Abstract
Aging is the single most important risk factor that increases susceptibility to many forms of diseases. As such, much effort has been put forward to elucidate the mechanisms behind the processes of aging and to discover novel compounds that retain antiaging activities. Korean red ginseng has been used for a variety of medical purposes in eastern countries for several thousands of years. It has been shown that Korean red ginseng affects a number of biological activities including, but not limited to, anti-inflammatory, anti-oxidative and anti-diabetic pathways. However, few studies have been performed to evaluate its anti-aging effects with an in vivo system. Here Drosophila melanogaster as an in vivo model organism demonstrates that Korean red ginseng tonic extends lifespan, increases resistance to starvation stress and prevents weight gain. This data suggest that Korean red ginseng may regulate organisms’ metabolism in favor of extending lifespan.
Keywords: Panax ginseng, Drosophila, Aging, Lifespan, Animal model
INTRODUCTION
Susceptibility to and the incidence of many forms of diseases increases with age. For example, it is expected that nearly half of people over 85 years of age will be affected by Alzheimer’s disease (Hebert et al., 2003). Cancer and diabetes are not the exceptions in that accumulated DNA mutations in somatic cells and decline in beta cell functions are inevitable consequences that accompany aging (Gunasekaran and Gannon, 2011; Kennedy et al., 2012). Therefore, the healthcare and social costs incurred with during the late stages of disease for affected individuals can be unacceptably high unless curative and preventive measures are found that effectively manage the aforementioned maladies. One attractive approach to cope with these formidable diseases is to slow down the progression of disease pathogenesis. With this approach, patients may suffer from age-related ailments over a shorter time or never experience recognizable medical symptoms during their lifetime. To achieve this goal, much effort has been put forward to elucidate mechanisms behind the processes involved in aging, with the goal to extend healthy lifespan by identifying novel agents or signaling pathways that affect the biology of aging.
Panax ginseng Meyer, as known as Korean red ginseng, is often considered one of the most effective forms of ginseng present today and has been widely used in eastern countries for a number of medical purposes including, but not limited to anti-inflammatory, anti-oxidant, and anti-fatigue therapy for thousands of years. Among the ginsenosides which are believed to play as active compounds that confer its biologically beneficial effects, Rg3, Rf, and Rh2 are the major forms of saponins that are isolated from Korean red ginseng (Bae et al., 2006). Accumulated scientific evidence has demonstrated that practical applications of Korean red ginseng enhances resistance to many diseases since it has been shown that Korean red ginseng reduces oxidative stress by restoring the scavenger systems (Ramesh et al., 2012), preserving immune homeostasis in a diabetic mouse model (Hong et al., 2012) and suppressing metastasis in human hepatoma cell lines (Ho et al., 2012). However, there is a paucity of current literature that examines Korean red ginseng’s anti-aging effects with an in vivo model system. Hence, it would be worth investigating if Korean red ginseng possesses biological activity in modulating organisms’ lifespans.
In this report, Drosophila melanogaster was utilized as an in vivo model organism given its successful track record in providing insights on human diseases (Mackay and Anholt, 2006). With this model organism the anti-aging effects of Korean red ginseng was described. By feeding fruit flies with diets supplemented with Korean red ginseng, it was found that Korean red ginseng extends lifespan, enhances resistance to starvation stress and prevents weight gain. Taken together, these results suggest that Korean red ginseng may modulate biological activities that alter organisms’ metabolic pathways toward extending healthy lifespan.
MATERIALS AND METHODS
Fly husbandry
Flies were maintained on a Drosophila stock food which was composed of 1.5 g of live yeast, 5 g of sucrose, 0.46 g of agar and 8.5 g of corn meal in 100 ml distilled water in a temperature (25℃) and humidity (60%) controlled fly room with a 12-hours light/dark cycle. Propionic acid (final v/v concentration was 1%) was added to the diets to prevent mold growth. For setting up crosses, 15 virgin flies were put into bottles containing 3-5 male flies and parents were removed after 4-5 days. Although progeny flies typically eclose 10 days after mating they were allowed to stay in bottles for an additional four days to ensure that all females were mated. While sorting flies, virginity was double-checked and only mated females were selected and used for the any experiments. Unless otherwise mentioned, all experiments were performed in the temperature and humidity controlled fly room.
Lifespan assay and starvation assay
Sorted adult flies were reared in vials containing a standard fly food which was made of 5 g of yeast extract, 5 g of sucrose, 0.46 g of agar and 8.5 g of corn meal in 100 ml distilled water. 1% final v/v concentration of propionic acid was added to the diet to inhibit fungus growth. This diet is called Ad libitum (AL) hereafter. To examine the effects of Korean red ginseng on the biology of aging, AL was supplemented with Korean red ginseng tonic as final w/v concentrations of 0.12 μg/ml, 1.2 μg/ml or 12 μg/ml of ginsenosides . For lifespan assays, fly survival was scored when transferring to fresh AL or AL diets supplemented with Korean red ginseng tonic every 2-3 days. For starvation assays, flies were reared on the designated diet conditions for 12 days, followed by transfer to the starvation media made of 1% agar. Survivorships were scored regularly and dead flies were eliminated when possible. Prism 4 (Graphpad, La Jolla, USA) was used for data analysis and statistical significance was tested with log-rank statistical methods. Mean (the sum of all values divided by the number of values added) and median (the central point of a sample set) values were presented to compare results.
Fly stain
w1118 flies, which were obtained from the Bloomington Drosophila Stock Center (Indiana, IN, USA) were used throughout the experiment.
Reagents
Korean red ginseng tonic (Hongsamton Mild®) was purchased from Korean Tobacco & Ginseng Cooperation. The active compound and its concentration are ginsenosides (Rg1+Rb1) and 60 μg/ml, respectively.
RESULTS
Korean red ginseng tonic (KRGT) extends lifespan in Drosophila
In this report, adult flies were fed on AL or AL diet supplemented with KRGT. Three different concentrations of KRGT (0.12 μg/ml, 1.2 μg/ml or 12 μg/ml final ginsenosides concentrations) were used to investigate its biological effects in a dose-dependent fashion. Only age-matched female flies were used throughout the experiments since female flies response much greater to changes in the composition of diet, and thus have been utilized extensively for studies on examining dietary effects (Partridge et al., 2005). In the current study it was found that the mean and median lifespan for the flies reared on the AL were 31.8 days and 33 days, respectively, results consistent with the published literatures (Zid et al., 2009; Katewa et al., 2012). However, when 0.12 μg/ml KRGT was supplemented into an AL diet, no statistically significant change in lifespan was observed (Fig. 1A, mean lifespan 33.6 days, median lifespan 34 days). This negative result could be explained if the low amount of KRGT taken by the flies was not sufficiently high enough to affect biology related to longevity. Higher doses of KRGT were therefore applied to examine if KRGT retains any ability to influence longevity. When the concentrations of KRGT were increased to 1.2 μg/ml and 12 μg/ml, significant extension in lifespan was observed (Fig. 1B, C). For the flies fed on 1.2 μg/ml KRGT, the mean and the median lifespan were 36.4 days and 38 days, respectively (Fig. 1B, p<0.01, Log-rank test) and for the flies reared on 12 μg/ml KRGT, the mean and the median lifespan were 36.1 days and 35 days, respectively (Fig. 1C, p<0.01, Log-rank test). It was notable that 12 μg/ml KRGT did not further increase lifespan compared to 1.2 μg/ml KRGT supplementation, suggesting a possible ceiling effect. Table 1 described these results in detail.
Fig. 1. KRGT supplementation extends lifespan in Drosophila. Agematched mated adult flies were fed with AL or AL supplemented with 0.12 μg/ml, 1.2 μg/ml or 12 μg/ml KRGT. Dead flies were scored every 2-3 days when transferring to fresh media. (A) 0.12 μg/ml KRGT supplementation had no effect on lifespan. (B, C) 1.2 μg/ml (B) and 12 μg/ml KRGT. (C) supplementation significantly extended lifespan. AL trace used in A was replotted in B and C. Lifespan curves were constructed with Prism 4 software and statistical significance was determined with Log-rank test. **p<0.01, N.S.: not significant.
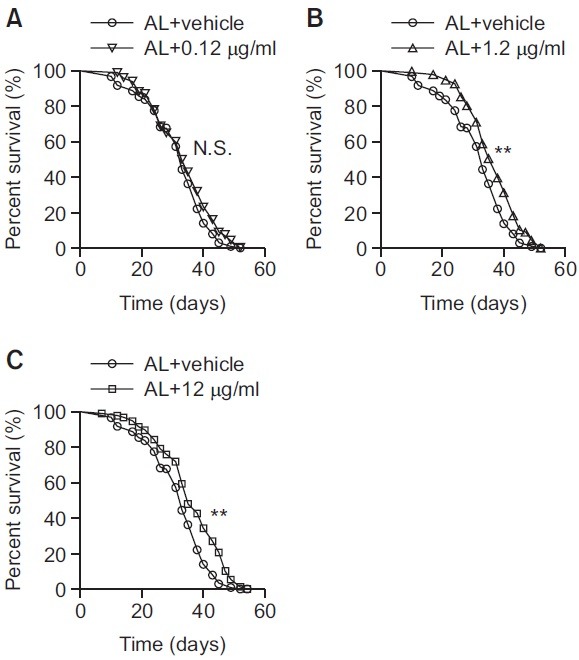
Table 1.
Summary of lifespan assay analyses
| Number of flies | Mean lifespan (days) | Median lifespan (days) | Remarks | Statistics | |
|---|---|---|---|---|---|
|
| |||||
| Standard diet | 99 | 31.8 | 33 | Control | |
| +0.12 μg/ml KRGT | 100 | 33.6 | 34 | 5.6% increase in mean lifespan | Not significant |
| 3% increase in median lifespan | |||||
| +1.2 μg/ml KRGT | 98 | 36.4 | 38 | 14.4% increase in mean lifespan | p<0.01, |
| 15.2% increase in median lifespan | Log-rank test | ||||
| +12 μg/ml KRGT | 96 | 36.1 | 35 | 13.5% increase in mean lifespan | p<0.01, |
| 6% increase in median lifespan | Log-rank test | ||||
Detailed information for the lifespan experiments shown in Fig. 1 is described.
KRGT increases resistance to starvation stress
Although it was found that KRGT extended lifespan in Drosophila, extension in lifespan does not necessarily mean prolongation of a healthy life. Since it is generally agreed that healthy flies have greater capacity to resist environmental stress it was decided to monitor flies’ responsiveness to starvation stress as a simple assay to address the issue. For the starvation assay, mated female flies were used and four types of diets (AL and AL supplemented with 0.12 μg/ml, 1.2 μg/ ml and 12 μg/ml KRGT) were prepared as described previously. Flies were reared on the designated diets for 12 days, followed by being transferred to media containing 1% agar. In this protocol despite the absence of nutrients, flies are not dehydrated since they are able to obtain water from agar (Tatar, 2007). After flies were transferred to the agar media, dead flies were scored every 4-8 hours and survivorship curves were generated. For flies fed with AL, mean and median survivals were 45.5 and 44.5 hours, respectively (Fig. 2 and Table 2). Contrary to 0.12 μg/ml and 1.2 μg/ml, 12 μg/ml KRGT supplementation significantly increased flies’ capacity to resist starvation stress by over 15% (17.4% increase in mean survival and 18.0% increase in median survival). In this particular set of experiments shown in the Fig. 2 and Table 2, 1.2 μg/ml KRGT slightly but significantly decreased the values, which led to another set of identical experiments to validate the result. In this biological replicate, increase in the capacity to resist starvation stress was consistently found in flies fed on 12 μg/ml KRGT. However, the previously observed effect with 1.2 μg/ml KRGT was not seen (Fig. 3, Table 3). Taken together, this data imply that a relatively high dose of KRGT helps flies handle environmental stresses, in particular starvation pressure.
Fig. 2. KRGT supplementation affects flies’ capacity to resist starvation stress. The effect of KRGT supplementation on influencing flies’ ability to resist starvation stress was examined. Flies were fed with three different doses of KRGT (0.12 μg/ml, 1.2 μg/ml or 12 μg/ml) for 12 days after eclosion, followed by being transferred to a starvation media. Prism 4 software was utilized to construct survivorship curves by scoring dead flies every 4-8 hours. Statistical significance was determined with Logrank testing by comparing to AL. ***p<0.001.
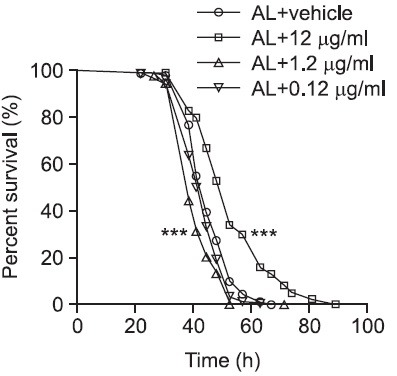
Table 2.
Summary of starvation assay analyses
| Number of flies | Mean survival hours | Median survival hours | Remarks | Statistics | |
|---|---|---|---|---|---|
|
| |||||
| Standard diet | 91 | 45.5 | 44.5 | Control | |
| +0.12 μg/ml KRGT | 94 | 43.5 | 42.8 | 4.2% decrease in mean survival | Not significant |
| 3.8% decrease in median survival | |||||
| +1.2 μg/ml KRGT | 91 | 41.9 | 38.5 | 7.7% decrease in mean survival | p<0.001, |
| 13.5% decrease in median survival | Log-rank test | ||||
| +12 μg/ml KRGT | 100 | 53.3 | 52.5 | 17.4% increase in mean survival | p<0.001, |
| 18.0% increase in median survival | Log-rank test | ||||
Detailed information for the starvation assay shown in Fig. 2 is described.
Fig. 3. KRGT supplementation increases resistance to starvation stress. Eclosed adult flies were reared on diets either with or without KRGT supplementation (0.12 μg/ml, 1.2 μg/ml or 12 μg/ml KRGT). 12 days later, flies were transferred to 1% agar media and survivorship curves were constructed by scoring dead flies every 4-8 hours with Prism 4 software. Statistical significance was determined with Log-rank testing by comparing to AL. ***p<0.001.
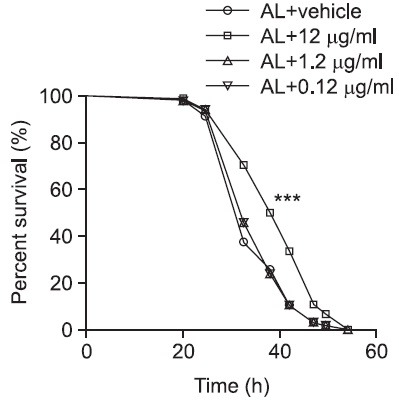
Table 3.
Summary of starvation assay analyses
| Number of flies | Mean survival hours | Median survival hours | Remarks | Statistics | |
|---|---|---|---|---|---|
|
| |||||
| Standard diet | 93 | 35.4 | 32.5 | Control | |
| +0.12 μg/ml KRGT | 99 | 35.1 | 32.5 | 1% decrease in mean survival | Not significant |
| 0% increase in median survival | |||||
| +1.2 μg/ml KRGT | 96 | 36.1 | 32.5 | 2% increase in mean survival | Not significant |
| 0% increase in median survival | |||||
| +12 μg/ml KRGT | 92 | 40.2 | 40 | 13.6% increase in mean survival | p<0.001, |
| 23.1% increase in median survival | Log-rank test | ||||
Detailed information for the starvation assay shown in Fig. 3 is described.
KRGT prevents weight gain
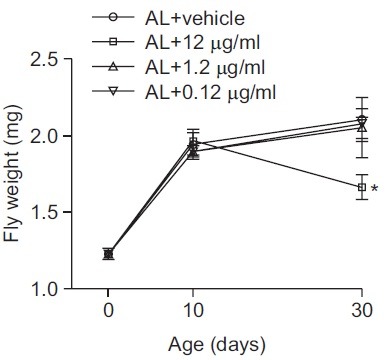
Results from the lifespan and starvation resistance assays suggest that KRGT affects an Drosophila metabolism to improve its ability to resist environmental stress, which potentially constitutes a mechanisms underlying the observed extension in lifespan. As an alternative approach to examine KRGT’s effect on metabolism, changes in flies’ weights was monitored at the age of 1, 10 and 30 day by rearing them on diets supplemented with different amounts of KRGT as described above. On day 1, mean fly weight was 1.23 mg. The weight of flies fed on AL kept increasing to 1.94 mg at age 10 day and to 2.10 mg at age 30 day. Until age 10 day, no difference in weight was found among the groups fed with diets supplemented with KRGT. However, at age 30 days, although low concentrations of KRGT (0.12 μg/ml and 1.2 μg/ml) did not impart any apparent effect on weight gain, the highest level of KRGT supplementation (12 μg/ml) significantly blocked weight gain (Fig. 4, mean weight was 1.66 mg, p<0.05).
Fig. 4. KRGT supplementation on AL diet prevents weight gain. Flies were maintained on either control diet (AL) or diets supplemented with KRGT (0.12 μg/ml, 1.2 μg/ml or 12 μg/ml KRGT). Flies’ weights were measured in each group when they reached 1, 10 and 30 days of age. Data are presented as mean ± s.e.m (N = 4-8). Statistical significance was determined with one way ANOVA with Dunnet post-hoc testing by comparing to AL. *p<0.05.
DISCUSSION
To examine the effects of KRGT on longevity with an in vivo system, Drosophila melanogaster was chosen as an ideal model organism since it is characterized by many significant advantages as an in vivo model animal to address agingrelated issues. These include inexpensive cost to maintain colonies, ease by which genetic, pharmacologic and dietary manipulation can be applied, no need for animal protocol approval, existence of many functionally characterized human orthologous genes and short lifespan which allows observation of entire pathological processes. As such, Drosophila has been extensively utilized to understand the pathogenesis of many diseases and in particular has become a preferred animal model organism in the field of aging research (Partridge and Barton, 1993; Helfand and Rogina, 2003; Katewa and Kapahi, 2011). Currently only a few publications utilizing aged rodents have been published for investigating Korean red ginseng’s specific biological effects (i.e. oxidative stress and wrinkle formation induced by UVB irradiation) (Kang et al., 2009; Ramesh et al., 2012). Hence, to my best knowledge, this report is the first examining the anti-aging effect of Korea red ginseng by analyzing the entire lifespan with an in vivo model organism.
Organisms are known to utilize stored fat to sustain ATP production in states of starvation, which depletes fuel sources. Flies are not the exception in this regard. Flies store fat in multiple places. One of well-known fat storages is the fat body, the organ equivalent to the mammalian liver, white adipose tissue. The extent of fat storage have been reported in the literature to be affected by dietary composition. For example, restricted diet (i.e. calorie restriction) increases fat storage, which constitutes a main mechanism to potentiate an organism’s capacity to resist starvation stress (Piper et al., 2005). It is also worth noting that calorie restriction is a well-known manipulation that increases lifespan of numerous organisms including yeast, worms, flies and even monkeys (Piper and Bartke, 2008; Fontana et al., 2010). Thus it would be an intriguing question to investigate whether supplementation with KRGT could mimic calorie restriction effects by measuring total fat content and monitoring signaling pathways that have been reported to be regulated by restricted diets in the fly system (i.e. Insulin/IGFlike/ mTOR signaling pathways) (Kapahi et al., 2004; Giannakou and Partridge, 2007; Partridge et al., 2011; Johnson et al., 2013). In addition, it is also possible that enzymatic activities associated with fat turnover (fat metabolism and synthesis) could be regulated by KRGT. These two hypotheses and other possibilities (i.e. regulation of reactive oxygen species, inflammation, etc) should be the subject of future experiments.
Thus far, several previous studies have highlighted ginseng as a critical player in regulating obesity. For instance, ginseng interrupts adipogenesis by regulating matrix metallopeptidases in a cell culture model and angiogenesis in an obese mouse model (Oh et al., 2012; Lee et al., 2013). With this in vivo Drosophila model organism, the data presented here add additional evidence supporting ginseng’s emerging role as a potentially attractive agent that could prevent obesity.
Taken together, in this report, Korean red ginseng’s novel effects on regulating lifespan and its capacity to mitigate environmental stresses and weight gain are described using Drosophila as an in vivo model organism. Considering that it has become one of most widely administered health food supplements in Korea and is attracting much interest in western countries, this report would spur further research to discover ginseng’s poorly characterized health benefits, thereby eventually helping pave new avenues to utilize ginseng as a key component in medical regimens in order to prevent and cure many forms of diseases.
Acknowledgments
This work was supported by the Research Settlement Fund for the new faculty of Inje University. I thank Thomas Chi, MD (University of California, San Francisco) for critically reading this manuscript.
References
- 1.Bae E. A., Han M. J., Shin Y. W., Kim D. H. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol. Pharm. Bull. (2006);29:1862–1867. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L., Partridge L., Longo V. D. Extending healthy life span--from yeast to humans. Science. (2010);328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannakou M. E., Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. (2007);32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Gunasekaran U., Gannon M. Type 2 diabetes and the aging pancreatic beta cell. Aging (Albany NY) (2011);3:565–575. doi: 10.18632/aging.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. (2003);60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 6.Helfand S. L., Rogina B. From genes to aging in Drosophila. Adv. Genet. (2003);49:67–109. doi: 10.1016/s0065-2660(03)01002-2. [DOI] [PubMed] [Google Scholar]
- 7.Ho Y. L., Li K. C., Chao W., Chang Y. S., Huang G. J. Korean red ginseng suppresses metastasis of human hepatoma SK-Hep1 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen activator. Evid. Based Complement. Alternat. Med. (2012);2012:965846. doi: 10.1155/2012/965846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong Y. J., Kim N., Lee K., Hee Sonn C., Eun Lee J., Tae Kim S., Ho Baeg I., Lee K. M. Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J. Ethnopharmacol. (2012);144:225–233. doi: 10.1016/j.jep.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Johnson S. C., Rabinovitch P. S., Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. (2013);493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang T. H., Park H. M., Kim Y. B., Kim H., Kim N., Do J. H., Kang C., Cho Y., Kim S. Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J. Ethnopharmacol. (2009);123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. (2004);14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katewa S. D., Demontis F., Kolipinski M., Hubbard A., Gill M. S., Perrimon N., Melov S., Kapahi P. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. (2012);16:97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katewa S. D., Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp. Gerontol. (2011);46:382–390. doi: 10.1016/j.exger.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy S. R., Loeb L. A., Herr A. J. Somatic mutations in aging, cancer and neurodegeneration. Mech. Ageing Dev. (2012);133:118–126. doi: 10.1016/j.mad.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H., Park D., Yoon M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat dietinduced obese C57BL/6J mice. Food Chem. Toxicol. (2013);53:402–408. doi: 10.1016/j.fct.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Mackay T. F., Anholt R. R. Of flies and man: Drosophila as a model for human complex traits. Annu. Rev. Genomics Hum. Genet. (2006);7:339–367. doi: 10.1146/annurev.genom.7.080505.115758. [DOI] [PubMed] [Google Scholar]
- 17.Oh J., Lee H., Park D., Ahn J., Shin S. S., Yoon M. Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid. Based Complement. Alternat. Med. (2012);2012:265023. doi: 10.1155/2012/265023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partridge L., Alic N., Bjedov I., Piper M. D. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp. Gerontol. (2011);46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partridge L., Barton N. H. Evolution of aging: testing the theory using Drosophila. Genetica . (1993);91:89–98. doi: 10.1007/BF01435990. [DOI] [PubMed] [Google Scholar]
- 20.Partridge L., Piper M. D., Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. (2005);126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Piper M. D., Bartke A. Diet and aging. Cell Metab. . (2008);8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Piper M. D., Skorupa D., Partridge L. Diet, metabolism and lifespan in Drosophila. Exp. Gerontol. (2005);40:857–862. doi: 10.1016/j.exger.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh T., Kim S. W., Hwang S. Y., Sohn S. H., Yoo S. K., Kim S. K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr. Res. (2012);32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Tatar M. Diet restriction in Drosophila melanogaster. Design and analysis. Interdiscip. Top. Gerontol. (2007);35:115–136. doi: 10.1159/000096559. [DOI] [PubMed] [Google Scholar]
- 25.Zid B. M., Rogers A. N., Katewa S. D., Vargas M. A., Kolipinski M. C., Lu T. A., Benzer S., Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell . (2009);139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


