Abstract
Avenin-like b proteins may contribute to the viscoelastic properties of wheat dough via inter-chain disulphide bonds, due to their rich cysteine residues. In order to clarify the effect of the avenin-like b proteins on the functional properties of wheat flour, the functional and biochemical properties of wheat flour were analyzed in three transgenic wheat lines overexpressing the avenin-like b gene using the sodium dodecyl sulfate sedimentation (SDSS) test, Mixograph and size exclusion-high performance liquid chromatography (SE-HPLC) analysis. The results of the SDSS test and Mixograph analysis demonstrated that the overexpression of avenin-like b proteins in transgenic lines led to significantly increased SDSS volume and improved flour mixing properties. The results of SE-HPLC analysis of the gluten proteins in wheat flour demonstrated that the improvement in transgenic line flour properties was associated with the increased proportion of large polymeric proteins due to the incorporation of overexpressed avenin-like b proteins into the glutenin polymers. These results could help to understand the influence and mechanism of avenin-like b proteins on the functional properties of wheat flour.
Electronic supplementary material
The online version of this article (doi:10.1007/s11032-013-9913-1) contains supplementary material, which is available to authorized users.
Keywords: Transgenic wheat, Avenin-like b protein, Mixing properties, Glutenin polymers
Introduction
Wheat (Triticum aestivum L.) is the dominant crop in temperate countries, being used for human food and livestock feed. Wheat flour forms the basis of a variety of food products, largely because of the unique viscoelastic properties of wheat dough conferred by the major storage gluten proteins in the seed endosperm (Shewry 1995, 2009; Shewry et al. 1997). Gluten proteins are classified into gliadins and glutenins. Gliadins are classified as α-, β-, γ- and ω-gliadins on the basis of their electrophoretic mobility in A-PAGE (pH 3.1). Gliadins are monomeric proteins having intra-chain disulphide bonds, with the exception of ω-gliadins, which have no cysteine in their structure (Müller and Wieser 1995, 1997). Glutenins are further divided into high-molecular-weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS) on the basis of their mobilities on sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Shewry et al. 2003). The numbers and distributions of the cysteine residues in glutenin subunits available for inter-chain disulphide bonds are critical in determining the rheological properties of wheat dough (Shewry and Halford 2002; Shewry et al. 1997).
It has been known that the viscoelastic properties of wheat dough are primarily determined by the glutenin fraction of the gluten proteins (Huebner and Wall 1976; Shewry 2009), which comprises HMW and LMW glutenin subunits, linked by inter-chain disulphide bonds to form polymers with a broad molecular-weight distribution. However, the existence of a further class of storage proteins, which were named avenin-like proteins by Kan et al. (2006), may contribute to the functional properties of wheat flour. Kan et al. (2006) characterized two classes of cDNAs encoding avenin-like a and b proteins, based on their nearest relatives identified in databases. The distinguishing feature of avenin-like proteins is that they contain more cysteine residues with the potential to form intra-chain and inter-chain disulphide bonds.
The molecular mass of avenin-like a proteins is about 18 kDa; each avenin-like a protein contains 14 cysteine residues (Kan et al. 2006). Because of the high homology sequence similar to a previously reported “low-molecular weight gliadin” monomer, it may be assumed that cysteine residues of avenin-like a protein mediate seven intra-chain disulfide bonds (Anderson et al. 2001; Clarke et al. 2003; Salcedo et al. 1979). In contrast, avenin-like b proteins contain 19 (typ-b1 and -b2) or 18 (typ-b3) cysteine residues, and do not correspond to any known protein sequences. They differ from avenin-like a proteins in that the mature avenin-like b proteins contain a duplicated sequence of about 120 residues (Kan et al. 2006). The first 18 amino acid residues of each avenin-like b protein have been proved to be a signal peptide while the mature proteins have an average molecular mass of 30 kDa (De Caro et al. 2010). A few years ago, avenin-like b proteins were detected in the glutenin fraction of durum wheat cultivar Svevo (Mamone et al. 2009). The identification of avenin-like b proteins was supported by acquisition of the sequence with a reasonable number of tryptic peptides and the matches between measured and expected molecular weight and pI (Mamone et al. 2009). The detection of avenin-like b proteins in the glutenin fraction together with their high contents of cysteine residues suggested that this type of protein could be integrated into the glutenin polymers by inter-chain disulphide bonds, and possibly contributes to the functional properties of wheat flour.
To support such a hypothesis, Chen et al. (2010) incorporated 10 or 15 mg of the heterologously expressed avenin-like b proteins (containing 19 cysteine residues) into 2 g of flour to evaluate the effect on dough functional properties by the 2 g Mixograph test. They confirmed that incorporation of the heterologously expressed avenin-like b proteins into flours resulted in significant improvement in flour mixing properties [measured as increased mixing time (MT) and peak resistance (PR) and as decreased breakdown in resistance], and provided a preliminary result regarding the relationships between avenin-like b proteins and functional properties of dough. When the avenin-like b proteins (containing 18 cysteine residues) were overexpressed specifically in wheat grain, whether these proteins could improve the functional properties of wheat flour still remained unclear.
In the present study, in order to confirm the effects of increasing the in vivo levels of avenin-like b proteins on the functional properties of wheat flour, the expression vector pLRPT-avel expressing specifically in the endosperm was successfully constructed and transformed into an elite wheat variety (T. aestivum L. cv. Zhengmai 9023) by particle bombardment. The Mixograph analysis and sodium dodecyl sulfate sedimentation (SDSS) test were performed to determine the functional properties of wheat flour using three transgenic wheat lines overexpressing the avenin-like b gene. Here we demonstrated the effects of the avenin-like b proteins overexpressed in wheat grain on the mixing properties of wheat flour.
Materials and methods
Plant material
Common wheat (T. aestivum) variety Zhengmai 9023 is a medium-strong gluten cultivar of the Yangzi River down-central area in China, with the genome constitution of AABBDD. It is a weak spring-type genotype containing four HMW-GS: Bx7, By8, Dx2 and Dy12.
Vector construction
Genomic DNA was extracted from wheat leaves using a CTAB method (Stacey and Isaac 1994). Primers specific to the avenin-like b locus were designed since the sequences in the N- and C-terminal domains were perfectly conserved in the b-type proteins. The avenin-like b gene was amplified from the genomic DNA of wheat (T. aestivum cv. Zhengmai 9023) using primers containing the restriction sites SalI and BamHI and having the following sequences: forward primer: 5′-CGCTGTCGACATGAAGGTCTTCATCCTGGCTC-3′ (SalI site underlined); reverse primer: 5′-TCGAGGATCCCTAGCACGCACCACCAGTGTA-3′ (BamHI site underlined). Plasmid pLRPT was used for the construction of the transgenic expression cassette (He et al. 2005; Tosi et al. 2004). The amplified products were cloned, sequenced, digested with SalI and BamHI, and finally cloned into the transformation vector pLRPT, using the same restriction enzymes, resulting in its insertion between the endosperm-specific 1Dx5 promoter and the CaMV35S terminator. The recombinant vector [Electronic Supplementary Material (ESM) Fig. S1] named pLRPT-avel was co-bombarded with the plasmid pAHC25 (Christensen and Quail 1996) containing the β-glucuronidase (uidA) gene and the selectable marker gene bar that confers tolerance to phosphinothricin (PPT) under the control of maize ubiquitin promoter.
Genetic transformation and plant regeneration
The transformation procedure was performed based on the bombardment method described by Sparks and Jones (2004). Immature scutella of wheat cultivar Zhengmai 9023 were used as targets for transformation by particle bombardment with the plasmids pLRPT-avel and pAHC25 at a 2:1 molar ratio. Plants were regenerated and selected under the herbicide phosphinotricin (3 mg/L) (Barro et al. 1998). Regenerated plants that survived selection were transferred to soil and grown to maturity under greenhouse conditions with air temperatures of 20/15 °C (day/night), a relative humidity of 50–70 % under ca. 350 μmol m−2 s−1 irradiance with a period of 16 h.
PCR and Southern blotting analysis
As the uidA gene and CaMV35S terminator were the unique sequences in the pAHC25 vector and pLRPT-avel vector, respectively, that did not have any similarity with the genomic DNA of common wheat, PCR amplifications for the uidA gene (primer pair: 5′-AGTGTACGTATCACCGTTTGTGTGAAC-3′, 5′-ATCGCCGCTTTGGACATACCATCCGTA-3′) and the CaMV35S terminator (primer pair: 5′-CGCTGAAATCACCAGTCT-3′, 5′-TCCTTCCTTCCGTCCACT-3′) were used to identify transgenic plants. The amplified fragment lengths were as follows: uidA, 1,056 bp and CaMV35S terminator, 417 bp.
Southern blotting analysis was performed to determine the transgene copies of T0 plants and to indicate the independent transgenic lines. Genomic DNA was extracted from leaves of T0 transgenic plants by the CTAB method (Stacey and Isaac 1994) and digested with HindIII (which cuts twice within the pLRPT-avel vector). Digested genomic (10 μg) and plasmid (5 pg) DNA were separated by electrophoresis using 0.8 % (w/v) agarose gel and transferred by capillary blotting onto positively charged nylon membrane, according to the manufacturer’s instructions (Roche). The membrane was hybridized with DIG-labelled 417 bp probe generated by PCR using primers designed to the CaMV35S terminator sequence in the pLRPT-avel vector.
SDS-PAGE and Western blotting analysis
Total proteins of wheat seeds were extracted using the method described by He et al. (1999) and separated by SDS-PAGE on 15 % polyacrylamide. Western blotting was performed using a rabbit anti-avenin-like b protein polyclonal antibody raised from the recombinant avenin-like b proteins expressed in E. coli. The heterologous expression of avenin-like b protein and its purification were carried out following the method reported in our previous study (Chen et al. 2008). After separation by SDS-PAGE, total proteins of wheat seeds were electro-blotted onto polyvinylidene fluoride (PVDF) membrane described by Fido et al. (1995). Primary rabbit anti-avenin-like b protein anti-serum was diluted 1:2 × 105 in TBST/5 % BSA and incubated at 25 °C for 2.5 h. The membrane was washed with TBST four times and incubated with 1:4 × 103 dilution of alkaline phosphatase conjugated goat anti-rabbit secondary antibody at 25 °C for 1 h, then detected according to the manufacturer’s instructions. Antibody against housekeeping protein GAPDH was used to normalize for equal amounts of proteins and to calculate the relative loading volume for each sample. The relative amounts of avenin-like b proteins in the transgenic wheat plants, compared to the non-transformed line, were measured by densitometry analysis of the Western blotting results in three biological replications using Bio-Rad Quantity One software (Bio-Rad, Hercules, CA, USA).
Single seed descent was used to obtain homozygotes for lines containing the avenin-like b transgene by PCR screening of T3 lines. In the following generation, SDS-PAGE analysis of avenin-like b proteins from 10 to 15 seeds of each T4 line was used to confirm the non-segregation of the avenin-like b gene and to determine the expression of avenin-like b proteins.
Seed storage protein characterization
To characterize storage proteins from each line, gliadins, glutenins and other proteins were sequentially extracted from 100 mg of flour from each sample according to DuPont et al. (2005). For densitometry, the gliadin, glutenin and albumin/globulin fractions from 15 flour samples per line were separated by SDS-PAGE as described previously and quantified by densitometry method using Bio-Rad Quantity One 1-D software version 4.6.2. The densitometry method was selected because of its higher reproducibility in characterization of storage proteins than that of HPLC (Shewry et al. 2006).
Quality tests
The protein contents and moisture contents of flour were measured by the near-infrared reflectance spectroscopy (NIRS) method using an Infratec TM1241 Grain Analyzer (Foss North America, Silver Spring, MD, USA) and samples were conditioned to 14 % moisture content and milled with a Chopin CD1 mill. The moisture contents and the protein contents of the transgenic and control lines were averaged from four replications. The water absorption was estimated by approved methods (AACC 1995) using the protein and moisture contents of flour. SDSS volumes (mL) were determined using a Brabender Quadrumat Sedimat (Brabender OHG, Duisburg, Germany) and the procedures described by ICC No. 169 (ICC Standard). Three technical replicates were carried out for each biological sample. Dough mixing properties were determined with a 10 g Mixograph (National Manufacturing Co., Lincoln, NE, USA) based on the AACC method 54-40A. Mixing was carried out in triplicate. The mixing parameters determined were MT (min), the maximum height of the midline trace (PR [arbitrary units, AU]), bandwidth at peak resistance (BWPR [AU]), percentage decrease in dough resistance 3 min after the peak (resistance to breakdown, RBD [%]), the maximum bandwidth during the mixing (MBW [AU]), the bandwidth of the midline after MT (MRW [AU]) and the midline integral at 8 min (MTxI [AU]).
Extraction of monomeric proteins, soluble and insoluble polymeric glutenin
Alcohol-soluble gluten proteins were extracted with 50 % (v/v) propan-1-ol in a 1:10 (mg:μL) ratio using the method described by Tosi et al. (2005). Insoluble glutenin proteins were extracted from the residue using a buffer containing 0.07 M Tris–HCl pH 6.8, 2 % (w/v) SDS, 10 % (w/v) glycerol, 0.002 % (w/v) bromophenol blue and 1 % (w/v) dithiothreitol in the same weight:volume ratio. Ten μL aliquots of the soluble and insoluble extracts were analyzed by SDS-PAGE using the standard method with 10 % separating gels, and Western blotting analysis.
SE-HPLC
Size exclusion-high performance liquid chromatography (SE-HPLC) was carried out using an Agilent 1100 Series chromatography station with a UV detector set at 214 nm. The total proteins were extracted following the method described by Tosi et al. (2005). The supernatants were filtered through a 0.45 μm membrane and 20 μL were injected into a ZORBAX GF-250 size-exclusion column, with temperature controlled at 40 °C. A 1:1 mixture of 0.05 % trifluoroacetic acid (TFA) in acetonitrile and 0.05 % TFA in deionized water was used as eluting solvent at a flow rate of 0.5 mL/min. Samples were extracted in three replicates and two separations of each extraction made.
The soluble proteins and the insoluble proteins in flour were extracted according to the method described by Tosi et al. (2005). In both cases the supernatants were filtered through 0.45 μm PVDF filters and 20 μL were injected into a ZORBAX GF-250 size-exclusion column, under the same condition described for total proteins. Three replicate separations were performed on each flour sample. The proportion of total polymeric proteins (%UPP) was determined according to Gupta et al. (1993).
Statistical analysis
Statistical analysis was performed using SPSS for Windows 15.0 statistical software (SPSS Inc.,). Significance of means within each data set was determined by Fisher’s least significant difference (LSD) test with p = 0.05.
Results
Production of transgenic wheat plants
The avenin-like b gene sequence in this research was 855 bp long with the GenBank accession number HM027637 and encoded a protein with 284 amino acid residues containing 18 cysteine residues, being grouped into avenin-like b (typ-b3) proteins (Kan et al. 2006). The phylogenetic relationships of avenin-like b proteins have been analyzed in our previous study (Chen et al. 2008). The first 18 amino acid residues corresponded to the signal peptide and the mature protein contained 266 amino acid residues having an average relative molecular mass of 30 kDa (De Caro et al. 2010). Plasmid pAHC25 conferring bialaphos resistance and plasmid pLRPT-avel encoding avenin-like b protein were used for transformation of the wheat variety Zhengmai 9023.
The positive transgenic T0 plants were confirmed by PCR to amplify the uidA gene and CaMV35S terminator sequence (Fig. 1a, b). A total of 30 positive transgenic T0 plants were obtained from about 3,000 bombarded immature scutella. The overall transformation efficiency was 1 %. The offspring of some transgenic T0 plants were further confirmed on the basis of Southern and Western blotting analyses.
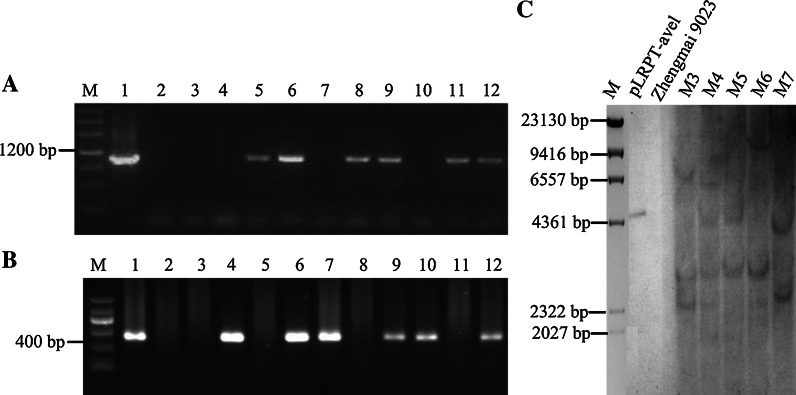
Fig. 1.
PCR (a, b) and Southern blotting analysis (c) of the transgenic wheat plants. Left PCR amplification results of uidA gene (a) and CaMV35S terminator sequence (B). Lane M DNA marker III (a) or marker II (b); lane 1 plasmid pAHC25 (a) or pLRPT-avel (b) for positive control; lane 2 water for negative control; lane 3 DNA of cv. Zhengmai 9023 for negative control; lanes 4–12 DNA of regenerated wheat plants. The samples in lanes 4–12 of b correspond to the samples in lanes 4–12 of a, respectively. Right Southern blotting analysis (c) of HindIII-cut genomic DNA from transgenic wheat lines (M3–M7) and from non-transformed control line (cv. Zhengmai 9023), hybridized with a probe prepared by random priming of the CaMV35S terminator sequence. Lane M λDNA/HindIII marker. Positive control of pLRPT-avel digested with HindIII. The PCR results of transgenic wheat lines (M3–M7) in c are shown in lanes 4, 6, 7, 9 and 12 of b, respectively
Southern blotting analysis using HindIII to digest the recombinant plasmid pLRPT-avel and the genomic DNA of transgenic wheat lines and non-transformed line hybridized with a probe corresponding to the CaMV35S terminator sequence showed that the selected T0 transgenic wheat lines contained multiple insertion sites (Fig. 1c), resulting in two or more bands on the blot. The banding patterns, however, were different, confirming that the plants were derived from independent transformation events and could be therefore considered as independent lines (Fig. 1c). The corresponding relationships of the samples in Fig. 1a–c are indicated in the Fig. 1 caption.
Recombinant plasmid pLRPT-avel was digested with HindIII (cuts twice within the plasmid), known to excise a ~4.6 kbp fragment including the transgene and the probe sequence of CaMV35S terminator (ESM Fig. S1). As shown in Fig. 1c, hybridizing fragments of the expected size, which were presumed to correspond to full-length versions of the expression vector digested with HindIII (~4.6 kbp), were detected in genomic DNA from the transgenic M4 and M5 lines. Moreover, hybridizing bands of the larger or smaller sizes than the expected band (~4.6 kbp), probably derived from transgene rearrangement and/or modification, were also detected in the transgenic M4 and M5 lines.
However, hybridizing bands of larger or smaller sizes than the expected size (~4.6 kbp) were also detected in the transgenic M3, M6 and M7 lines. This was most likely because the expression plasmid insertions had undergone deletions or rearrangements resulting in the loss of either HindIII restriction sites or the sequence of the expression plasmid in the process of genetic transformation. The presence of the larger or smaller than expected sizes in Southern blotting was similar to the results of previous studies (Zhang et al. 2003; Tosi et al. 2004; He et al. 2005).
Selection of homozygous progeny with stable expression of avenin-like b proteins was carried out in four generations (T0–T3) by SDS-PAGE and Western blotting analysis. In addition, the presence of uidA gene and CaMV35S terminator were determined by PCR in four generations. Three homozygous transgenic wheat lines (designated M3, M6 and M7) in the T3 generation overexpressing avenin-like b proteins were obtained, while the other transgenic lines tested were still hemizygous. Mature T4 seeds were harvested separately from each plant and analyzed by SDS-PAGE and Western blotting to verify the stability of transgene expression and homozygosity. Three homozygous lines overexpressing avenin-like b gene (named M3, M6 and M7), together with one non-transgenic line (designated N-4) derived from T1 hemizygous plants, and one non-transformed line (cv. Zhengmai 9023), were used for further analysis.
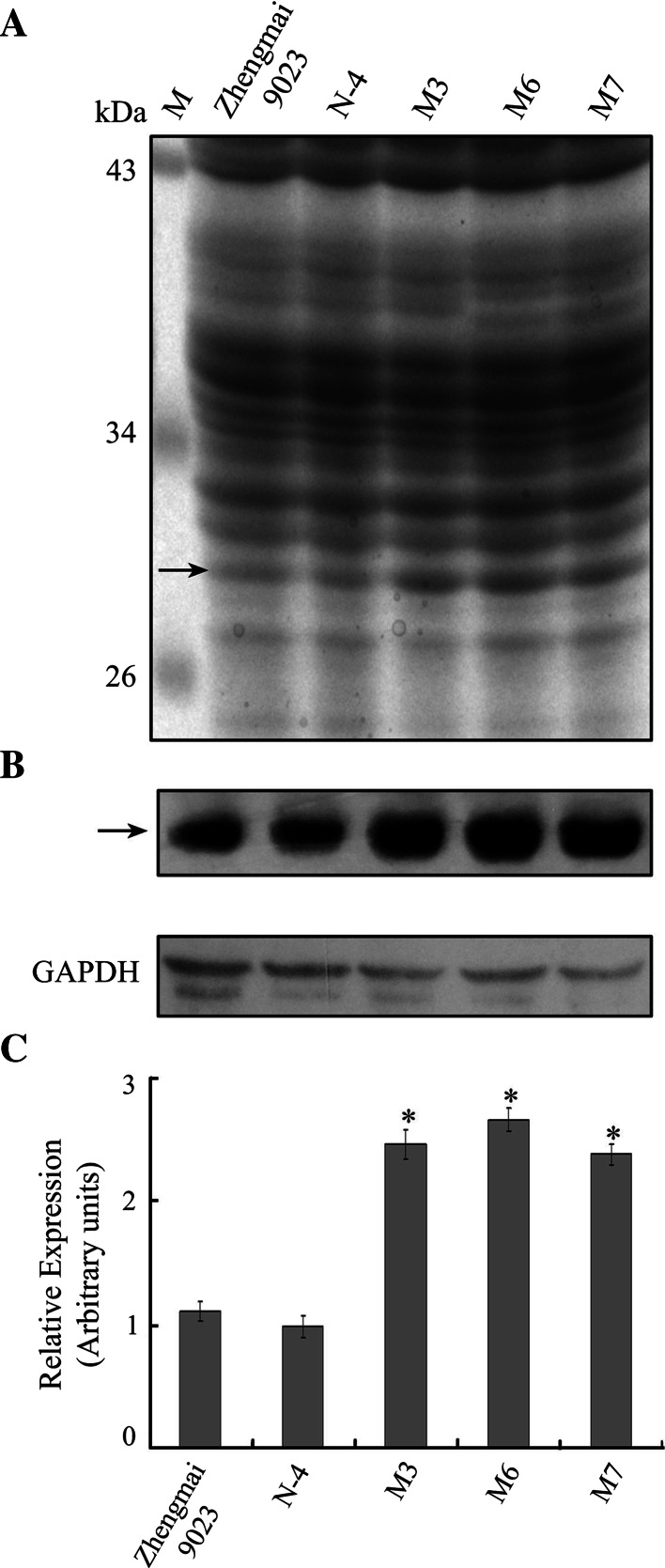
Total seed storage protein of the transgenic (M3, M6 and M7) lines and control (cv. Zhengmai 9023 and N-4) lines were separated by SDS-PAGE for identification of the transgenic subunits by staining with Coomassie Brilliant Blue R250. The amounts of avenin-like b proteins in the three transgenic wheat lines were increased compared with the non-transformed control line (Fig. 2a, arrow). Due to the high sensitivity of the Western blotting analysis, it has been possible to assess more accurately the avenin-like b proteins expressed in wheat grain. Western blotting analysis was carried out using polyclonal antibodies to confirm whether the amounts of avenin-like b proteins in the transgenic wheat lines were increased. A clear reactive band of the expected molecular mass (about 30 kDa) was observed in all seed protein extracts (Fig. 2b). The relative amounts of avenin-like b proteins in the transgenic wheat, compared to the non-transformed line, were measured by using GAPDH as control to normalize for equal amounts of proteins and to calculate the relative loading volume for each sample. Western blotting analysis showed that the relative amounts of avenin-like b proteins in three transgenic M3, M6 and M7 lines were increased 1.6-, 1.8- and 1.4-fold, respectively, when compared with the non-transformed line, as calculated by densitometry (Fig. 2c). However, no significant difference in the amount of avenin-like b proteins was observed between the non-transgenic line (N-4) and non-transformed control line (cv. Zhengmai 9023) (Fig. 2). These results demonstrated that the amount of avenin-like b proteins was not changed in line N-4 by possible somaclonal variations in the process of genetic transformation.
Fig. 2.
SDS-PAGE (a) and Western blotting analysis (b) of avenin-like b proteins in transgenic lines (M3, M6 and M7) and control lines (cv. Zhengmai 9023 and N-4). a Lane M protein marker. Arrow indicates the position of the transgenic avenin-like b proteins. b Western blotting results of avenin-like b proteins in transgenic and control lines. Housekeeping protein GAPDH was used as control to normalize for equal amounts of proteins and to calculate the relative loading volume for each sample. c Relative amounts of the avenin-like b proteins in the transgenic plants were densitometrically quantified with respect to the non-transformed control line (cv. Zhengmai 9023)
Characterization of the storage proteins in the transgenic and control lines
The flour protein contents varied from 13.57 % in the non-transformed lines and 13.79 % in the non-transgenic lines to 14.33, 14.61 and 14.47 % in the M3, M6 and M7 lines, respectively. Although flour protein contents for the transgenic lines were higher than those of the control lines, this increase in protein contents was not significant as determined by analysis of variance (ANOVA) analysis. Further, SDS-PAGE results of total storage protein did not show a difference in their expression patterns among transgenic and control lines (ESM Fig. S2A).
To compare the protein compositions of flour samples from these lines, we separated and quantified glutenins and gliadins according to DuPont et al. (2005). ANOVA analysis results showed that differences in the amounts of glutenins and gliadins were not significant between these lines (ESM Fig. S2B). As shown in ESM Fig. S2, overexpression of avenin-like b proteins did not influence the amounts and proportions of storage proteins. SDS-PAGE analysis of total proteins showed no marked difference in the endogenous HMW-GS (1Dx2, 1Bx7, 1By8 and 1Dy12) subunits of transgenic and control lines. The proportions of HMW-GS in the glutenin varied from 17.32 to 17.43 % for line M6 and the non-transformed control line, while those of LMW-GS ranged from 82.68 to 82.81 % for line M6 and the non-transformed control line. Furthermore, the ratios of HMW/LMW were similar between transgenic and control lines. In general, the expression of the avenin-like b proteins in the transgenic lines did not alter the amounts and proportions of glutenins (including both HMW- and LMW-GS) and gliadins in the endosperms of wheat.
Mixing properties analysis
The SDSS test showed no differences between the two control lines (N-4 and cv. Zhengmai 9023). The average SDSS volume was significantly higher in the three transgenic lines (M3, M6 and M7) than in the control lines (Table 1). The mixograms and mixing parameters of dough from the three transgenic wheat lines and control lines, measured using a 10 g Mixograph, with three replications, are shown in Fig. 3 and Table 1, respectively. No significant differences were found in the mixing parameters between the non-transgenic line (N-4) and non-transformed line (cv. Zhengmai 9023), suggesting that particle bombardment and tissue culture of wheat did not affect dough mixing properties (Table 1).
Table 1.
Comparisons of flour quality-related parameters of the transgenic and control wheat lines
| Parameters | Lines | LSD 0.05 | ||||
|---|---|---|---|---|---|---|
| Zhengmai 9023 | N-4 | M3 | M6 | M7 | ||
| Mixograph | ||||||
| Mixing time (min) | 3.42 ± 0.09aa | 3.35 ± 0.05a | 3.46 ± 0.04a | 3.56 ± 0.04a | 3.45 ± 0.03a | NS |
| Peak resistance (AU) | 40.28 ± 0.14a | 42.35 ± 0.37a | 45.67 ± 0.78b | 46.16 ± 0.67b | 45.38 ± 0.58b | 2.46 |
| Resistance breakdown (%) | 16.42 ± 0.76a | 17.34 ± 0.52a | 14.44 ± 0.67b | 13.16 ± 0.44b | 13.57 ± 0.36b | 1.68 |
| Bandwidth at peak resistance (AU) | 17.2 ± 0.43a | 15.54 ± 0.69a | 26.44 ± 0.65b | 24.92 ± 0.48b | 27.06 ± 0.51b | 2.17 |
| Bandwidth of the midline after mixing time (AU) | 18.62 ± 1.81a | 13.06 ± 0.78a | 28.98 ± 0.66b | 25.77 ± 0.74b | 28.33 ± 0.96b | 5.96 |
| Maximum bandwidth during the mixing (AU) | 21.17 ± 0.21a | 27.98 ± 1.35ac | 31.73 ± 1.26bc | 35.91 ± 3.5b | 33.64 ± 2.17b | 6.04 |
| Midline integral at 8 min (AU) | 282.48 ± 3.57a | 291.58 ± 0.78a | 314.23 ± 4.66b | 322.36 ± 3.74b | 319.35 ± 2.96b | 9.62 |
| SDSS test | ||||||
| Sedimentation (mL) at 14 % | 41.5 ± 0.1a | 41.7 ± 0.3a | 52.77 ± 0.1b | 54.12 ± 0.3b | 53.72 ± 0.1b | 1.62 |
| SE-HPLC | ||||||
| %F1 | 36.64 ± 0.3a | 37.69 ± 0.43a | 46.31 ± 1.63b | 47.29 ± 0.63b | 46.82 ± 0.78b | 2.17 |
| %F1/%F2 | 2.11 ± 0.17a | 2.15 ± 0.13a | 2.38 ± 0.11b | 2.6 ± 0.15b | 2.41 ± 0.19b | 0.25 |
| (%F3 + %F4)/%F1 | 1.19 ± 0.03a | 1.07 ± 0.02a | 0.74 ± 0.04b | 0.73 ± 0.03b | 0.79 ± 0.05b | 0.28 |
| %UPPb | 35.57 ± 0.41a | 36.56 ± 1.02a | 43.53 ± 1.32b | 44.1 ± 1.54b | 43.87 ± 1.28b | 3.52 |
Values within the same parameter followed by the same letter are not significantly different at 0.05 probability level
NS not significant, AU arbitrary units, LSD 0.05 least significant difference at p = 0.05
aMean ± SD among three replications
b%UPP (polymeric insoluble fraction/total polymeric protein) of the transgenic and control line flour
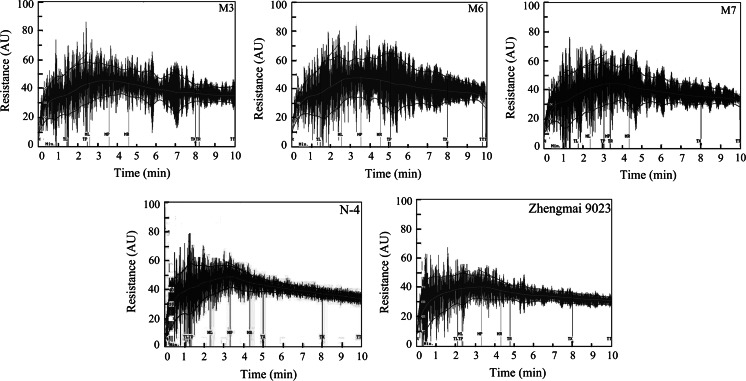
Fig. 3.
Mixograph curves of the dough prepared from three transgenic wheat lines (M3, M6 and M7) and control lines (N-4 and cv. Zhengmai 9023). TL time before peak of trace envelope, TP time to peak of trace envelope, TR time after peak of trace envelope, ML time before peak of the midline of the trace, MP time to peak of the midline of the trace, MR time after peak of the midline of the trace, TX time at 8 min of mixing, TTT time at 10 min of mixing
As shown in Table 1 and Fig. 3, the MBW were significantly higher in the three transgenic wheat lines M3 (31.73), M6 (35.91) and M7 (33.64) than in the non-transformed lines (21.17). The MRW varied from 18.62 in the non-transformed lines to 28.98, 25.77 and 28.33 in the transgenic lines M3, M6 and M7, respectively. All the dough from the transgenic wheat lines had higher PR and broader BWPR than the non-transformed lines (Fig. 3). The PRs of transgenic wheat M3, M6 and M7 lines were increased to 45.67, 46.16 and 45.38, respectively when compared to that of the non-transformed line (40.28). Moreover, the dough from transgenic lines had thicker BWPR than the dough from the non-transformed lines (Fig. 3), suggesting that the resistance to extension of the transgenic wheat dough was improved (Tosi et al. 2005). Based on the RBD (Table 1), the mixing tolerance of the transgenic wheat dough was improved. The RBD of transgenic wheat lines M3, M6 and M7 were decreased to 14.44, 13.16 and 13.57, respectively, compared to 16.42 in the non-transformed wheat lines. Furthermore, the increased MTxI for transgenic lines indicated the enhancement of dough strength in comparison with those for control lines. In addition, slight positive changes were observed in MT. The MT of transgenic lines M3, M6 and M7 was 0.04, 0.14 and 0.03 min higher, respectively, when compared with the non-transformed lines, but these differences were not statistically significant. It could be concluded that the overexpression of avenin-like b proteins generally led to improved dough elasticity, mixing tolerance and dough resistance to extension.
Analysis of glutenin polymers in the transgenic and control lines
Gluten proteins are classically divided into two groups, the gliadins and glutenins, based on their extractability (albumins, globulins and gliadins) or unextractability (glutenin) in aqueous alcohols (Shewry and Tatham 1997). However, small amounts of polymers related to the glutenins are also present in the gliadin fraction. These appear to differ from the alcohol-unextractable glutenins in having lower molecular mass and higher amounts of LMW subunits, and have been called ‘aggregated gliadins’ (Shewry et al. 1983), ‘high molecular-weight gliadin’ or ‘ethanol-soluble glutenin’ (Bietz and Wall 1980). In this study, monomeric, soluble and insoluble polymeric glutenin proteins of wheat flour were extracted following the method described by Tosi et al. (2005). Proteins were separated into fractions soluble (soluble gluten protein, 50PS) and insoluble (insoluble gluten protein, 50PI) in 50 % (v/v) propan-1-ol. The 50PI fraction was essentially free of monomeric proteins and comprised mainly glutenin, while the 50PS fraction was a mixture of monomeric proteins and soluble polymeric glutenin (Bean et al. 1998; Fu and Sapirstein 1998; Sapirstein and Fu 1996). We therefore initially determined the effects of the avenin-like b proteins on the proportions of alcohol-soluble and alcohol-insoluble proteins and on the distribution of transgenic subunits in these fractions.
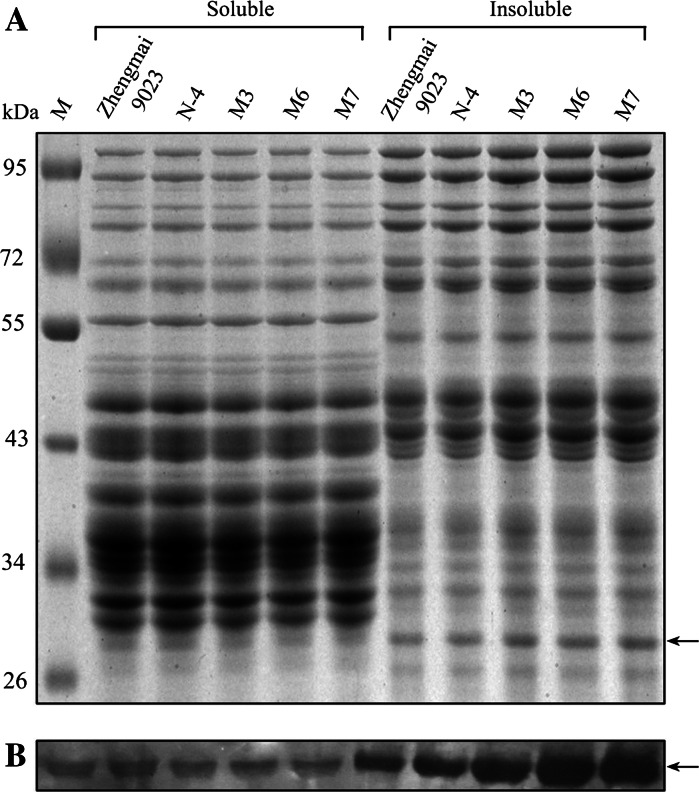
The SDS-PAGE patterns under reducing conditions of these fractions from the five flour samples are shown in Fig. 4. Gel scanning was used to determine the relative proportions of these two fractions (by determining the total absorbance of the tracks). The ratios of soluble to insoluble proteins were significantly lower in the three transgenic lines than in the control lines. The ratios of soluble to insoluble proteins varied from 1.42 in the non-transformed lines and 1.38 in the non-transgenic lines to 1.19, 1.12 and 1.08 in the M3, M6 and M7 lines, respectively. In our study, no significant difference in the amount of avenin-like b proteins was observed in the 50PS and 50PI fractions between the non-transgenic line (N-4) and non-transformed line (cv. Zhengmai 9023) (Fig. 4). This result met expectation, as line N-4 showed expression amounts of avenin-like b proteins in the wheat grain almost identical to the non-transformed line (Fig. 2; ESM Fig. S2).
Fig. 4.
SDS-PAGE (a) and Western blotting analysis (b) of soluble and insoluble gluten protein fractions extracted from flour of non-transformed line (cv. Zhengmai 9023), non-transgenic line (N-4) and transgenic lines (M3, M6 and M7). Lane M protein marker. Arrow indicates the position of the transgenic avenin-like b proteins
Figure 4a showed that no significant difference in the amount of avenin-like b proteins in the 50PS fraction was observed between the control lines and transgenic lines (M3, M6 and M7), while the amounts of avenin-like b proteins in the 50PI were increased in the transgenic lines compared with those of the control lines (Fig. 4, arrow). Especially in transgenic lines M6 and M7, avenin-like b proteins were much more abundant in the polymers than in the transgenic line M3. These results indicated that the overexpression of avenin-like b proteins in transgenic wheat lines was presumably incorporated into the polymers.
SE-HPLC can be used to fractionate gluten proteins based on their molecular masses without the reduction of the inter-chain disulphide bonds that stabilized the glutenin polymers. The chromatographic patterns were divided into four fractions corresponding to large polymeric proteins (F1), smaller polymeric proteins (F2), large monomeric proteins (F3) and smaller monomeric proteins (F4), respectively (Tosi et al. 2005). Each fraction was quantified by measurement of the peak area. The relative amounts of the individual fractions were quantified as the percentages of each peak relative to the total area and the four separated fraction amounts were expressed as %F1, %F2, %F3 and %F4, respectively. The relative amounts of the peaks do relate to dough strength, with %F1/%F2 and (%F3 + %F4)/%F1 showing particularly strong correlations (Morel et al. 2000). The SE-HPLC results for the transgenic and control lines are summarized in Table 1.
As judged from the SE-HPLC analysis, the %F1 was drastically increased in the transgenic wheat lines compared to the control lines. The %F1 amounts in the M3, M6 and M7 lines were increased 0.26, 0.29 and 0.28 times respectively. All transgenic lines had higher values for %F1/%F2 while the values for (%F3 + %F4)/%F1 were decreased in the three transgenic lines compared with the control lines (Table 1), indicating that all the transgenic lines had higher proportions of polymeric proteins. This observation was also supported by determination of the proportion of unextractable polymeric protein (%UPP), which represented an alternative way of measuring polymer size distribution. The %UPP in the three transgenic lines M3 (43.53 %), M6 (44.1 %) and M7 (43.87 %) were markedly higher than in the non-transformed wheat lines (35.57 %) (Table 1). Based on the above results, the increased amounts of avenin-like b protein resulted in a significant effect on the proportion of polymeric proteins.
Discussion
The objective of this study was to evaluate the effect of avenin-like b proteins on the functional properties of wheat flour. We have clearly demonstrated that all the transgenic lines that tested PCR-positive for the uidA gene and CaMV35S terminator were also positive for transgene expression, unequivocally determined by Western blotting with the anti-avenin-like b protein polyclonal antibody. After selection for four generations, three transgenic wheat lines overexpressing the avenin-like b gene (designated M3, M6, and M7), one non-transgenic line (designated N-4) and one non-transformed control line (cv. Zhengmai 9023) were used to investigate the relationships between avenin-like b proteins and the mixing properties of wheat flour.
Overexpression of avenin-like b proteins has positive effects on dough mixing properties
In this work, both total proteins and dough mixing properties were characterized (Fig. 3; ESM Fig. S2). Due to the dominant role of glutenins in dough functionality, we first determined whether any difference in the glutenin profiles existed between transgenic lines and control lines. As shown in ESM Fig. S2, three transgenic lines, the non-transgenic line and the non-transformed line showed almost identical SDS-PAGE patterns of storage proteins, indicating that overexpression of avenin-like b proteins did not alter any aspects of the glutenin profiles.
The potential bread-making quality of the flours was assessed with two small-scale tests: the SDSS test and Mixograph analysis (Carter et al. 1999; Lorenzo and Kronstad 1987; Martinant et al. 1998). SDSS volume is a predictor of baking quality that is often used by breeders to screen small flour samples (Dick and Quick 1983). The results reported here showed significant increases in the average of SDSS volumes of the transgenic lines. According to the SDSS values, the transgenic lines had better quality than their control lines. High SDSS volumes have been associated with stronger gluten and superior bread-making quality (Ayoub et al. 1993; Lorenzo and Kronstad 1987). Mixing properties of transgenic lines analyzed using the 10 g Mixograph provided information on dough gluten strength, closely correlated with baking quality. The relationships between Mixograph parameters and dough viscoelasticity have been explained in detail (Martinant et al. 1998) and associations of these parameters with other wheat quality traits have also been discussed previously (Bordes et al. 2008). In generally, MPW, MRW, BWPR and MBW are positively correlated with dough resistance to extension (Tosi et al. 2005), while RBD is negatively correlated with the mixing tolerance (Tosi et al. 2005; Piston et al. 2011). MT, MPV and MTxI are positively correlated with dough strength (León et al. 2009). Weak dough has higher RBD, shorter MT, lower PR and smaller MTxI when compared to strong dough (León et al. 2009; Piston et al. 2011; Li et al. 2012). In our study, no significant difference in mixing parameters was observed between the non-transgenic line (N-4) and non-transformed control line (cv. Zhengmai 9023) (Fig. 3; Table 1). This result met expectation, as line N-4 showed almost identical patterns of storage proteins with the non-transformed control line (ESM Fig. S2). These results demonstrated that no variations in storage proteins were caused in line N-4 by possible somaclonal variations in the process of genetic transformation.
We further compared the mixing parameters of transgenic lines (M3, M6 and M7), the non-transgenic line (N-4) and the non-transformed control line (cv. Zhengmai 9023). In transgenic lines, the significant increase of PR demonstrated that dough elasticity was increased by overexpression of the avenin-like b gene. Significantly lower RBD values for lines M3, M6 and M7 revealed that the mixing tolerance was also improved. In transgenic lines, all mixing parameters relating to the curve widths showed significant increases compared to the control lines, indicating that dough resistance to extension was improved. Furthermore, significant increases in MT, PR and MTxI were detected in the transgenic lines, suggesting that the overexpression of avenin-like b proteins caused an increase in dough strength. It could be concluded that overexpression of avenin-like b proteins generally led to improved dough elasticity and mixing tolerance and increased dough resistance to extension.
These results showed a similar trend to the results of our previous report, that incorporation of avenin-like b proteins into flour resulted in statistically significant increases in PR and mixing tolerance by in vitro assay (Chen et al. 2010), indicating that avenin-like b proteins had advantageous effects on mixing properties of wheat flour. In this study, the difference in MT between the transgenic wheat lines and control lines was not statistically significant, while Chen et al. (2010) reported that statistically significant increases were observed in MT by incorporation of avenin-like b proteins into flour. This difference might come from the different protein sequence, different wheat cultivars, the unknown (undetermined) amount of avenin-like b protein in transgenic wheat lines and different mixing equipment used for these studies.
Incorporation of avenin-like b proteins into the glutenin polymers
The avenin-like b proteins of this research contained 18 cysteine residues, speculated to form inter-chain disulphide bonds allowing incorporation into high-molecular-mass polymers (Kan et al. 2006). In order to confirm this possibility, monomeric, soluble and insoluble polymeric glutenin proteins of wheat flour were extracted using the method described by Tosi et al. (2005). Both the soluble and insoluble extracts were analyzed by SDS-PAGE and Western blotting analysis. These results showed that avenin-like b proteins existed in both 50PS (monomericn and soluble polymeric glutenin) and 50PI (insoluble polymer proteins). Moreover, the amount of avenin-like b proteins in the insoluble polymer proteins was increased in the transgenic lines (M3, M6 and M7) compared to the control lines, while no significant differences were found in the soluble fractions between the three transgenic lines and control lines (Fig. 4). Based on these results, it was reasonable to conclude that transgenic avenin-like b proteins were indeed incorporated into the glutenin polymers.
This observation was also supported by the results of SE-HPLC analysis which was used to analyze the polymer size distribution between transgenic and control lines. Based on the SE-HPLC analysis (Table 1), the overexpression of avenin-like b proteins in the transgenic lines could result in significant effects on the proportion of the polymeric gluten protein fractions, indicating that the avenin-like b proteins affected the degree of cross-linking. Furthermore, the %UPP in the three transgenic lines (M3, M6 and M7) were markedly higher than in the control lines (Table 1). On the basis of the above analysis, the increased amounts of avenin-like b proteins could result in significant effects on the proportion of the polymeric gluten protein fractions. These results demonstrate that the improvement in flour properties in the transgenic lines was associated with an increased proportion of polymer proteins.
Our results showed that the overexpression of avenin-like b proteins in transgenic wheat lines improved flour mixing properties and demonstrated that the overexpression of avenin-like b proteins in transgenic plants led to an increase in the proportion of large polymeric proteins, which should help to understand the influence and mechanism of avenin-like b proteins on the functional properties of wheat flour. These results also suggested that avenin-like b proteins play an important role in determining the mixing properties of wheat flour and that the avenin-like b gene could be a candidate gene for improving functional properties of wheat flour. It would be of interest to clarify whether different types of avenin-like b proteins with different numbers and/or positions of cysteine residues could improve the mixing properties of dough in the same way. Therefore, more work is required for a better understanding of their role in the functional properties of wheat flour in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1 Map of the plasmid pLRPT-avel with the positions of relevant restriction sites. The avenin-like b gene was inserted between the endosperm-specific 1Dx5 promoter and the CaMV35S terminator (TIFF 552 kb)
Characterization of storage proteins in transgenic (M3, M6 and M7) lines and control (cv. Zhengmai 9023 and N-4) lines. A. SDS-PAGE of seed protein extracts from transgenic lines, non-transgenic line and non-transformed control line. Arrow indicates the position of the transgenic Avenin-like b proteins. B. Characterization of storage proteins from the transgenic and control lines. HMW % glutenin and LMW % glutenin means quantities of HMW-GS and LMW-GS, respectively, expressed relative to total quantity of the glutenins (and the same for 1Dx2 %, 1Bx7 %, 1By8 %, and 1Dy12 %). HMW/LMW: ratio of the high and low molecular weight glutenin subunits. Glutenin %: quantity of the glutenins expressed relative to total proteins extracted by the sequential extraction methods (and the same for Gliadin %). Glu/Glia: ratio of the glutenins and gliadins. Data are given as mean ± SEM. Values within the same characteristics of storage proteins are not significantly different (P =0.05) (TIFF 1913 kb)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30871524, 31071403), the National Natural Science Foundation of Hubei, China (2010CBD02403), Wuhan Municipal S&T research project (201120922286), International S&T Cooperation Key Projects of MoST (Grant No. 2009DFB30340), and National Genetically Modified New Varieties of Major Projects of China (2013ZX08002-004, 2013ZX08010-004).
Abbreviations
- BWPR
Bandwidth at peak resistance
- MBW
Maximum bandwidth during the mixing
- MRW
Bandwidth of midline after mixing time
- MT
Mixing time
- MTxI
Midline integral at 8 min
- NFDM
Non-fat dry milk
- PR
Peak resistance
- PVDF
Polyvinylidene fluoride
- RBD
Resistance to breakdown
- SDSS
Sodium dodecyl sulfate sedimentation
- SE-HPLC
Size exclusion-high performance liquid chromatography
Contributor Information
Yuesheng Wang, Phone: +86-27-87792271, FAX: +86-27-87792272, Email: wysh@hust.edu.cn.
Guangyuan He, Phone: +86-27-87792271, FAX: +86-27-87792272, Email: hegy@hust.edu.cn.
References
- Anderson OD, Hsia CC, Adalsteins AE, Lew EJL, Kasarda DD. Identification of several new classes of low-molecular-weight wheat gliadin-related proteins and genes. Theor Appl Genet. 2001;103:307–315. doi: 10.1007/s001220100576. [DOI] [Google Scholar]
- Ayoub M, Fregeaureid J, Smith DL. Evaluation of the SDS-sedimentation test for the assessment of eastern Canadian bread wheat quality. Can J Plant Sci. 1993;73:995–999. doi: 10.4141/cjps93-130. [DOI] [Google Scholar]
- Barro F, Cannell ME, Lazzeri PA, Barcelo P. The influence of auxins on transformation of wheat and tritordeum and analysis of transgene integration patterns in transformants. Theor Appl Genet. 1998;97:684–695. doi: 10.1007/s001220050944. [DOI] [Google Scholar]
- Bean SR, Lyne RK, Tilley KM, Chung OK, Lookhart GL. A rapid method for quantitation of insoluble polymeric proteins in flour. Cereal Chem. 1998;75:374–379. doi: 10.1094/CCHEM.1998.75.3.374. [DOI] [Google Scholar]
- Bietz JA, Wall JS. Identity of high molecular weight gliadin and ethanol-soluble glutenin subunits of wheat: relation to gluten structure. Cereal Chem. 1980;57:415–420. [Google Scholar]
- Bordes J, Branlard G, Oury FX, Charmet G, Balfourier F. Agronomic characteristics, grain quality and flour rheology of bread wheats in a worldwide core collection. J Cereal Sci. 2008;48:569–579. doi: 10.1016/j.jcs.2008.05.005. [DOI] [Google Scholar]
- Carter BP, Morris CF, Anderson JA. Optimizing the SDS sedimentation test for end-use quality selection in a soft white and club wheat breeding program. Cereal Chem. 1999;76:907–911. doi: 10.1094/CCHEM.1999.76.6.907. [DOI] [Google Scholar]
- Chen P, Wang CD, Li KX, Chang JL, Wang YS, Yang GX, Shewry PR, He GY. Cloning, expression and characterization of novel avenin-like genes in wheat and related species. J Cereal Sci. 2008;48:734–740. doi: 10.1016/j.jcs.2008.04.002. [DOI] [Google Scholar]
- Chen P, Li R, Zhou R, He GY, Shewry PR. Heterologous expression and dough mixing studies of a novel cysteine-rich Avenin-like protein. Cereal Res Commun. 2010;38:406–418. doi: 10.1556/CRC.38.2010.3.11. [DOI] [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Clarke B, Phongkham T, Gianibelli M, Beasley H, Bekes F. The characterisation and mapping of a family of LMW-gliadin genes: effects on dough properties and bread volume. Theor Appl Genet. 2003;106:629–635. doi: 10.1007/s00122-002-1091-1. [DOI] [PubMed] [Google Scholar]
- De Caro S, Ferranti S, Addeo F, Mamone G. Isolation and characterization of avenin-like protein type-B from durum wheat. J Cereal Sci. 2010;52:426–431. doi: 10.1016/j.jcs.2010.07.005. [DOI] [Google Scholar]
- Dick JW, Quick JS. A modified screening test for rapid estimation of gluten strength in early-generation durum wheat breeding lines. Cereal Chem. 1983;60:315–318. [Google Scholar]
- DuPont FM, Chan R, Lopez R, Vensel WH. Sequential extraction and quantitative recovery of gliadins, glutenins, and other proteins from small samples of wheat flour. J Agric Food Chem. 2005;53:1575–1584. doi: 10.1021/jf048697l. [DOI] [PubMed] [Google Scholar]
- Fido RJ, Tatham AS, Shewry PR. Western blotting analysis. In: Jones H, editor. Methods in molecular biology. Totowa: Humana; 1995. pp. 423–437. [DOI] [PubMed] [Google Scholar]
- Fu BX, Sapirstein HD. Intercultivar variation in the quantity of monomeric proteins, soluble and insoluble glutenin, and residue protein in wheat flour and relationships to breadmaking quality. Cereal Chem. 1998;75:500–507. doi: 10.1094/CCHEM.1998.75.4.566. [DOI] [Google Scholar]
- Gupta RB, Khan K, MacRitchie F. Biochemical basis of flour properties in bread wheats. I. Effects of variation in the quantity and size distribution of polymeric protein. J Cereal Sci. 1993;18:23–41. doi: 10.1006/jcrs.1993.1031. [DOI] [Google Scholar]
- He GY, Rooke L, Steele S, Bekes F, Gras P, Tatham AS, Fido R, Barcelo P, Shewry PR, Lazzeri PA. Transformation of pasta wheat (Triticum turgidum L. var. durum) with high-molecular-weight glutenin subunit genes and modification of dough functionality. Mol Breed. 1999;5:377–386. doi: 10.1023/A:1009681321708. [DOI] [Google Scholar]
- He GY, Jones HD, D’Ovidio R, Masci S, Chen MJ, West J, Butow B, Anderson OD, Lazzeri P, Fido R, Shewry PR. Expression of an extended HMW subunit in transgenic wheat and the effect on dough mixing properties. J Cereal Sci. 2005;42:225–231. doi: 10.1016/j.jcs.2005.04.004. [DOI] [Google Scholar]
- Huebner FR, Wall JS. Fractionation and quantitative differences of glutenin from wheat varieties varying in baking quality. Cereal Chem. 1976;53:258–269. [Google Scholar]
- Kan YC, Wan YF, Beaudoin F, Leader DJ, Edwards K, Poole R, Wang DW, Mitchell RAC, Shewry PR. Transcriptome analysis reveals differentially expressed storage protein transcripts in seeds of Aegilops and wheat. J Cereal Sci. 2006;44:75–85. doi: 10.1016/j.jcs.2006.04.004. [DOI] [Google Scholar]
- León E, Marin S, Gimenez MJ, Piston F, Rodriguez-Quijano M, Shewry PR, Barro F. Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. J Cereal Sci. 2009;49:148–156. doi: 10.1016/j.jcs.2008.08.002. [DOI] [Google Scholar]
- Li Y, Wang Q, Li XY, Xiao X, Sun FS, Wang C, Hu W, Feng ZJ, Chang JL, Chen MJ, Wang YS, Li KX, Yang GX, He GY. Coexpression of the high molecular weight glutenin subunit 1Ax1 and Puroindoline improves dough mixing properties in durum wheat (Triticum turgidum L. ssp. durum) PLoS One. 2012;7(11):e50057. doi: 10.1371/journal.pone.0050057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Kronstad WE. Reliability of two laboratory techniques to predict bread wheat protein quality in nontraditional growing areas. Crop Sci. 1987;27:247–252. doi: 10.2135/cropsci1987.0011183X002700020025x. [DOI] [Google Scholar]
- Mamone G, De Caro S, Di Luccia A, Addeo F, Ferranti P. Proteomic-based analytical approach for the characterization of glutenin subunits in durum wheat. J Mass Spectrom. 2009;44:1709–1723. doi: 10.1002/jms.1680. [DOI] [PubMed] [Google Scholar]
- Martinant JP, Nicolas Y, Bouguennec A, Popineau Y, Saulnier L, Branlard G. Relationships between mixograph parameters and indices of wheat grain quality. J Cereal Sci. 1998;27:179–189. doi: 10.1006/jcrs.1997.0156. [DOI] [Google Scholar]
- Morel MH, Dehlon P, Autran JC, Leygue JP, Bar-L’Helgouac’h C. Effects of temperature, sonication time and power settings on size distribution and extractability of total wheat flour proteins as determined by size-exclusion high-performance liquid chromatography. Cereal Chem. 2000;77:685–691. doi: 10.1094/CCHEM.2000.77.5.685. [DOI] [Google Scholar]
- Müller S, Wieser H. Disulphide bonds of α-type gliadins. J Cereal Sci. 1995;22:21–27. doi: 10.1016/S0733-5210(05)80004-9. [DOI] [Google Scholar]
- Müller S, Wieser H. The location of disulphide bonds in monomeric γ-gliadins. J Cereal Sci. 1997;26:169–176. [Google Scholar]
- Piston F, Gil-Humanes J, Rodriguez-Quijano M, Barro F. Down-regulating γ-gliadins in bread wheat leads to non-specific increases in other gluten proteins and has no major effect on dough gluten strength. PLoS One. 2011;6(9):e24754. doi: 10.1371/journal.pone.0024754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo G, Prada J, Aragoncillo C. LMW gliadin-like proteins from wheat endosperm. Phytochemistry. 1979;18:725–727. doi: 10.1016/0031-9422(79)80003-5. [DOI] [Google Scholar]
- Sapirstein HD, Fu BX. Procedure for isolating monomeric proteins and polymeric glutenin of wheat flour. Cereal Chem. 1996;73:143–152. [Google Scholar]
- Shewry PR. Plant storage proteins. Biol Rev Camb Philos Soc. 1995;70:375–426. doi: 10.1111/j.1469-185X.1995.tb01195.x. [DOI] [PubMed] [Google Scholar]
- Shewry PR. Wheat. J Exp Bot. 2009;6:1537–1553. doi: 10.1093/jxb/erp058. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–958. doi: 10.1093/jexbot/53.370.947. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Tatham AS. Disulphide bonds in wheat gluten proteins. J Cereal Sci. 1997;25:207–227. doi: 10.1006/jcrs.1996.0100. [DOI] [Google Scholar]
- Shewry PR, Miflin BJ, Lew E-J-L, Kasarda DD. The preparation and characterization of an aggregated gliadin fraction from wheat. J Exp Bot. 1983;148:1403–1410. doi: 10.1093/jxb/34.11.1403. [DOI] [Google Scholar]
- Shewry PR, Tatham AS, Lazzeri P. Biotechnology of wheat quality. J Sci Food Agric. 1997;73:397–406. doi: 10.1002/(SICI)1097-0010(199704)73:4<397::AID-JSFA758>3.0.CO;2-Q. [DOI] [Google Scholar]
- Shewry PR, Halford NG, Tatham AS, Popineau Y, Lafiandra D, Belton PS. The high molecular weight subunits of wheat glutenin and their role in determining wheat processing properties. Adv Food Nutr Res. 2003;45:221–302. doi: 10.1016/s1043-4526(03)45006-7. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Powers S, Field JM, Fido RJ, Jones HD, Arnold GM, West J, Lazzeri PA, Barcelo P, Barro F, Tatham AS, Bekes F, Butow B, Darlington H. Comparative field performance over 3 years and two sites of transgenic wheat lines expressing HMW subunit transgenes. Theor Appl Genet. 2006;113:128–136. doi: 10.1007/s00122-006-0279-1. [DOI] [PubMed] [Google Scholar]
- Sparks CA, Jones HD. Transformation of wheat by biolistics. In: Curtis IS, editor. Transgenic crops of the world-essential protocols. Dordrecht: Kluwer; 2004. pp. 19–34. [Google Scholar]
- Stacey J, Isaac PG. Isolation of DNA from plants. In: Isaac PG, editor. Methods in molecular biology: protocols for nucleic acid analysis by nonradiactive probes. Totowa: Humana Press; 1994. pp. 9–15. [DOI] [PubMed] [Google Scholar]
- Tosi P, D’Ovidio R, Napier JA, Bekes F, Shewry PR. Expression of epitope-tagged LMW glutenin subunits in the starchy endosperm of transgenic wheat and their incorporation into glutenin polymers. Theor Appl Genet. 2004;108:468–476. doi: 10.1007/s00122-003-1459-x. [DOI] [PubMed] [Google Scholar]
- Tosi P, Masci S, Giovangrossi A, D’Ovidio R, Bekes F, Larroque O, Napier J, Shewry PR. Modification of the low molecular weight (LMW) glutenin composition of transgenic durum wheat: effects on glutenin polymer size and gluten functionality. Mol Breed. 2005;16:113–126. doi: 10.1007/s11032-005-5912-1. [DOI] [Google Scholar]
- Zhang Y, Darlington H, Jones HD, Halford NG, Napier JA, Davey MR, Lazzeri PA, Shewry PR. Expression of the gamma-zein protein of maize in seeds of transgenic barley: effects on grain composition and properties. Theor Appl Genet. 2003;106:1139–1146. doi: 10.1007/s00122-002-1162-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1 Map of the plasmid pLRPT-avel with the positions of relevant restriction sites. The avenin-like b gene was inserted between the endosperm-specific 1Dx5 promoter and the CaMV35S terminator (TIFF 552 kb)
Characterization of storage proteins in transgenic (M3, M6 and M7) lines and control (cv. Zhengmai 9023 and N-4) lines. A. SDS-PAGE of seed protein extracts from transgenic lines, non-transgenic line and non-transformed control line. Arrow indicates the position of the transgenic Avenin-like b proteins. B. Characterization of storage proteins from the transgenic and control lines. HMW % glutenin and LMW % glutenin means quantities of HMW-GS and LMW-GS, respectively, expressed relative to total quantity of the glutenins (and the same for 1Dx2 %, 1Bx7 %, 1By8 %, and 1Dy12 %). HMW/LMW: ratio of the high and low molecular weight glutenin subunits. Glutenin %: quantity of the glutenins expressed relative to total proteins extracted by the sequential extraction methods (and the same for Gliadin %). Glu/Glia: ratio of the glutenins and gliadins. Data are given as mean ± SEM. Values within the same characteristics of storage proteins are not significantly different (P =0.05) (TIFF 1913 kb)