Abstract
Background:
Hematological values of the newborn babies vary according to the gestational age and intrauterine growth.
Objective:
The objective of this study is to compare the iron status and red cell parameters in healthy term small for gestational age (SGA) and appropriate for gestational age (AGA) neonates.
Materials and Methods:
A prospective hospital based study was conducted in a tertiary care teaching institution of central India. 50 AGA and 50 SGA neonates were included in the study and serum iron, serum ferritin and red cell parameters (hemoglobin (Hb), red blood cell (RBC) count and hematocrit (Hct), mean corpuscular volume (MCV), means corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and red cell distribution width (RDW]) were estimated within 24 h of birth.
Results:
Serum ferritin levels were significantly low in SGA neonates as compared with AGA (mean 103 vs. 158 ng/ml; P=0.001) neonates. In SGA neonates, mean values of Hb (P=0.001), RBC count (P=0.018) and Hct (P=0.005) were significantly higher than in AGA neonates. Higher values of RDW and MCV were seen in SGA group in comparison with AGA neonates. Similarly, lower values of serum iron, MCH and MCHC were seen in the same group; although, these were non-significant.
Conclusion:
Despite higher Hb content, SGA neonates are deficient in iron store at birth as indicated by lower serum ferritin levels in them and early iron supplementation should be considered in them.
Keywords: Complete blood count, serum ferritin, serum iron, term appropriate for gestational age neonate, term small for gestational age neonates
INTRODUCTION
Intrauterine growth restriction (IUGR) is an important cause of neonatal morbidity and mortality and is also associated with the long-term sequelae. In India, IUGR is the major underlying cause of the low birth weight in neonates. Chronic intrauterine hypoxia stimulates erythropoiesis leading to polycythemia in these IUGR neonates at birth; however, this occurs at the cost of loss of the iron stores to fulfill the demands of increased erythropoiesis. Approximately, 50% of IUGR neonates are iron deficient at birth as suggested by low serum ferritin concentration. The liver and brain iron concentration are decreased in IUGR neonates without a significant effect on hemoglobin (Hb) at birth. Iron is an essential micronutrient that plays a significant role in critical cellular functions in all organ systems in all species. Iron is particularly vital for early brain growth and function in humans since it supports neuronal and glial energy metabolism, neurotransmitter synthesis and myelination.[1,2,3]
A wide spectrum of measures is used to diagnose iron deficiency. Hb, mean corpuscular volume (MCV) and red cell distribution width (RDW) are late markers of iron deficiency and may not reflect tissue iron status in the newborn infant. Serum ferritin concentration has been used as a standard measurement of iron stores in neonates, children and adults.[4,5,6] Despite being major neonatal problem, limited data on iron status is available for the Indian infants. Therefore, a prospective study was conducted to compare red blood cell (RBC) parameters, serum ferritin and serum iron values between full term small for gestational age (SGA) and full term appropriate for gestational age (AGA) neonates.
MATERIALS AND METHODS
This prospective study was conducted in Gandhi Medical College and associated Kamla Nehru and Hamidia Hospital, Bhopal, Madhya Pradesh from September 2011 to August 2012 to assess iron status in healthy full term SGA and AGA neonates. This study was approved by ethical committee of Gandhi Medical College, Bhopal. Informed consent obtained from mothers of all the recruited newborns. SGA newborn was defined as having birth weight below the 10th percentile for their gestational age.[7] Weight of newborns was taken over an electronic weighing machine with an error of ±5 g. Gestational age was assessed by New Ballard scoring.[8] Details of maternal and perinatal medical history and anthropometry were recorded for each neonate. As per our hospital policy, early umbilical cord clamping was performed in all neonates (i.e., within 15 s). Neonates were excluded if they were large for gestational age (birth weight ≥90th percentile), preterm, delivered as one of the multiple pregnancies, had intrapartum complications or low Apgar scores (≤7) within the first 5 min, gross congenital anomalies, born to mother with chronic medical illness and/or moderate to severe anemia. Venous blood samples were collected within 24 h of birth from dorsal hand veins for red cell parameters, serum iron and ferritin. Red cell parameters were assessed using fully automated blood cell counter (ERMA-INC) and different parameters including Hb, RBCs count, hematocrit (Hct), means corpuscular hemoglobin (MCH), MCV, mean corpuscular hemoglobin concentration (MCHC) and RDW were assessed. Serum ferritin was measured by fully automated bidirectional interfaced chemi-luminescent immunoassay and serum iron by photometry method. Statistical analysis was performed using the Statistical Package for Social Sciences versions 17.0. Parameters were described as mean ± standard deviation and range. Mean values between SGA and AGA group were compared using the unpaired Student′s t-test. P<0.05 was considered to be significant.
RESULTS
During the study period, total 100 neonates were recruited and out of these hundred neonates, 50 were SGA and 50 were AGA. Male to female ratio was 0.72:1. Demographic data of the study population have been shown in Table 1. Hematological results for the two groups are summarized in Table 2. Mean Hb was significantly high in SGA group as compared with AGA group (P=0.001). Hct (P=0.005) and RBC count (P=0.018) were also significantly higher in SGA group. SGA group had significantly lower mean serum ferritin than AGA group (P=0.001) and similar pattern was observed in mean values of serum iron, but difference was statistically not significant (P=0.40).
Table 1.
Demographic profile of neonates
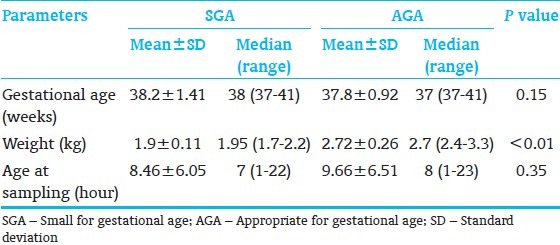
Table 2.
Hematological parameters in neonates
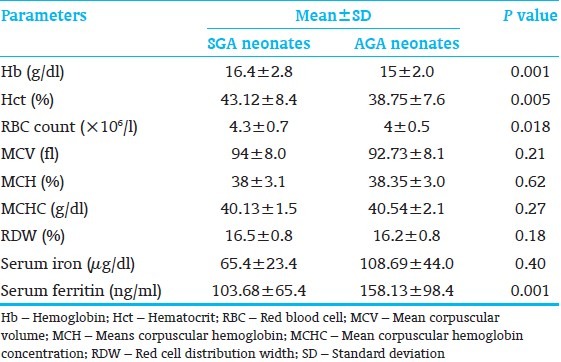
A non-significantly higher mean values for MCV and RDW were seen in SGA group as compared with AGA group (P=0.21 and 0.18 respectively). However, MCH and MCHC exhibit a different pattern and SGA neonates showed lower mean values of MCH and MCHC than AGA neonates, but difference was statistically not significant (P=0.62 and 0.27 respectively).
DISCUSSION
In the present study, mean values of Hb, Hct and RBC count were significantly higher in SGA group than AGA group. Similar results were observed by Nunes et al., [9] Maconi et al.[10] and Karaduman et al.[11] This can be explained by the fact that chronic fetal hypoxia due to poor placental function leads to increased erythropoiesis in the SGA neonates. Many previous studies showed the importance of assessment of serum ferritin levels at birth as an indicator of iron stores in neonates.[4,5] In this study, SGA neonates had significantly lower mean values of serum ferritin and these results are similar to the previous observations by Mukhopadhyay et al.[12] and Chockalingam et al., [13] concluding that term SGA neonates have lesser total iron store than gestational age matched AGA neonates at birth. Intrauterine growth retardation may lead to iron deficiency due to placental vascular insufficiency mediated impairment in iron transport and an increased iron requirement for the augmented fetal erythropoiesis secondary to chronic hypoxia. On the contrary, Nunes et al.[9] observed higher serum ferritin level in term SGA neonates as compared with term AGA neonates at birth. In the present study, mean value of serum iron was low in SGA group as compared to AGA group, which is corroborative with the results found in previous study done by Karaduman et al.[11] Higher mean values of MCV in SGA group may be due to release of large numbers of young erythrocytes from the bone marrow into peripheral blood during increased fetal erythropoietic activity. Lower MCH and MCHC values in SGA babies may be due to relatively higher RBC mass and MCV in this group.
In the present study, we evaluated another parameter to assess iron status, i.e., RDW, which was not evaluated in most of the other studies. In the present study, mean RDW was higher in SGA group as compared with AGA group (P=0.18); although, difference was not statistically significant in the present study. This is consistent with the only study, which evaluated RDW in anemia.[13] RDW is a measure of degree of variation in red cell size and some causes of microcytic anemia, especially iron deficiency, are characterized by an increase in RDW. RDW can be a useful tool to assess the iron status as it is the first parameter to increase in response to iron depletion followed by decrease in MCH and MCV. RDW can also be used to differentiate the iron deficiency anemia from thalassemia, where red cells are uniformly microcytic without a concomitant increase in RDW. However, few thalassemia syndromes, e.g. Hb H disease and δ b-thalassemia minor are associated with increase in RDW. Similarly, in cases with coexistent iron deficiency and thalassemia, RDW alone may be misleading. Therefore, the RDW can be useful as an adjunct to diagnosis, but is not useful as a lone indicator.[14]
We would like to highlight few study limitations, which include small sample size and further studies with larger sample size may help confirming these findings. Secondly, maternal iron status is another factor that can affect the iron status of neonates and which we could not assess due to financial constraints. However, this factor is less likely to affect the study outcome as we excluded all the neonates born to moderately or severely anemic (Hb < 8 g%) mothers. This is in accordance with previous studies which showed that iron status of pregnant women with mild anemia seemed to have no significant impact on the iron status of their children.[9,15,16,17]
Lastly, timing of umbilical cord clamping after birth is another factor, which could affect the iron status of neonates and recent studies have shown improved iron stores in neonates on delayed cord clamping.[18,19] However, in our study this variable also has little effect on the results as early cord clamping (within 15 s) was carried out in both SGA as well as AGA neonates as per our hospital policy.
CONCLUSION
Despite higher Hb content, SGA neonates are deficient in iron store at birth, as indicated by lower serum ferritin level in them and early iron supplementation should be considered in them.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: Involvement of dopamine-opiate system. Cell Mol Biol (Noisy-le-grand) 2000;46:491–500. [PubMed] [Google Scholar]
- 2.Connor JR, Menzies SL. Altered cellular distribution of iron in the central nervous system of myelin deficient rats. Neuroscience. 1990;34:265–71. doi: 10.1016/0306-4522(90)90320-4. [DOI] [PubMed] [Google Scholar]
- 3.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–76. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Siimes MA, Addiego JE, Jr, Dallman PR. Ferritin in serum: Diagnosis of iron deficiency and iron overload in infants and children. Blood. 1974;43:581–90. [PubMed] [Google Scholar]
- 5.Rios E, Lipschitz DA, Cook JD, Smith NJ. Relationship of maternal and infant iron stores as assessed by determination of plasma ferritin. Pediatrics. 1975;55:694–9. [PubMed] [Google Scholar]
- 6.Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213–6. doi: 10.1056/NEJM197405302902201. [DOI] [PubMed] [Google Scholar]
- 7.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 8.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 9.Nunes MF, Assis AM, Pinheiro SM, da Rocha MF. Erythrocyte indices and serum ferritin in newborns. Rev Bras Hematol Hemoter. 2010;32:365–70. [Google Scholar]
- 10.Maconi M, Rolfo A, Cardaropoli S, Brini M, Danise P. Hematologic values in healthy and small for gestational age newborns. Lab Hematol. 2005;11:152–6. doi: 10.1532/LH96.04076. [DOI] [PubMed] [Google Scholar]
- 11.Karaduman D, Ergin H, Kiliç I. Serum ferritin, iron levels and iron binding capacity in asymmetric SGA babies. Turk J Pediatr. 2001;43:121–4. [PubMed] [Google Scholar]
- 12.Mukhopadhyay K, Yadav RK, Kishore SS, Garewal G, Jain V, Narang A. Iron status at birth and at 4 weeks in term small-for-gestation infants in comparison with appropriate-for-gestation infants. J Matern Fetal Neonatal Med. 2011;24:886–90. doi: 10.3109/14767058.2010.536866. [DOI] [PubMed] [Google Scholar]
- 13.Chockalingam UM, Murphy E, Ophoven JC, Weisdorf SA, Georgieff MK. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J Pediatr. 1987;111:283–6. doi: 10.1016/s0022-3476(87)80088-4. [DOI] [PubMed] [Google Scholar]
- 14.Hedlund B. Hemoglobins of human embryos, fetuses, and neonates. In: Fairbanks VF, editor. Hemoglobinopathies and Thalassemias. New York: Brian C. Decker; 1980. pp. 14–7. [Google Scholar]
- 15.Paiva Ade A, Rondó PH, Pagliusi RA, Latorre Mdo R, Cardoso MA, Gondim SS. Relationship between the iron status of pregnant women and their newborns. Rev Saude Publica. 2007;41:321–7. doi: 10.1590/s0034-89102007000300001. [DOI] [PubMed] [Google Scholar]
- 16.Mamoury GH, Hamedy AB, Akhlaghi F. Cord hemoglobin in newborns in correlation with maternal hemoglobin in northeastern Iran. Iran J Med Sci. 2003;28:166–8. [Google Scholar]
- 17.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, et al. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–9. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson O, Hellström-Westas L, Andersson D, Domellöf M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: A randomised controlled trial. BMJ. 2011;343:d7157. doi: 10.1136/bmj.d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaleel R, Deeba F, Khan A. Timing of umbilical cord clamping and neonatal haematological status. J Pak Med Assoc. 2009;59:468–70. [PubMed] [Google Scholar]


