care and Use Guidelines of School of Medicine, Ewha Womans University, Korea.
Passive avoidance test
Passive avoidance test was carried out in identical light and dark boxes (Gemini Avoidance System, San Diego, CA, USA). The light compartment (20×20×20 cm) contained a 100 W bulb, and the floor of dark compartment (20×20×20 cm) was composed of 2 mm grid stainless steel bars spaced 1 cm apart. These compartments were separated by a small guillotine door (5×5 cm). One hour before the acquisition trial, mice were administered Pcer (5-50 mg/kg, p.o.). Memory impairment was induced by scopolamine treatment (1 mg/kg, i.p.) 30 min after the administration of Pcer. For the acquisition trial, mice were initially placed in the light compartment and the door between the two compartments was opened 10 s later. When mice entered the dark compartment, the door closed and the mouse stepped down onto the grid floor, it received an electrical foot shock (0.25 mA, 2.5 sec). After twenty four hours, the mice were again placed in the light compartment for the retention trials. The time taken for a mouse to enter the dark compartment was measured after door opening as latency times in both acquisition and retention trials. If a mouse did not enter the dark compartment within 300 sec, it was assumed that the mouse had remembered the single training trial.
Y-Maze task
The Y-maze is a three-arm triangle-shaped maze (40 cm-long and 3 cm-wide with 12 cm-high walls) in which the arms separated by 120o angles each other. The maze floor and walls were constructed from black polyvinyl plastic. Mice were initially placed on the central platform, and the sequence (i.e., ABCAB, etc.) and number of arm entries were recorded for each mouse with all four paws over 8-min period. An actual alternation was calculated as entries into all three arms on consecutive choices (i.e., ABC, CAB, or BCA but not BAB). Maze arms were thoroughly cleaned between tasks to remove residual odors. One hour after the last administration of Pcer or vehicle alone, memory impairment was induced by scopolamine treatment (1 mg/kg, i.p.). The percentage of alternations was defined according to the following equation: % alternation=[(number of alternations)/(total arm entries-2)] ×100. The number of arm entries served as an indicator of locomotor activity.
Bromodeoxyuridine (BrdU) administration and immunohistochemistry
To evaluate the effect of cell proliferation, the thymidine analog 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) was injected intraperitoneally (50 mg/kg, dissolved in PBS) twice daily with a 2 h interval after the last drug administration. The animals were sacrificed 16 h after the last injection. All procedures were based on information from Cameron and McKay (2001). The animals were anesthetized and perfused first with ice-cold PBS followed by a ice cold 4% paraformaldehyde (PFA) solution (in 0.1 M phosphate buffer, pH 7.4). The brains were removed and postfixed in the same fixation solution for 24 h at 4℃. Following post fixation, brains were overnight in 30% sucrose solution (in 0.1 M PBS) at 4℃ until they sank. For immunohistochemical staining of BrdU, frozen brains were coronally sectioned on a microtome at 30 μmthick between 1.70 and 1.94 mm posterior to the bregma as defined in mouse brain atlas for each brain and then stored in storage solution (30% ethylene glycol and 30% glycerin in a 0.02 М phosphate buffer) at 4℃. Free-floating brain sections were incubated in 0.3% H2O2 at room temperature to eliminate endogenous peroxidase for 15 min. Then they were rinsed with PBS followed by 0.3% Triton-X for 15 min. To detect BrdU labeled cell, free-floating sections were rinsed with PBS, and to denature DNA, sections were incubated in 2N HCl at 37℃ for 30 min. Sections were neutralized by incubating them for 15 min in PBS. Then the sections were incubated with rat anti-BrdU antibody (1:1,000, Abcam, USA) overnight at 4℃. After rinsed with PBS, the sections were incubated with biotinylated anti-rat IgG second antibody (1:200, Vector, CA, USA), and then with ABC complex (1:100, Vector, CA, USA ) for 1 h at room temperature. BrdU-labeled cells were visualized with nickel ammonium sulfate in DAB solution for about 3 min. The number of BrdU-positive cells in the hippocampal granular layer of the DG was counted in four slides per mouse using a computerized image analysis system (Leica Microsystems AG, Wetzlar, Germany) and results were averaged.
Statistical analysis
The results were subjected to an analysis of the variance (ANOVA) using the Newman-Keuls multiple comparison test. Differences with *p<0.05 and **p<0.01 were considered as statistically significant to analyze the difference.
RESULTS
Phytoceramide ameliorated the scopolamine-induced memory impairment
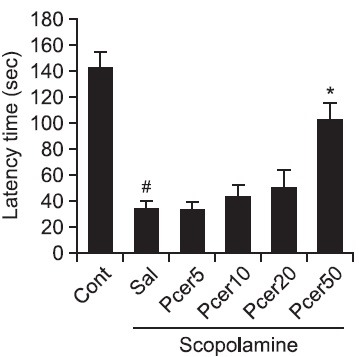
The effect of Pcer on scopolamine-induced memory deficit was measured by the passive avoidance test. Scopolamineinduced memory deficits during the passive avoidance test are dependent on long-term memory. Phytoceramide was administered to mice orally for 7 days, and memory recovery was observed on step-through latency in the retention trial (Fig. 1). The mean step-through latency of Pcer-treated (50 mg/kg, p.o.) mice was significantly longer than that of scopolaminetreated mice. This result showed that Pcer ameliorated the memory impairment.
Fig. 1. Effects of Pcer on the scopolamine-induced memory impairment in the passive avoidance test. Memory impairment was induced by scopolamine treatment (1 mg/kg, i.p.) in mice. At 60 min before acquisition trials, Pcer (5, 10, 20, 50 mg/kg) or saline was administered (p.o.) to mice. At 24 h after last acquisition trials, the retention trials were carried out for 5 min. Data were analyzed by one-way ANOVA for multiple comparison and Student-Newman- Keuls test as post hoc test. All values are expressed as mean ± S.E.M. from three independent experiments (n=6). #p<0.05 in comparison with control group, *p<0.05 in comparison with saline treated group.
Phytoceramide enhanced the memory in the Y-maze task
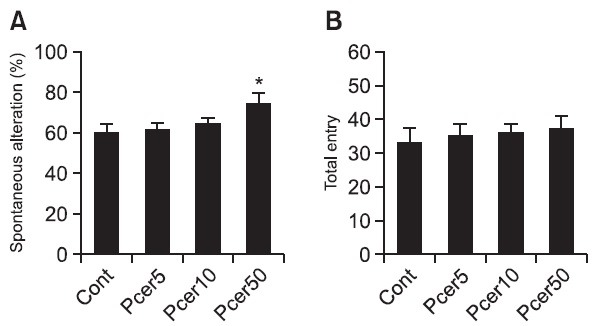
To determine whether Pcer modulate the working memory function, mice were tested in the Y-maze. Pcer was administered to mice orally for 7 days, and memory enhancing was observed in the Y-maze task. Pcer-treated group enhanced working memory since 50 mg/kg Pcer-treated group spent significantly high spontaneous alteration in the novel arm than controls (Fig. 2A). However, administration of Pcer did not affect the total entry in Y-maze task (Fig. 2B).
Fig. 2. The memory enhancing effect of Pcer in the Y-maze test. Mice were orally administered with Pcer (5, 10, 50 mg/kg) for 7 days. Spontaneous alteration behavior and number of entries during 8 mim sessions were measured. Data were analyzed by oneway ANOVA for multiple comparison and Student-Newman-Keuls test as post hoc test. All values are expressed as mean ± S.E.M. from independent experiments (n=6).*p<0.05 in comparison with control group.
Phytoceramide enhanced the neurogenesis in hippocampal area of mice
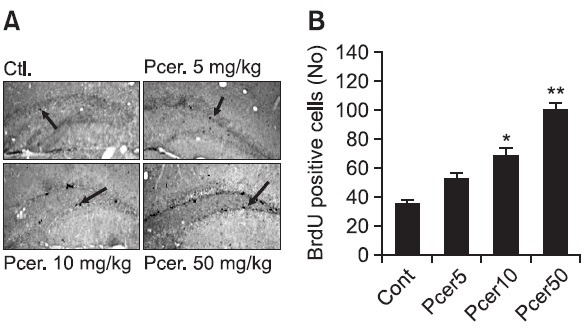
To determine the neurogeneration during memory enhancing after treatment of Pcer, the Pcer-related neurogenic effects was investigated by using the BrdU staining. Bromodeoxyuridine was administered to control or Pcer-treated mice, and 24 h later mice were anesthetized and perfused for immunohistochemical analysis. Representative sections of BrdU immunohistochemistry for the dentate gyrus were determined. A number of BrdU-positive cells were seen along the subgranular zone. The effect of Pcer on the cell proliferation was shown in the dentate gyrus after 7 days treatment (Fig. 3). The number of BrdU-positive cells in the dentate gyrus (DG) was increased after Pcer treatment in a dose dependent manner compared with the control group.
Fig. 3. Effect of Pcer on the neurogenesis by BrdU staining. Various doses of Pcer (5, 10, 50 mg/kg) were administered orally to mice for 7 days. BrdU was injected (50 mg/kg, i.p.) twice per day 2 h interval after last administration of Pcer. Immunohistochemistry with free-floating brain sections were stained in DAB solution. All values are expressed as mean ± S.E.M. from three independent experiments (n=6). *p<0.05, **p<0.01 in comparison with control group.
Phytoceramide increased the BDNF and CREB Expression
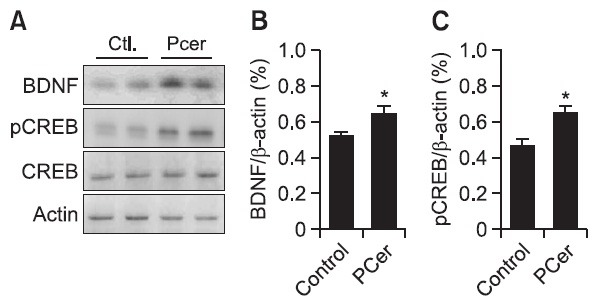
Hippocampal area of ICR mice were collected after oral administration of Pcer (50 mg/kg) for 7 days. The protein expression levels of the BDNF and phosphorylated CREB were increased by treatment with Pcer (Fig. 4). However, the expression of CREB was not changed by the treatment with Pcer. These results implied that the neurogenesis by Pcer might be due to the increasing of BDNF and pCREB expression.
Fig. 4. Effect of Pcer on pCREB and BDNF expression in hippocampus of mice. Mice were treated with PCer (50 mg/kg, p.o.) for 7 days. BDNF and phosphorylated CREB was measured by immunoblot analysis. Phytoceramide increased the BDNF and pCREB in mice. β-Actin was used as an internal control. All values are expressed as mean ± S.E.M. from three independent experiments (n=6).*p<0.05 in comparison with control group.
DISCUSSION
The results of this study demonstrate that Pcer (50 mg/kg) treatment for 7 days ameliorate the scopolamine-induced memory impariment in passive avoidance test. And Pcer administration for 7 days showed memory enhancing in Y-maze task. Y-maze task is largely dependent on working memory, a form of short-term memory (Sarter et al., 1988). Furthermore, the expressions of BDNF and pCREB were increased in the hippocampus of Pcer-treated mice. In addition, BrdU-immunopositive cells were increased in hippocampus by the Pcertreatment. And a number of researches have been made to find a relationship between memory and neurogenesis. It has been found that neurogenesis arises with age (Gage, 2000) in two specific regions, in the subventricular zone of the lateral ventricle and in the subgranular zone (SGZ) of the dentate gyrus (DG), in the adult brain (Christie and Cameron, 2006; Zhao et al., 2008). Constantly dividing progenitor cells inhabit in the SGZ of the DG, and these progenitor cells are generated to new neurons and granule cells (Weiss et al., 1996; Eriksson et al., 1998). It has been suggested that newborn cell proliferation enhances memory formation (Gould et al., 1999). Recently, it was also proved that newly generated neurons in the hippocampus are considered to play important roles in learning and memory (Taupin, 2005), and neurogenesis in the DG is well known to be controlled by stimuli. Therefore, an increased neuronal cell proliferation or immature neuronal survival in the DG might support memory enhancement by Pcer. To determine whether cell survival or proliferation is increased by Pcer, we injected BrdU to observe changes in proliferation of immature neuronal cells in the hippocampus. This study implicates that, repetitive administration Pcer produces cell proliferation in the DG of the mice hippocampus in a dosedependent manner. Thus, this result indicates that administration of Pcer increases neurogenesis in the DG region of hippocampus. The enhancement of hippocampal neurogenesis by Pcer could underlie the Pcer-induced memory enhancement.
Also many studies have shown that BDNF infusions raise neurogenesis and the induction of neurogenesis is known to be blocked in BDNF deletion mutant mice (Duman and Monteggia, 2006). BDNF has been shown to promote the survival and differentiation of neurons during development, in adult brain and in cultured cells (Memberg and Hall, 1995; Palmer et al., 1997; Takahashi et al., 1999). BDNF increases the syntheses of a many translational proteins (Liao et al., 2007) which is required for memory formation (Gruest et al., 2004). In addition, the pCREB is essentially needed for memory consolidation and storage in the hippocampus (O'Connell et al., 2000; Pittenger et al., 2002; Bozon et al., 2003). It has been informed that the CREB has a function in the modulation of cell proliferation using a dominant negative CREB over expessed mutant transgenic mice (Nakagawa et al., 2002). They reported that many BrdU-labeled cells were significantly reduced in the CREB negative mutant mice, it suggest that CREB induces a positive effect on cell proliferation in the adult hippocampus (Nakagawa et al., 2002). In addition, it has been also determined that an increasing CREB activity may enhance cell proliferation and memory (Walton et al., 1999). It has been recommended that the activation of the BDNF-CREB signaling may accompany in the memory enhancement. Similarly, our findings also showed that Pcer increased the expression of BDNF and pCREB in the hippocampus. Therefore, it might be suggested that the activation of the BDNF and CREB signaling pathways could mediate the effects of Pcer on neurogenesis. In conclusion, the present study revealed that Pcer stimulates progenitor cell proliferation and suggested that the increase in neurogenesis by Pcer could be associated with its memory enhancing effects.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (MRC, 2010-0029355) fundend by the Korean Government (MEST).
References
- 1.Ambrogi Lorenzini C. G., Baldi E., Bucherelli C., Sacchetti B., Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res. . (1997);768:242–248. doi: 10.1016/S0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- 2.Bartus R. T., Dean R. L., Beer B., Lippa A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science. (1982);217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 3.Bozon B., Kelly A., Josselyn S. A., Silva A. J., Davis S., Laroche S., Bozon B., Kelly Á., Josselyn S. A., Silva A. J. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philol. Trans. R. Soc. Lond. B. Biol. Sci. (2003);358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron H. A., McKay R. D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. (2001);435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 5.Christie B. R., Cameron H. A. Neurogenesis in the adult hippocampus. Hippocampus. (2006);16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- 6.Duman R. S., Monteggia L. M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry . (2006);59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Dupret D., Revest J. M., Koehl M., Ichas F., De Giorgi F., Costet P., Abrous D. N., Piazza P. V. Spatial relational memory requires hippocampal adult neurogenesis. PloS one . (2008);3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand G. M., Kovalchuk Y., Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. (1996);381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- 9.Ennaceur A., Meliani K. Effects of physostigmine and scopolamine on rats' performances in object-recognition and radialmaze tests. Psychopharmacology. (1992);109:321–330. doi: 10.1007/BF02245880. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson P. S., Perfi lieva E., Björk-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. Neurogenesis in the adult human hippocampus. Nat. Med. . (1998);4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 11.Gage F. H. Mammalian neural stem cells. Science. (2000);287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 12.Garcia J., Shea J., Alvarez-Vasquez F., Qureshi A., Luberto C., Voit E. O., Del Poeta M. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol. Syst. Biol. (2008);4:183. doi: 10.1038/msb.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg J. L., Barres B. A. The relationship between neuronal survival and regeneration. Annu. Rev. Neurosci. (2000);23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 14.Gould E., Beylin A., Tanapat P., Reeves A., Shors T. J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. (1999);2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 15.Gruest N., Richer P., Hars B. Memory consolidation and reconsolidation in the rat pup require protein synthesis. J. Neurosci. (2004);24:10488–10492. doi: 10.1523/JNEUROSCI.2984-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hait N. C., Oskeritzian C. A., Paugh S. W., Milstien S., Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. (2006);1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Hannun Y. A., Obeid L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. (2008);9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 18.Jung J.-C., Lee Y., Moon S., Ryu J. H., Oh S. Phytoceramide shows neuroprotection and ameliorates scopolamineinduced memory impairment. Molecules. (2011);16:9090–9100. doi: 10.3390/molecules16119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao L., Pilotte J., Xu T., Wong C. C. L., Edelman G. M., Vanderklish P., Yates III J. R. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J. Proteome Res. (2007);6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- 20.Mao C., Xu R., Szulc Z. M., Bielawska A., Galadari S. H., Obeid L. M. Cloning and characterization of a novel human alkaline ceramidase. A mamalian enzyme that hydrolyzes phytoceramide. J. Biol. Chem. . (2001);276:26577–26588. doi: 10.1074/jbc.M102818200. [DOI] [PubMed] [Google Scholar]
- 21.Memberg S. P., Hall A. K. Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol. Cell. Neurosci. (1995);6:323–335. doi: 10.1006/mcne.1995.1025. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa S., Kim J. E., Lee R., Malberg J. E., Chen J., Steffen C., Zhang Y. J., Nestler E. J., Duman R. S. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J. Neurosci. (2002);22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connell C., Gallagher H. C., O'Malley A., Bourke M., Regan C. M. CREB phosphorylation coincides with transient synapse formation in the rat hippocampal dentate gyrus following avoidance learning. Neural Plast. (2000);7:279–289. doi: 10.1155/NP.2000.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer T. D., Takahashi J., Gage F. H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. (1997);8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 25.Patschan S., Chen J., Polotskaia A., Mendelev N., Cheng J., Patschan D., Goligorsky M. S. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am. J. Physiol. Heart Circ. Physiol. (2008);294:H1119–H1129. doi: 10.1152/ajpheart.00713.2007. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger C., Huang Y. Y., Paletzki R. F., Bourtchouladze R., Scanlin H., Vronskaya S., Kandel E. R. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. (2002);34:447–462. doi: 10.1016/S0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- 27.Posse de Chaves E. I. Sphingolipids in apoptosis, survival and regeneration in the nervous system. Biochim. Biophys. Acta. (2006);1758:1995–2015. doi: 10.1016/j.bbamem.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Renner U. D., Oertel R., Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther. Drug Monit. (2005);27:655–665. doi: 10.1097/01.ftd.0000168293.48226.57. [DOI] [PubMed] [Google Scholar]
- 29.Sarter M., Bodewitz G., Stephens D. N. Attenuation of scopolamine- induced impairment of spontaneous alternation behaviour by antagonist but not inverse agonist and agonist β-carbolines. Psychopharmacology. (1988);94:491–495. doi: 10.1007/BF00212843. [DOI] [PubMed] [Google Scholar]
- 30.Shors T. J., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. (2001);410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi J., Palmer T. D., Gage F. H. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J. Neurobiol. . (1999);38:65–81. doi: 10.1002/(SICI)1097-4695(199901)38:1&lt;65::AID-NEU5&gt;3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Taupin P. Adult neurogenesis in the mammalian central nervous system: functionality and potential clinical interest. Med. Sci. Monit. (2005);11:RA247–252. [PubMed] [Google Scholar]
- 33.van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. Functional neurogenesis in the adult hippocampus. Nature. (2002);415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walton M., Woodgate A. M., Muravlev A., Xu R., During M. J., Dragunow M. CREB phosphorylation promotes nerve cell survival. J. Neurochem. (1999);73:1836–1842. [PubMed] [Google Scholar]
- 35.Weiss S., Reynolds B. A., Vescovi A. L., Morshead C., Craig C. G., der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. (1996);19:387–393. doi: 10.1016/S0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhao C., Deng W., Gage F. H. Mechanisms and functional implications of adult neurogenesis. Cell. (2008);132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]


