Abstract
Objective:
To correlate electroencephalogram (EEG), computed tomography (CT), and magnetic resonance imaging (MRI) brain with neurological outcome at 12 months in term neonates with hypoxic ischemic encephalopathy.
Design:
Prospective observational study.
Setting:
Neonatal intensive care unit (NICU) in a tertiary care teaching hospital.
Materials and Methods:
The study was conducted between June 2010 and November 2011. Consecutive term neonates with perinatal asphyxia and hypoxic ischemic encephalopathy were the subjects. All babies were managed as per standard protocol. EEG was done as soon as the baby was stable and CT brain within 7 days. MRI was done at 3 months. Neurodevelpmental assessment was done at 12 months.
Results:
Of the 31 babies, four died and one was lost to follow-up. Neurodevelopmental at 12 months of age was normal in 15 babies. EEG was normal in six babies and all of them had a normal neurodevelopment. Thirteen of the 14 babies with burst suppression pattern were abnormal (P<0.001). CT brain was normal in 14 and all of them had normal neurodevelopment (P<0.001), while 11 of the 12 with cerebral edema had abnormal outcome (P<0.001). Of the 16 babies with normal MRI, 14 were normal, while all six babies with abnormal signals in the cortex and thalamus had abnormal outcome (P=0.002).
Conclusions:
A normal EEG and CT brain in a term newborn with hypoxic ischemic encephalopathy (HIE) is associated with good neurological outcome. Burst suppression pattern in EEG, bleeds, or hypodensities in the CT and involvement of basal ganglia/thalamus in the MRI are predictors of abnormal outcome.
Keywords: Electroencephalogram, hypoxic ischemic encephalopathy, magnetic resonance imaging, magnetic resonance imaging brain, neurodevelopment
INTRODUCTION
In spite of an improved understanding of its pathogenesis, hypoxic ischemic encephalopathy (HIE) is still the most dreaded neurological disease of the newborn. The reported incidence of HIE vary from one to four per 1,000 live births. Published data show that 25-60% of the babies who survive, suffer from permanent neurodevelopmental handicaps including cerebral palsy, seizures, mental retardation, and learning disabilities. Assessment of severity of HIE would help proper parent counseling and early institution of stimulation therapy for better development of the infant. In this study, an attempt has been made to associate the electroencephalogram (EEG) and CT done in the newborn period and MRI brain done at 3 months of age with the neurological outcome at 1 year in term asphyxiated newborns.
MATERIALS AND METHODS
This was a prospective observational study conducted from June 2010 to November 2011 at the PSG Institute of Medical Sciences and Research, Coimbatore, India. The study population included term newborns with perinatal asphyxia and HIE admitted to the newborn intensive care unit of PSG Hospital. Perinatal asphyxia was defined as presence of two or more of the following: [1] (a) Signs of fetal distress as indicated by one or more of the following: Fetal bradycardia (≤100 beats/min), thick meconium staining of liquor, abnormal cardiotocography recordings, arterial cord pH <7.2 or base deficit >15 mmol/L. (b) Apgar score <6 at 5 min of life. (c) Need for >10 min of positive pressure ventilation before occurrence of sustained respiration. All newborn babies who were born with gestational age ≤36+6 or had major congenital anomalies/inborn error of metabolism/low Apgar score as a result of maternal sedation were excluded from the study. Newborn babies who fulfilled the inclusion criteria were admitted to the neonatal intensive care unit (NICU) and included in the study. The study was approved by the institutional ethics committee. Informed written consent was obtained from the parents. Details regarding the mother′s medical history, antenatal illnesses, mode of delivery, duration of labor, and drugs administered were obtained. The neonates were classified into one of the three stages of Sarnat and Sarnat classification for HIE.[2] The babies were all managed as per the standard management protocol of the neonatal unit which did not include hypothermia. All babies had an EEG recorded during the first 72 h of life or as soon as the baby was stable. EEG recording was done using recorders and medicare system (RMS) recorder and the electrodes were placed according to 10-10 system which is the internationally recommended system for infants. The EEG was reported by a single trained neurologist, who was blinded to the clinical status of the baby. The different patterns in EEG were classified into normal continuous activity, isolated temporal spikes, transient discontinuous activity, and permanent discontinuous activity/suppression burst.[1,3] Computed tomography (CT) of the brain was done using Siemens Somatom sensation 64 slice machine. The CT scan was done between 72 h of life and the 7th day, depending on the clinical condition. The findings were documented as normal/cerebral edema and other changes such as intracranial or extra cranial bleeds, and areas of hypodensities. Magnetic resonance imaging (MRI) was done at 10–12 weeks of age by using Siemens Avantom 1.5 Tesla machine using multichannel head coils under sedation. The MR sequences that were employed were flair, T2, T1, diffusion, susceptibility weighted, and inversion recording imaging. The MRI findings were classified according to basal ganglia/watershed pattern as described in a study by Barkovich et al.[4] Normal was classified as score 0, abnormal signal in basal ganglia/thalamus as score 1, abnormal signal in cortex as score 2, abnormal signal in areas of cortex and basal nuclei as score 3, and abnormal signal in entire cortex and basal nuclei as score 4. The CT and MRI scans were reported by qualified radiologists who were blinded to the clinical details.
The babies were followed-up at 3, 6, and 12 months of age. Parents were told to report in between if there were any abnormal sudden neurological deterioration or seizures. During the follow-up period, seizure recurrences and developmental milestones were noted and neurological examination carried out. At 12 months of age, a complete neurological assessment was done by a pediatrician and developmental assessment was done by the authors. Developmental screening was done by two authors separately, using Denver Developmental Screening Test II (DDST II). While doing DDST II the items intersected by and just adjacent to the age line were tested. The items were denoted as P for pass, F for failed, No for no opportunity, and R for refused to cooperate or attempt. The interpretation of the individual items was made as follows:
Advanced: Child passes item that falls completely to the right of the age line
Normal: Child passes, fails, or refuses item on which the age line falls between the 25th and 75th percentile
Caution: Child fails or refuses item on which the age line falls between the 75th and 90th percentile
Delayed: Child fails or refuses item that falls completely to the left of the age line
No opportunity: Child has had no chance to perform the item (taken only for report items).
DDST II test interpretation was done as
Normal: Child with no delays and a maximum of 1 caution
Suspect: Two or more cautions and or one or more delays
Untestable: Refusal scores on 1 or more items completely to the left of age line or; more than one item intersected by the age line in the 75-90th percentile area. These children were rescreened again.
Infants with a normal neurological examination and normal DDST II were considered as normal neurodevelopmental outcome and those who had an abnormal neurological examination and or untestable DDST II were taken as poor neurodevelopmental outcome. Those with suspect DDST II had a reevaluation after 2 weeks.
Descriptive statistical analysis was carried out. Results on continuous measurements are presented on mean ± SD (min–max) and results on categorical measurements are presented in number (%). Significance is assessed at 5% level of significance. Chi-square/Fisher exact test was used to find the significance of study parameters on categorical scale between two or more groups. Diagnostic statistics viz. sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were computed to find the correlation of ECG, CT, and MRI brain with abnormal outcome.
RESULTS
There were 42 newborns with birth asphyxia admitted during the study period. Two of them had inborn errors of metabolism and were not included. Two were excluded due to major congenital anomalies. Seven were preterm and so not included. Among the remaining 31, there were 24 males and seven females. One was lost to follow-up. Four infants (13.3%) died. While 18 babies had birth weights between 2.5 and 3 kg, three were over 3.5 kg, and one weighed less than 2.5 kg. Seven were born normally, while 11 were instrumental, and 18 were born by lower segment caesarian section (LSCS). While nine babies had Sarnat stage 1, nine were in stage 3. EEG showed normal continuous activity in six (19.4%), isolated temporal spikes in four (12.9%), transient discontinuous activity in seven (22.6%), and permanent discontinuous activity in 14 (45.2%).
The CT brain was normal in 14 (45.2%), while in 12 (38.7%) there was cerebral edema, and in five (16.1%) there were bleeds or hypodensities. The MRI was normal in 16 (61.5%). There were abnormal signals in basal ganglia in two (7.7%) in the cerebral cortex and scattered signal abnormalities in both cortex and basal ganglia in six (23.1%). MRI could not be done in the four babies who died and the one who was lost to follow-up.
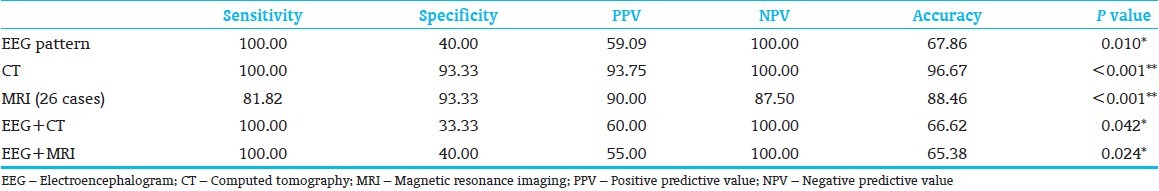
Neurological examination at 12 months was normal in 17, while there were neurological deficits in nine babies. DDST II was normal in 15, while in 11 it was suspect. The correlation of neurological outcome with EEG is shown in Table 1, with CT brain in Table 2 and with MRI in Table 3. The value of the three modalities in predicting an abnormal outcome is shown in Table 4. The predictive value of EEG in combination with CT and MRI in predicting abnormal outcome is shown in Table 4.
Table 1.
Correlation of electroencephalogram with neurodevelopmental outcome

Table 2.
Correlation of computed tomography scan with neurodevelopmental outcome

Table 3.
Correlation of magnetic resonance imaging scan with neurodevelopmental outcome

Table 4.
Value of EEG, CT and MRI alone and in combination in predicting the abnormal outcome
DISCUSSION
This study attempted to associate EEG and neuroimaging with neurological outcome in term babies with HIE. Out of the 31 cases, six (19.4%) had normal EEG pattern and all of them had a normal outcome (P value 0.017). This is similar to the finding by El-Ayouty et al., who reported that normal EEG background activity was associated with normal neurological outcome at 12 months of age [5] was associated with all the 14 babies who showed permanent discontinuous activity or suppression burst pattern had Sarnat stage 2 or 3 HIE. Thirteen out of these 14 cases with suppression burst pattern (one case lost to follow-up) had an abnormal outcome; which is either death, neurological deficits, or suspect cases as shown by the DDST II assessment (P<0.001). In predicting an abnormal outcome, EEG has a sensitivity of 100%, specificity of 40%, PPV of 59.09%, NPV of 100%, accuracy of 67.86, and P value of 0.010. Ong et al., has reported that in asphyxiated newborns, EEG has a PPV of 100%, NPV of 80.6%, and sensitivity of 53%, and specificity of 100%.[1] El-Ayouty et al., have reported that for the prediction of poor outcomes, abnormal EEG background activity had a sensitivity, specificity, PPV, and NPV of 100%.[6] Early EEG (within 7 days of life) done on 77 asphyxiated infants in a study done by Caravale et al., showed that out of the 52 who had normal EEG, 83% had a normal outcome at 1 year, 17% had mild abnormalities and none of them had any severe abnormalities.[7] Presslan et al., have documented the usefulness of serial EEG in the newborn period in predicting the neurological outcome.[8] A suppression burst pattern recording obtained on any day of life is associated with a very high likelihood of an unfavorable outcome.[3,9,10,11,12,13,14] A group of 15 term infants with burst suppression on EEG were followed up by Grigg et al., who reported that 14 of them had poor outcome.[11] The NPV of a normal EEG was emphasized by Murray et al., who concluded that a normal or mildly abnormal EEG within 6 h of life was associated with normal neurodevelopmental outcome at 24 months.[15] Our study has shown a 100% NPV for a normal EEG.
Imaging studies are usually done in all neonates with HIE. MRI is difficult to perform during the acute stage, since it takes almost an hour and needs deep sedation which is risky in asphyxiated babies. CT is easier to perform and helps in practical management by detecting intracranial hemorrhage, infarction, and cerebral edema.
Of the 31 CT scans, 14 (45.2%) were normal, 12 (38.7%) had cerebral edema of varying degree, and five (16.1%) had other findings (left frontoparietal and temporal lobe wedge-shaped hypodensity, white matter hypodensity, subarachanoid hemorrhage, subgaleal bleed, and thalamic hypodensity with loss of gray and white matter differentiation). All neonates with normal CT scan had normal outcome (P<0.001). Eleven cases with cerebral edema had abnormal outcome (P<0.001) and four cases with other findings in CT had an abnormal outcome (P value=0.1, not significant). Our study showed that CT brain has a sensitivity of 100%, specificity of 93.3%, PPV of 93.75%, NPV of 100%, and accuracy of 96.67 in predicting an abnormal outcome at 1 year. Our results are consistent with the observation made by Volpe that infants with normal CT scans rarely exhibit major neurological deficits on follow-up and infants with scans demonstrating marked diffuse hypodensity are rarely normal on follow-up.[16]
Of the 31 cases in the study, only 26 could undergo MRI brain (four deaths, one lost to follow-up). Among the 26 cases 16 (61.5%) had normal MRI, two (7.7%) showed abnormal signal in the basal ganglia/thalamus, and two (7.7%) showed abnormal signal in the cortex. Six (23.1%) cases showed abnormal signal in the cortex and the basal ganglia. Of the 16 infants with normal MRI, 14 had a normal outcome. Two infants had a normal neurological assessment, but an abnormal DDST score and were labeled as suspects. El-Ayouty et al., in their study of 25 newborns have reported that all infants with a normal MRI in the first 4 weeks were neurologically normal at 12 months of age.[5] All the six cases which had shown abnormal signals in the cortex and thalamus had an abnormal outcome (P=0.002). MRI brain has a sensitivity of 81.82%, specificity of 93.3%, PPV of 90%, NPV of 87.5%, and accuracy of 88.46 in predicting abnormal outcome at 1 year of age (P<0.001). If an assumption is made that the MRI results of the four infants who died were abnormal; the sensitivity, specificity, PPV, NPV, and accuracy are higher (86.67, 93.33, 92.86, 87.50, and 90%, respectively). El-Ayouty et al., have reported that abnormal MRI scans had a sensitivity of 100%, specificity of 43%, PPV of 82%, and NPV of 100% in predicting an abnormal outcome at 18 months.[6]
Many studies have found the basal ganglia watershed score to be an excellent predictor of the neurological outcome.[4,17,18,19] The result of our study is also similar, though the MRI in this study was done later than in the former. Studies based on the topographic pattern of neuronal injury have shown that term infants with predominant injury to basal ganglia and thalamus have an unfavorable neurological outcome.[20,21,22,23] Rutherford et al., compared MRI and cranial ultrasonographic findings with the outcome at 1 year of age and found a poor outcome if both of the investigations showed a basal ganglia or thalamic lesion.[21] Mercuri et al., have reported that discrete lesions in basal ganglia were associated with normal motor outcome at 1 year of age in 57% cases, but Barnett et al., followed up seven cases of asphyxia with such lesion till school age and reported that only one out of seven had a completely normal motor outcome.[22,24] Kaufman et al., have shown that more basal ganglia involvement in MRI correlates with more severe encephalopathy.[25] In our study, it was seen that all infants with lesions in the basal ganglia and/or thalamus had an abnormal outcome, which is consistent with the findings of other studies.
An attempt was made to see if EEG in addition to an imaging study improves the predictive ability. In our study, combining EEG with CT brain did not result in better prediction. However, an early EEG followed by an MRI at 3 months predicts neurological outcome with the highest statistical significance. Biagioni et al., have reported very good correlation between EEG and MRI findings in neonatal encephalopathy and affirmed their value in predicting the neurological outcome.[26]
Our study shows that in a term newborn with HIE, a normal EEG and/or CT scan of brain during the acute phase of illness is associated with good neurological outcome. Burst suppression pattern in the EEG and bleeds or hypodensities in the CT in the acute stage indicates a poor outcome. Involvement of the basal ganglia/thalamus in the MRI at 3 months of age also indicates a poor prognosis. EEG in the acute phase of HIE, combined with an MRI at around 3 months during follow-up is most useful in predicting the neurological outcome at 1 year. Similar studies using larger numbers of patients and follow-up for longer duration are needed to confirm the findings of our study.
SUMMARY
This study attempted to correlate EEG and CT brain during the acute phase and an MRI at 3 month follow-up of term newborn babies who had HIE with their neurodevelopmental outcome at 1 year. While a normal EEG and CT brain is associated with normal neurological outcome, Burst suppression pattern in EEG and bleeds/hypodensities in the CT scan were predictive of adverse outcome. While a normal MRI at 3 months has a specificity of over 90% in predicting a normal outcome, involvement of the thalamus or basal ganglia indicates a poor outcome. Clinicians can judiciously use this data to reassure some of the anxious parents of babies with HIE who are worried about neurodevelopmental outcome.
Footnotes
Source of Support: Research funds of the PSG Institute of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Ong LC, Kanaheswari Y, Chandran V, Rohana J, Yong SC, Boo NY. The usefulness of early ultrasonography, electroencephalography, and clinical parameters in predicting adverse outcome in asphyxiated term infants. Singapore Med J. 2009;50:705–9. [PubMed] [Google Scholar]
- 2.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 3.Selton D, André M. Prognosis of hypoxic-ischaemic encephalopathy in full-term newborns-value of neonatal electroencephalography. Neuropediatrics. 1997;28:276–80. doi: 10.1055/s-2007-973714. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: Evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 5.El Ayouty M, Abdel-Hady H, El-Mogy S, Zaghlol-H, El-Beltagy M. Prognosis of term infants with Hypoxic ischemic encephalopathy: A clinical, EEG and MRI study. Int J Child Neuropsychiatr. 2005;2:56–73. [Google Scholar]
- 6.El-Ayouty M, Abdel-Hady H, El-Mogy S, Zaghlol H, El-Beltagy M, Aly H. Relationship between electroencephaolography and magnetic resonance imaging findings after hypoxic-ischemic encephalopathy at term. Am J Perinataol. 2007;24:467–73. doi: 10.1055/s-2007-986686. [DOI] [PubMed] [Google Scholar]
- 7.Caravale B, Allemand F, Libenson MH. Factors predictive of seizures and neurologic outcome in perinatal depression. Pediatr Neurol. 2003;29:18–25. doi: 10.1016/s0887-8994(03)00046-8. [DOI] [PubMed] [Google Scholar]
- 8.Presslan RM, Boylan GB, Morton M, Binnie CD, Rennie JM. Early serial EEG in hypoxic ischemic encephalopathy. Clin Neurophysiol. 2001;112:31–7. doi: 10.1016/s1388-2457(00)00517-4. [DOI] [PubMed] [Google Scholar]
- 9.Berger R, Garnier Y. Perinatal brain injury. J Perinat Med. 2000;28:261–85. doi: 10.1515/JPM.2000.034. [DOI] [PubMed] [Google Scholar]
- 10.Biagioni E, Ferrari F, Boldrini A, Roversi MF, Cioni G. Electroclinical correlation in neonatal seizures. Eur J Paediatr Neurol. 1998;2:117–25. doi: 10.1016/s1090-3798(98)80027-5. [DOI] [PubMed] [Google Scholar]
- 11.Grigg-Damberger MM, Coker SB, Halsey CL, Anderson CL. Neonatal burst suppression: Its developmental significance. Pediatr Neurol. 1989;5:84–92. doi: 10.1016/0887-8994(89)90032-5. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T, Watanabe K. The EEG evolution and neurological prognosis of neonates with perinatal hypoxia [corrected] Brain Dev. 1989;11:115–20. doi: 10.1016/s0387-7604(89)80079-8. [DOI] [PubMed] [Google Scholar]
- 13.van Lieshout HB, Jacobs JW, Rotteveel JJ, Geven W, v′t Hof M. The prognostic value of the EEG in asphyxiated newborns. Acta Neurol Scand. 1995;91:203–7. doi: 10.1111/j.1600-0404.1995.tb00435.x. [DOI] [PubMed] [Google Scholar]
- 14.Biagioni E, Bartalena L, Boldrini A, Pieri R, Cioni G. Constantly discontinuous EEG patterns in full-term neonates with hypoxic-ischaemic encephalopathy. Clin Neurophysiol. 1999;110:1510–5. doi: 10.1016/s1388-2457(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 15.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–67. doi: 10.1542/peds.2008-2190. [DOI] [PubMed] [Google Scholar]
- 16.Volpe JJ. Neurology of the Newborn. Philadelphia: WB Saunders; 2008. Hypoxic-ischemic encephalopathy; pp. 400–80. [Google Scholar]
- 17.Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–30. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. AJNR Am J Neuroradiol. 1992;13:959–72. [PMC free article] [PubMed] [Google Scholar]
- 19.Westmark KD, Barkovich AJ, Sola A, Ferriero D, Partridge JC. Patterns and implications of MR contrast enhancement in perinatal asphyxia: A preliminary report. AJNR Am J Neuroradiol. 1995;16:685–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:543–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford MA, Pennock JM, Dubowitz LM. Cranial ultrasound and magnetic resonance imaging in hypoxic ischaemic encephalopathy: A comparison with outcome. Dev Med Child Neurol. 1994;36:813–25. doi: 10.1111/j.1469-8749.1994.tb08191.x. [DOI] [PubMed] [Google Scholar]
- 22.Mercuri E, Ricci D, Cowan FM, Lessing D, Frisone MF, Haataja L, et al. Head growth in infants with hypoxic-ischemic encephalopathy: Correlation with neonatal magnetic resonance imaging. Pediatrics. 2000;106(2 Pt 1):235–43. doi: 10.1542/peds.106.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998;102:323–8. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- 24.Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, et al. Neurological and perceptual-motor outcome at 5-6 years of age in children with neonatal encephalopathy: Relationship with neonatal brain MRI. Neuropediatrics. 2002;33:242–8. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman AS, Miller PS, Ferriero DM, Glidden DH, Barkovich AJ, Partridge JC. Encephalopathy as a predictor of magnetic resonance imaging abnormalities in asphyxiated newborns. Pediatr Neurol. 2003;28:342–6. doi: 10.1016/s0887-8994(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 26.Biagioni E, Mercuri E, Rutherford M, Cowan F, Azzopardi D, Frisone MF, et al. Combined use of electroencephalogram and magnetic resonance imaging in full-term neonates with acute encephalopathy. Pediatrics. 2001;107:461–8. doi: 10.1542/peds.107.3.461. [DOI] [PubMed] [Google Scholar]


