Abstract
Monoamine oxidase inhibitors (MAOI) have been widely used as antidepressants. Recently, there has been renewed interest in MAO inhibitors. The activity-guided fractionation of extracts from Angelica keiskei Koidzumi (A. keiskei K.) led to the isolation of two prenylated chalcones, xanthoangelol and 4-hydroxyderricin and a flavonoid, cynaroside. These three isolated compounds are the major active ingredients of A. keiskei K. to inhibit the MAOs and DBH activities. Xanthoangelol is a nonselective MAO inhibitor, and a potent dopamine β-hydroxylase (DBH) inhibitor. IC50 values of xanthoangelol to MAO-A and MAO-B were calculated to be 43.4 μM, and 43.9 μM. These values were very similar to iproniazid, which is a nonselective MAO inhibitor used as a drug against depression. The IC50 values of iproniazid were 37 μM, and 42.5 μM in our parallel examination. Moreover, IC50 value of xanthoangelol to DBH was calculated 0.52 μM. 4-Hydroxyderricin is a potent selective MAO-B inhibitor and also mildly inhibits DBH activity. The IC50 value of 4-hydroxyderricin to MAO-B was calculated to be 3.43 μM and this value was higher than that of deprenyl (0.046 μM) used as a positive control for selective MAO-B inhibitor in our test. Cynaroside is a most potent DBH inhibitor. The IC50 value of cynaroside to DBH was calculated at 0.0410 μM. Results of this study suggest that the two prenylated chalcones, xanthoangelol and 4-hydroxyderricin isolated from A. keiskei K., are expected for potent candidates for development of combined antidepressant drug. A. keiskei K. will be an excellent new bio-functional food material that has the combined antidepressant effect.
Keywords: Angelica keiskei Koidzumi, Monoamine oxidase inhibitor, Dopamine β-hydroxylase inhibitor, Xanthoangelol, 4-hydroxyderricin, Cynaroside
INTRODUCTION
Monoamine oxidase (MAO) inhibitors were the first antidepressants introduced, but their use has dwindled because of their reported side effects, their food and drug interactions, and the introduction of other classes of agents. However, there has been renewed interest in MAO inhibitors (Wimbiscus et al., 2010). Recently, Meyer group reported the relationship between MAO-A levels and selective serotonin reuptake inhibitor (SSRI) treatment, recovery, and recurrence in major depressive disorder (MDD). They concluded that from the perspective of monoamine theory, SSRI raise serotonin levels vigorously whereas elevated MAO-A levels would be expected to metabolize serotonin, norepinephrine, and dopamine excessively. The mismatch between monoamine levels raised by treatment and monoamine levels lowered by disease processes might, at times, contribute to lack of response to SSRI treatment (Meyer et al., 2009). Consequently, A MAO-A inhibitor helps on the treatment of depression with a SSRI. On the other hand, Kitaichi group reported that the combined treatment with a MAO-A inhibitor and MAO-B inhibitor strengthens antidepressant effects because the combined treatment increases extracellular norepinephrine levels more than MAO-A inhibitor alone through increases in β-phenylethylamine (Kitaichi et al., 2010). Angelica keiskei Koidzumi (Umbelliferae), which is growing mainly along the Pacific coast of Asia is a hardy perennial herb. It has been used traditionally as a diuretic, laxative, analeptic and galactagogue, and has recently gained attention as a health food in Korea. It has been studied as folklore medicine for many metabolic diseases, such as hypertension, hepatosis and neuralgia (Kim et al., 1992), and is reported to have numerous biological benefits, such as anti-hyperlipidemic activity (Park et al., 1997), lowering blood pressure (Shimizu et al., 1999), antitumor action (Okuyama et al., 1991), and suppression of gastric acid secretion (Fujita et al., 1992). Various chalcones, coumarins and flavonoids have so far been isolated and characterized from this plant (Baba et al., 1990; Park et al., 1995; Akihisa et al., 2003).
Although Ogawa group reported the dietary 4-hydroxyderricin produces suppression in the elevation of systolic blood pressure (Ogawa et al., 2005), it remains unclear whether A. keiskei effects to the nervous system, such as nervous sedative effect, antidepression, and dementia treatment, which could be controlled by MAO or DBH inhibitors.
MAO inhibitors were among the first drugs used in the treatment of depression (Quitkin et al., 1979), Parkinson’s disease (PD) (Birkmayer et al., 1983; Riederer et al., 1983; Stern et al., 1983) and schizophrenia (Rigal and Zarifian, 1983). MAO-B inhibitors have been used in the treatment of Parkinson disease. Especially, selective MAO-B inhibitor, selegiline does show success as an adjunctive treatment for Parkinson disease and does not necessitate any dietary restriction. Particularly, DBH inhibitors can provide the added advantage of increasing levels of dopamine (DA) with MAO-B inhibitors.
In the present study, we showed that the two prenylated chalcones, xanthoangelol and 4-hydroxyderricin isolated from A. keiskei have inhibitory activities against MAO-A and MAOB. We also investigated the inhibitory activities of a flavonoid, cynaroside on DBH activities in in vitro assay.
MATERIALS AND METHODS
Plant materials
Fresh leaves and stems of A. keiskei were collected from Yangju, Korea, and identified by Dr. KW Park, botanist at the Korea National Arboretum. The voucher specimen (NP20-204) has been deposited in the specimen room of Duksung Women’s university, Seoul, Korea.
Chemicals and instruments
NMR experiments were performed on a Bruker/Advance- 600 (600 MHz). The chemical shifts were reported in parts per million, and the coupling constants (J values) were in Hertz. Exact masses were measured by a Hewlett Packard 5890 series II (EI-MS) or Jeol JMSAX505WA Mass spectrometer. Column chromatography was carried out on silica gel 60 (0.063-0.200 μm; Merck 7734) and Lichroprep RP-18 (particle size 40-60 μm, Merck). TLC analyses were carried out on silicagel 60 F254 (Merck 7734) and RP-18 F254s (Merck 15685) plates. In bioassay experiments, UV absorbance was measured by a UVIKON XS UV/Vis spectrometer of SECOMAN. Serotonin creatinine sulfate, Iproniazid, deprenyl Tyramine- HCl, Dowex 50W 8 and Amberlite CG50 were purchased from Sigma Co., USA, and Benzylamine-HCl from Tokyo Kasei Co., Japan.
Animals
Male Sprague-Dawley rats weighing 180-200 g were obtained from the Orient Animal Laboratoty (Seoul, Korea) and were maintained on a 12 h light-dark cycle (light phase: 06:30-18:30) in a temperature-controlled environment (22 ± 1℃) with free access to food and water. Experiment began after 10 day period of acclimatization. All procedures were approved by the Kunkuk University Animal Care and Use Committee. They complied with the Guide for the Care and Use of Laboratory Animals, Bio-Food and Drug Research Center Kunkuk University.
Procedures of MAO-A assay in vitro
Rat brain mitochondrial MAO was prepared by the method of Kim et al. (2012). The activity of MAO-A was measured according to Han et al. (2001) using serotonin as the substrate. The reaction mixture containing 0.5 ml of enzyme solution in 10 mM phosphate buffered saline (pH 7.1) and 1 ml of test solution was preincubated at 37℃ for 15 min, after which 0.5 ml of 1 mM solution of buffered serotonin creatinine sulfate (Sigma, USA) was added. Following incubation at 37℃ for 90 min, the enzyme reaction was terminated by heating the reaction mixture for 3 min in a boiling water bath. After being centrifuged, 1.6 ml of the supernatant, was applied to an Amberlite CG50 column (0.8 i.d.×3 cm). The column was washed with 40 ml of distilled water and eluted with 3 ml of 4N acetic acid. The absorbance of the metabolite that produced after being reacted with MAO-A was measured at 277 nm. Purely isolated compounds were dissolved in DMSO and then suspended in water for testing their inhibitory activities on MAO-A. Final concentration of DMSO in enzymatic reaction mixture was below 5%. Iproniazid was used as positive control for nonselective inhibitor.
Procedures of MAO-B assay in vitro
Rat liver mitochondrial MAO was prepared by Zeller’s method (Kim et al., 2012). The activity of MAO-B was measured according to Han et al. (2001), using benzylamine as the substrate. The reaction mixture containing 0.5 ml of enzyme solution in 10 mM phosphate buffered saline (pH 7.1) and 1 ml of test solution was preincubated at 37℃ for 15 min and then cooled in an ice bath. 0.5 ml of 4 mM benzylamine HCl (Tokyo Kasei Co., Japan) in buffer was added to it. This mixture was further incubated at 37℃ for 90 min in a shaking water bath. The reaction was terminated by addition of 0.2 ml of 60% perchloric acid. After extraction with 3 ml of cyclohexane, the organic layer was taken and the absorbance of benzaldehyde produced was measured at 242 nm. Purely isolated compounds were dissolved in DMSO and then suspended in water for testing their inhibitory activities on MAO-A. Final concentration of DMSO in enzymatic reaction mixture was below 5%. Deprenyl was used as positive control for selective MAOB inhibitor.
Procedures of DBH assay in vitro
The enzyme activity of DBH was determined according to Kim et al. (2012). Using tyramine as the substrate. The following were sequentially added to 0.3 ml of enzyme solution in 0.25 M sucrose: 1 ml of test solution; 0.2 ml of 3 mg/ml catalase; 0.5 ml of 1 M acetate buffer (pH 5.0); and 0.5 ml of a reaction aid, prepared by dissolving fumaric acid, Nethylmaleimide, iproniazide phosphate and ascorbic acid to concentrations of 0.06, 0.06, 0.006 and 0.06 M, respectively, in distilled water. The solution was allowed to stir at 37℃ for 15 min, and then 0.5 ml of 0.12 M tyramine HCl solution was added and the resulting mixture was allowed to stir for 90 min. Next, 0.4 ml of 3 M solution of trichloroacetic acid was added to the reaction mixture to terminate the enzyme reaction. Immediately thereafter, the solution was centrifuged and 3 ml of the supernatant was poured onto a Dowex 50 W×8 column (0.8 i.d.×3 cm, H+ form, 200-400 mesh) and the column was washed with 30 ml of distilled water. Three milliliters of 4 N NH4OH solutions were then added to the column. The eluate was collected in a test tube and 0.2 ml of 4% sodium metaperiodate solution was added. The test tube was allowed to stand for 10 min, before 0.2 ml of 20% sodium metabisulfite solution was added. The absorbance of the resulting mixture was measured at 330 nm.
Isolation of xanthoangelol, 4-hydroxyderricin and cynaroside from A. keiskei
The dried aerial parts (10 kg) of A. keiskei were extracted three times with a mixture of methanol and distilled water (7:3, v/v: 25 L) at 80℃ for 4 hr. The combined MeOH extract was concentrated in vacuo at 45℃ to give 2.8 kg residue. The purification and isolation of two prenylated chalcones and a flavonoid from the EtOAc and n-BuOH extract were performed according to general chromatographic methods. Briefly, the EtOAc and BuOH extract was chromatographed on a silica gel column (Silica gel 60, 70-230 mesh: Merck Japan, Tokyo, Japan) using stepwise gradient elution with the hexanedihloromethane (1:3), dichloromethane-methanol (30:1), dichloromethane- methanol (3:1) to yield fraction I to VI. The xanthoangelol (3.1 g, 99% purity, yellow needles) was finally purified with a LiChroprep RP-18 Lobar column (Merck, Japan) with 80% methanol from the fraction I (15 g). The 4-hydroxyderricin (400 mg, 98% purity, yellow powders) was finally purified with a LiChroprep RP-18 Lobar column with 80% methanol from the fraction II (6 g). The fraction VI (13 g) was subjected to silica gel column chromatography using dicloromethane-methanol (5:1) to afford cynaroside (300 mg, 99% purity, yellowneedles). These three compounds were identified by direct comparison with an authentic sample (Park et al., 1995; Akihisa et al., 2003). The structures of the isolated compounds are given in Fig. 1.
Fig. 1. The Structures of the two prenylated chalcones, xanthoangelol (1) and 4-hydroxyderricin (2) and a flavonoid, cynaroside (3) isolated from Angelica keiskei.
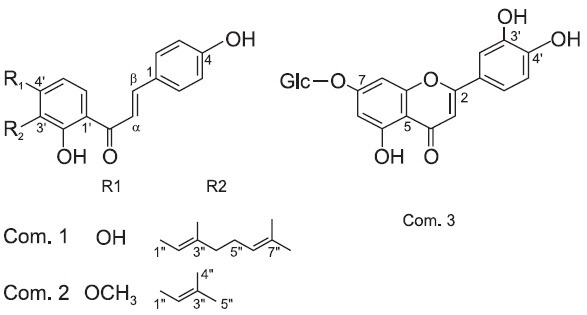
Xanthoangelol (1)
Yellowish needles, EI-MS : m/z 392[M]+(C25H28O4), 1HNMR (600 MHz, CDCl3) : δ 1.59 (3H, s, 7''-CH3), 1.63 (3H, s, 8''-CH3), 1.85 (3H, s, 3''-CH3), 2.08 (2H, m, 4''-H), 2.10 (2H, m, 5''-H), 3.49 (1H, d, J=7.1 Hz, 1''-H), 5.05 (1H, m, 6''-H), 5.30 (1H, t, J=7.1 Hz, 2''-H), 6.43 (1H, d, J=8.9 Hz, 5'-H), 6.88 (2H, d, J=8.5 Hz, 3,5-H), 7.46 (1H, d, J=15.4 Hz, α), 7.55 (2H, d, J=8.5 Hz, 2,6-H), 7.72 (1H, d, J=8.9 Hz, 6'-H), 7.83 (1H, d, J=15.4 Hz, b), 13.88 (1H, s, 2'-OH).
4-Hydroxyderricin (2)
Yellowish powders, EI-MS: m/z 338[M]+(C21H22O4), 1HNMR (600MHz, CDCl3) : δ 1.60 (3H, s, 4''-CH3), 1.71 (3H, s, 5''-CH3), 3.31 (1H, d, J=7.0 Hz,1''-H), 3.82 (3H, s, 4-OCH3), 5.30 (1H, t, J=7.0 Hz, 2''-H), 6.41 (1H, d, J=9.0 Hz, 5'-H), 6.79 (2H, d, J=8.5 Hz, 3,5-H), 7.36 (1H, d, J=15.4 Hz, α), 7.70 (2H, d, J=9.4 Hz, 2,6-H), 7.70 (1H, d, J=9.4 Hz, 6'-H), 7.72 (1H, d, J=15.4 Hz, b), 13.38 (1H, s, 2'-OH).
Cynaroside (3)
Yellowish needles, 1H-NMR (600MHz, DMSO d6+D2O) δ 5.04 (1H, d, J=7.5 Hz, G1), 6.44 (H, d, J=2.0 Hz, 6-H), 6.73 (1H, s, 3-H), 6.80 (1H, d, J=2.1 Hz, 8-H), 6.91 (1H, d, J=8.4 Hz, 5'-H), 6.91 (1H, d, J=8.4 Hz, 5'-H), 7.40 (1H, d, J=2.2 Hz, 2'-H), 7.44 (1H, d, J=8.4 Hz, 6'-H). 13C-NMR (150MHz, DMSO d6 + D2O) : δ 60.8 (G6-C), 69.8 (G4-C), 73.3 (G2-C), 76.4 (G3-C), 77.3 (G5- C), 95.3 (C-8), 99.9 (6-C), 100.2 (G1-C), 105.7 (10-C), 113.7 (2'-C), 116.2 (3-C), 116.3 (5'-C), 119.6 (6'-C), 121.7 (1'-C), 145.9 (3'-C), 150.1 (4'-C), 151.1 (5-C), 157.3 (9-C), 163.2 (7- C), 164.8 (2-C), 182.2 (4-C).
RESULTS
The inhibitory activities of xanthoangelol on MAOs
Each solvent fraction and isolated compounds from Fr. I to VI, concentrated EtOAc and butanol extracts of A. keiskei were examined for MAOs inhibitory activities. As shown in Table 1, total MeOH extract and each solvent fraction have inhibitory potential on MAO-A and MAO-B activities. Especially, Dichloromethane and EtOAc fractions showed most potent inhibitory activities against of both enzymes. BuOH fraction also shows the inhibitory activities on both enzymes. EtOAc and BuOH fractions were chosen for elucidating their active principles. Table 1 shows the inhibitory activities of each solvent fraction on MAO-A and MAO-B. The IC50 values of the dichloromethane fraction were 0.30 mg/ml for MAO-A, 0.06 mg/ml for MAO-B. The IC50 values of the EtOAc and BuOH fractions were 0.09 mg/ml and 1.3 mg/ml for MAO-A, 0.13 mg/ml and 0.85 mg/ml for MAO-B, respectively. Table 2 shows the inhibitory activities of the isolated compounds on MAO-A and MAOB. Xanthoangelol exhibited the inhibitory activities on the both enzymes potentially. The IC50 value of xanthoangelol was 43.4 μM for MAO-A, 43.9 μM for MAO-B. In our examinations, the IC50 value of the iproniazid, positive control of the nonselective MAO inhibitor was 37 μM for MAO-A and 42.5 μM for MAOB, respectively. Iproniazid is a nonselective inhibitor of MAOs and deprenyl (selegiline) is a selective MAO-B inhibitor. Iproniazid and deprenyl were used as positive controls for nonselective and selective inhibitors.
Table 1.
The IC50 values of each solvent extract of A. keiskei against MAO-A, MAO-B and DBH activities
| Fractions | Amounts of extract (g) | IC50 values (mg/ml) | ||
|---|---|---|---|---|
|
| ||||
| MAO-A | MAO-B | DBH | ||
|
| ||||
| MeOH | 1,000 | 0.51 | 0.33 | 0.68 |
| Hexane | 110 | - | 1.90 | - |
| CH2Cl2 | 56 | 0.30 | 0.06 | 0.17 |
| EtOAc | 45 | 0.09 | 0.13 | 0.08 |
| BuOH | 138 | 1.30 | 0.85 | 0.22 |
| Water | 400 | - | 2.75 | - |
Table 2.
The IC50 values of the isolated compounds from A. keiskei against MAO-A, MAO-B and DBH activities
| Compounds | IC50 values (micro mole) | ||
|---|---|---|---|
|
| |||
| MAO-A | MAO-B | DBH | |
|
| |||
| Xanthoangelol | 43.4 | 43.9 | 516 |
| 4-hydroxyderricin | 3,520 | 3.43 | 12.0 |
| Cynaroside | 400 | 268 | 0.041 |
| Iproniazida | 37 | 42.5 | - |
| Deprenylb | 3.3 | 0.046 | - |
aUsed as a positive control drugs for nonselective MAO inhibitor.
bUsed as a positive control drug for selective MAO-B inhibitor.
The inhibitory activities of xanthoangelol on DBH
As shown in Table 1, total MeOH extract and each solvent fraction have inhibitory potential on DBH activities. Except for the hexane fraction, CH2Cl2, EtOAc and BuOH fractions showed lowest IC50 values against DBH enzyme. Among them, EtOAc fraction and BuOH fraction were chosen for elucidating their active principles. Table 2 shows the inhibitory activities of the isolated compounds on DBH. Xanthoangelol exhibited the very weak inhibitory activities on DBH. The IC50 value of xanthoangelol was 516 μM for DBH.
The inhibitory activities of 4-hydroxyderricin on MAOs
Table 2 shows the inhibitory activities of the isolated compounds on MAO-A and MAO-B. 4-hydroxyderricin exhibited the inhibitory activities on the both enzymes potentially. The IC50 value of 4-hydroxyderricin was 3.52 mM for MAO-A, 3.43 μM for MAO-B. In our examinations, the IC50 value of the deprenyl, positive control of the selective MAO-B inhibitor was 3.3 μM for MAO-A and 0.046 μM for MAO-B, respectively. 4-Hydroxyderricin was the strongest and selective MAO B inhibitor among the isolated compounds. Its specific activity on MAO-B was about 15 more than that of xanthoangelol, about 150 times more than that of cynaroside, and about 1,000 and 5,000 times more than its own specific activity on MAO-A and DBH. In addition, it exhibited about 1,000 times less IC50 value on MAO-B than that on MAO-A, deprenyl showing about 70 times less that on MAO-B than that on MAO-A. This result indicates that 4-hydroxyderricin is a more selective MAO-B inhibitor than deprenyl as a selective MAOB inhibitor.
The inhibitory activities of 4-hydroxyderricin on DBH
As shown in Table 2,4-hydroxyderricin exhibited the inhibitory activities on DBH mildly. The IC50 value of 4-hydroxyderricin was 12.0 μM for DBH.
The inhibitory activities of cynaroside on MAOs
Table 2 shows the inhibitory activities of cynariside on MAOA and MAO-B. Cynaroside was not a good inhibitor for MAO-A and MAO-B. Even though it was very weak, cynaroside exhibited the inhibitory activities on both enzymes. The IC50 values of cynaroside were 0.4 mM for MAO-A, 0.27 mM for MAO-B.
The inhibitory activities of cynaroside on DBH
As shown in Table 1, total MeOH extract and each solvent fraction have inhibitory potential on DBH activities. Except the hexane fraction, CH2Cl2, EtOAc and BuOH fractions showed potent inhibitory activities against DBH enzyme. Among them, EtOAc fraction and BuOH fraction were chosen for elucidating their active principles. In Table 2, we show the inhibitory activities of the isolated compounds on DBH. Cynaroside exhibited strongest inhibitory activities on DBH among all of the isolated compounds. The IC50 value of cynaroside was 0.041 μM for DBH.
DISCUSSION
The activity-guided fractionation of extracts from Angelica keiskei Koidzumiled to the isolation of two prenylated chalcones, xanthoangelol and 4-hydroxyderricin and a flavonoid, cynaroside. Three compounds were exhibited the inhibitory activities against MAO-A, MAO-B and DBH respectively. A. keiskei is a major vegetable used as a fresh salad. As described in the introduction, traditional use of this plant is not well known, except for some medicinal purposes, such as hypertension, hepatosis and neuralgia (Kim et al., 1992). Reported studies about bioactivities of A. keiskei are few. There are some reports such as an anti-hyperlipidemic (Park et al., 1997), lowering blood pressure (Shimizu et al., 1999), antitumor action(Okuyama et al., 1991), and suppression of gastric acid secretion (Fujita et al., 1992). Some chalcones, coumarins and flavonoids have so far been isolated and characterized from this plant (Baba et al., 1990; Park et al., 1995; Akihisa et al., 2003). In this study, we can find out that this plant has also antidepressant effect. Each isolated compound showed different inhibitory pattern.
One of the isolated compounds, xanthoangelol was found to be nonselective MAO inhibitor, because of its inhibitory activities on MAO-A and MAO-B were very similar to iproniazid, nonselective MAO inhibitor. Although its activity was not great, xanthoangelol also inhibited the DBH activity. The other one, 4-hydroxyderricin was a potent selective MAO-B inhibitor. Its IC50 value was higher than that of deprenyl, a selective MAOB inhibitor used as a positive control, but this plant mainly is used as food, this value is significantly low enough. On the other hands, cynaroside showed potent inhibitory activity on DBH. This compound showed very weak inhibitory activity on MAO-A, and exhibited low activity on MAO-B.
Several reports have described the MAO-A inhibition contributes to the mechanism of antidepressant effects of MAO inhibitors more than MAO-B inhibition (Lipper et al., 1979; Mann et al., 1989). Larsen et al. (1991) reported that reversible monoamine oxidase inhibitor (RIMA) has equal antidepressant effects to those of irreversible MAO inhibitors. However, Lotufo-Neto et al. (1999) examined antidepressant effects of MAO inhibitors in a meta-analysis and described the possibility that non-selective MAO inhibitors are more effective than RIMA. Consequently, it is likely that MAO-B inhibition also contributes to an antidepressant effects. Kitaichi et al. (2010) measured extracellular noradrenaline and serotonin levels after administration of RIMA and reversible MAO-B inhibitor in the medial prefrontal cortec of rats using the in vivo micro dialysis method. And they suggested that the combined treatment with a MAO-A inhibitor and a MAO-B inhibitor strengthens antidepressant effects because the combined treatment increases extracellular noradrenaline levels more than a MAO-A inhibitor alone through increases in β-phenylethylamine. According to the results of this study, A. keiskei is an excellent combined MAO inhibitor for treatment for depression, because its major bioactive compounds, xanthoangelol, 4-hydroxyderricin and cynaroside were selective, nonselective MAO inhibitor and DBH inhibitor, respectively. Xanthoangelol is a nonselective MAO inhibitor, 4-hydroxyderricin is a selective MAO-B inhibitor, and cynaroside is a selective DBH inhibitor.
There are more MAO-B than MAO-A in the human brain, but more MAO-A than MAO-B in the rat brain (Riederer et al., 1983). The distribution of MAO-A and MAO-B is different between the human brain and the rat brain. The role of MAO-B in antidepressant effects might be greater in human than in rat; stronger antidepressant effects of combined treatment with a MAO-A inhibitor and a MAO-B inhibitor might be likely to be induced in humans (Kitaichi et al., 2010). Results of this study suggest that xanthoangelol, as a nonselective MAO inhibitor; can be potentially used as a drug candidates against depression. 4-Hydroxyderricin, as a selective MAO-B inhibitor, can be potentially used as a drug candidate for this kind of disease. Cynaroside, as a selective DBH inhibitor, can effectively elevate the level of released dopamine (DA) by preventing DBH from converting DA to norepinephrine and being destroyed by oxidative deamination effect of MAO. Thus, that seems to be useful materials for antidepressant drug for human.
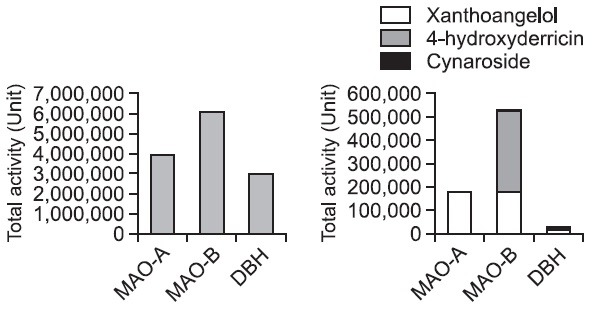
As shown in Fig. 2, the degree and the way of inhibition against each enzyme of the isolated compound was different. Xanthoangelol was nonselective between MAO-A and MAOB. Its inhibitory potentials (IC50 value, total activity, and specific activity) on MAO-A were no less than those on MAO-B and about 10 times more than those on DBH. In addition, Its IC50 values on MAOs were at a rate comparable with those of iproniazid used as positive control. 4-Hydroxyderricin was the strongest selective MAO B inhibitor among the isolated compounds. Its specific activity on MAO-B was about 15 times more than that of xanthoangelol, about 150 times more than that of cynaroside, and about 1,000 and 5,000 times more than its own specific activity on MAO-A and DBH. In addition, it exhibited about 1,000 times less IC50 value on MAO-B than that on MAO-A, deprenyl showing about 70 times less that on MAO-B than that on MAO-A. As Fig. 3 shows, against MAOs and DBH, total activities of the methanol extract (the left graph) were about 10 to 100 times more than accumulated total activities of the isolated compounds (the right one). However, the both graphs showed similar tendency of the total activities. The activities on MAO-B were more than those on the other enzymes and the activities on DBH were lowest. These results strongly suppose that the isolated compounds are the major active components of Angelica keiskei against MAOs and DBH. This result indicates that 4-hydroxyderricin is more selective MAO-B inhibitor than deprenyl used as selective MAO-B inhibitor. Cynaroside was a selective DBH inhibitor. It exhibited inhibitory activity against DBH at concentrations below 0.9 μM, but did not on MAO-A and MAO-B.
Fig. 2. Inhibitory activities on MAO-A, MAO-B and DBH of the two prenylated chalcones, xanthoangelol (1) and 4-hydroxyderricin (2) and a flavonoid, cynaroside (3) isolated from Angelica keiskei. Each compound was tested at concentrations of 1-500 ug/ml) to derive IC50 values, and these data obtained mean value from repeated experiments 3 to 5 times of duplicated tests. Specific activity expressed as unit/g, one unit is defined as a sample amount to give 50% inhibition against enzyme activity.
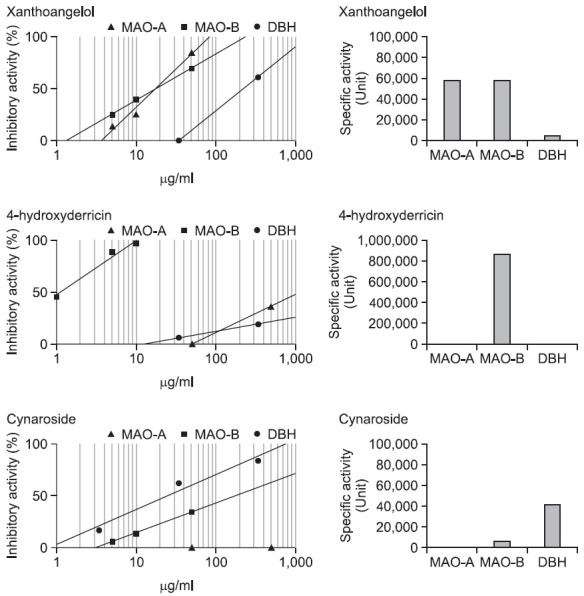
Fig. 3. Total activities of each compound on MAO-A, MAO-B and DBH. The left graph is showing the total activity of the MeOH extract of A. keiskei and the right one, a cumulative graph, showing cumulative total activity of the isolated compounds. Each compound was tested at concentrations of 1-500 μg/ml) to derive IC50 values, and these data obtained mean value from repeated experiments 3 to 5 times of duplicated tests. Total activity expressed as unit, one unit is defined as a sample amount to give 50% inhibition against enzyme activity.
In conclusion, two prenylated chalcones, xanthoangelol and 4-hydroxyderricin and a flavonoid, cynaroside isolated from A. keiskei were major active principles of this plant. The possibility exists that these three compounds isolated from A. keiskei are expected for potent candidates for development of combined antidepressant drug, and the plant A. keiskei contained xanthoangelol, 4-hydroxyderricin and cynaroside, as major active components, and will be an excellent functional food material for combined antidepressant effect.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0021753).
References
- 1.Akihisa T., Tokuda H., Hasegawa D., Ukiya M., Kimura Y., Enjo F., Suzuki T., Nishino H. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. (2003);201:133–137. doi: 10.1016/S0304-3835(03)00466-X. [DOI] [PubMed] [Google Scholar]
- 2.Baba K., Nakata K., Taniguchi M., Kido T., Kozawa M. Chalcones from Angelica keiskei. Phytochemistry. (1990);29:3907–3910. doi: 10.1016/0031-9422(90)85357-L. [DOI] [Google Scholar]
- 3.Birkmayer W., Knoll J., Riederer P., Youdim M. (-)-Deprenyl leads to prolongation of L-dopa efficacy in Parkinson's disease. Mod. Probl. Pharmacopsychiatry. (1983);19:170–176. doi: 10.1159/000407513. [DOI] [PubMed] [Google Scholar]
- 4.Fujita T., Sakuma S., Sumiya T., Nishida H., Fujimoto Y., Baba K., Kozawa M. The effects of xanthoangelol E on arachidonic acid metabolism in the gastric antral mucosa and platelet of the rabbit. Res. Comun. Chem. Pathol. Pharmacol. (1992);77:227–240. [PubMed] [Google Scholar]
- 5.Han Y. N., Choo Y. S., Lee Y. C., Moon Y. I., Kim S. D., Choi J. W. Monoamine oxidase B inhibitors from the fruits of Opuntia ficus-indica var. saboten. Arch. Pharm. Res. (2001);24:51–54. doi: 10.1007/BF02976493. [DOI] [PubMed] [Google Scholar]
- 6.Kim O. K., Kung S. S., Park W. B., Lee M. W., Ham S. S. The nutritional components of aerial whole plant and juice of Angelica keiskei. Korean J. Food Sci. Technol. (1992);25:592–596. [Google Scholar]
- 7.Kim J. H., Kim G. H., Hwang K. H. Monoamine oxadase and dopamine β-hydroxylaseinhibitors from the fruits of gardenia jasminoides. Biomol. Ther. (2012);20:214–219. doi: 10.4062/biomolther.2012.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitaichi Y., Inoue T., Nakagawa S., Boku S., Izumi T., Koyama T. Combined treatment with MAO-A inhibitor increases extracellular noradrenaline levels more than MAO-A inhibitor alone through increases in β-phenylethylamine. Euro. J. Pharmacol. . (2010);637:77–82. doi: 10.1016/j.ejphar.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Larsen J. K., Gjerris A. P., Anderson J., Bille A., Christensen E. M., Hoyer E., Hensen H., Mejlhede A., Langagergaard A., Laursen A. L., Nilkantan B., Olafsson K., Severin B., Rafaelsen O. J. Moclobemide in depression: a randomized, multicentre trial against isocarboxazide and clomipramine emphasing atypical depression. Acta Psychiatr. Scand. . (1991);84:564–570. doi: 10.1111/j.1600-0447.1991.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 10.Lipper S., Murphy D. L., Slater S., Buchsbaum M. S. Comparative behavioral effects of clorgyline and pargyline in man: a preliminart evaluation. Psychopharmacol. (Berl) (1979);62:123–128. doi: 10.1007/BF00427124. [DOI] [PubMed] [Google Scholar]
- 11.Lotufo-Neto F., Trivedi M., Thase M. E. Meta-analysis of the reversible inhibitors of monoamine oxidase type A moclobemide and brofarnmine for the treatment of depression. Neuropsychopharmacology. (1999);20:226–247. doi: 10.1016/S0893-133X(98)00075-X. [DOI] [PubMed] [Google Scholar]
- 12.Mann J. J., Aarons S. F., Wilner P. J., Keilp J. G., Sweeney J. A., Pearlstein T., Frances A. J., Kocsis J. H., Brown R. P. A controlled study of the antidepressant efficacy and side effects of (-) deprenyl. A selective monoamine oxidase inhibitor. Arch. Gen. Psychiatry. (1989);46:45–50. doi: 10.1001/archpsyc.1989.01810010047007. [DOI] [PubMed] [Google Scholar]
- 13.Meyer J. H., Wilson A. A., Sagrati S., Miler L., Rusjan P., Bloomfi eld P. M., Clark M., Sacher J., Voineskos A. N., Houle S. Brain monoamine oxidase a binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Psychiatry. (2009);66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa H., Ohno M., Baba K. Hypotensive and lipidregulatory actions of 4-hydroxyderricin, a chalcone from Angelca keiskea, in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. (2005);32:19–23. doi: 10.1111/j.1440-1681.2005.04147.x. [DOI] [PubMed] [Google Scholar]
- 15.Okuyama T., Takata M., Takayasu J., Hasegawa T., Tokuda H., Nishino A., Nishino H., Iwahima A. Anti-tumor-promotion by principles obtained from Angelica keiskei. Planta Med. (1991);57:242–246. doi: 10.1055/s-2006-960082. [DOI] [PubMed] [Google Scholar]
- 16.Park J. C., Cho Y. S., Park S. K., Park J. R., Chun S. S., Ok K. D., Choi J. W. Isolation of flavone-7-O-glycosides from the aerial parts of Anglica keiskei and anti-hyperlipidmic effect. Korean J. Pharmacogn. (1995);26:337–343. [Google Scholar]
- 17.Park J. R., Park S. K., Cho Y. S., Chun S. S., Choi S. H., Park J. C. Effects of Angelica keiskei on lipid metabolism in rats. J. Korean Soc. Food Sci. Nutr. (1997);26:308–313. [Google Scholar]
- 18.Quitkin F., Rifkin A., Klein D. F. Monoamine oxidase inhibitors: a review of antidepressant effectiveness. Arch. Gen. Psychiatry. (1979);36:749–760. doi: 10.1001/archpsyc.1979.01780070027003. [DOI] [PubMed] [Google Scholar]
- 19.Riederer P., Reynolds G. P., Jellinger K., Seemann D., Danielczyk W. Tranylcypromine isomers in Parkinson's disease: effect of low doses on monoamine oxidase inhibition and blood pressure response. Mod. Probl. Pharmacopsychiatry. (1983);19:154–161. [PubMed] [Google Scholar]
- 20.Rigal F., Zarifi an E. MAO inhibitors in psychiatric therapy: effects and side effects. Mod. Probl. Pharmacopsychiatry. (1983);19:162–169. doi: 10.1159/000407512. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu E., Hayashi A., Takahashi R., Aoyagi Y., Murakami T., Kimoto K. Effects of angiotensin I-converting enzyme inhibitor from Ashitaba (Angelica keiskei) on blood pressure of spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. (1999);45:375–383. doi: 10.3177/jnsv.45.375. [DOI] [PubMed] [Google Scholar]
- 22.Stern G. M., Lees A. J., Hardie R., Sandler M. Clinical and pharmacological aspects of (-)-deprenyl treatment in Parkinson's disease. Acta Neurol. Scand. Suppl. (1983);95:113–116. doi: 10.1111/j.1600-0404.1983.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 23.Wimbiscus M., Kostenko O., Malone D. MAO inhibitors: Risks, benefits, and lore. Cleve. Clin. J. Med. (2010);77:859–882. doi: 10.3949/ccjm.77a.09103. [DOI] [PubMed] [Google Scholar]


