Abstract
Opioid use disorders present with distressing withdrawal symptoms at the time of detoxification. The pharmacological agents and methods currently in use for detoxification mainly include buprenorphine, methadone, and clonidine. Many other pharmacological agents have been tried for opioid detoxification. This review takes a look at the newer pharmacological options, both opioid agonists and non-agonist medications that have been utilized for detoxification. Peer reviewed articles were identified using PubMed and PsychInfo databases. The keywords included for the search were a combination of ‘opioid’ and ‘detoxification’ and their synonyms. All the articles published in the last 10 years were screened for. Relevant data was extracted from identified studies. Many newer pharmacological agents have been tried in detoxification of opioids. However, the quest for a safe, efficacious, cost-effective pharmacological option which requires minimal monitoring still continues. The role of non-pharmacological measures and alternative medicine needs further evaluation.
Keywords: Alternatives, detoxification, modifications, newer techniques, opioid
Opioids have been in use by mankind for centuries. These act primarily on the opioid receptors in the body and carry a very high potential for dependence.[1,2] Their abuse has been world-wide; India is home to an estimated 2 million opioid dependent subjects.[3] Opioid abuse leads to health costs for the individual and socio-economic costs for the society;[4] while cessation of illicit opioid use is associated with improvement in physical health and social functioning.[5,6,7] Thus, opioid abuse is a big challenge for the entire humanity, and there is a social desire that abusers must come off this abuse. However, stopping of opioid use is associated with discomforting withdrawal symptoms: Muscle aches and pains, loose stools, piloerection, irritability, sleep disturbances, sympathetic activation, rhinorrhoea and lacrimation. Detoxification involves the process of withdrawing an individual from a specific psychoactive substance in a safe and effective manner, thereby minimizing the withdrawal symptom.[8]
Various methods used for opioids detoxification are: Abrupt cessation without using withdrawal syndrome ameliorating medication (“cold Turkey”), and cessation using either withdrawal syndrome ameliorating medications, and/or techniques from alternative medicines. The medications used, and gradually tapered off, are either opioid agonists or other non-agonist medications for symptomatic management. The opioid agonists used commonly for opioid detoxification include methadone, buprenorphine. The non-opioid medications used include alpha-2 adrenergic agents.[9] Additional medications often required and used concurrently, especially with non-opioid medications, include hypnotics and analgesics.
The detoxification can be carried out either on inpatient or outpatient basis. The detoxification can be ultra-rapid (under general anesthesia or heavy sedation), rapid (over 3-6 days), short-term (1-3 weeks) or long-term (over months). The present methods of detoxification pose some challenges and concerns. The side-effects of the medications, persistence of withdrawal symptoms, potential of diversion, and abuse, and need for monitoring are some of the problems encountered. These contribute to high rates of dropout and relapse. Hence, the search for an ideal pharmacological agent for detoxification continues.
An ideal agent for opioid detoxification should relieve withdrawal symptoms effectively, require minimal monitoring, and have negligible side effects and no abuse potential. In the last decade or so many medications and non-medication approaches have been tried for opioids detoxification. This review looks at these approaches.
SEARCH STRATEGY
The relevant peer reviewed articles were identified using PubMed and PsychInfo databases. The search was conducted in October 2011. The keywords included for the search were a combination of ‘opioid’ and ‘detoxification’ and their synonyms. All the articles published in the last 10 years were screened. A total of 659 abstracts were identified. The abstracts were further looked into for description of a newer or an alternate technique of detoxification. Those using buprenorphine, methadone or clonidine were excluded. For sake of summarizing, only a review article relating to lofexidine was included. The data regarding the methodology and results were further extracted from the identified studies, which are mentioned in this review.
Newer opioid agonist regimen of opioid detoxification
In the last decade or so various opioid agonists have been introduced for opioid detoxification in the newer formulations [Table 1]. These opioid agonist act on the opioid receptors (primarily μ receptors, but also κ and δ receptors), and act as replacement for illicit opioids. They prevent significant withdrawals are tapered off over a course of days to weeks. The newer formulations use proven and effective detoxification agents, and intend to minimize the potential for diversion.
Table 1.
Alternative opioid agonist regimen/formulation for detoxification
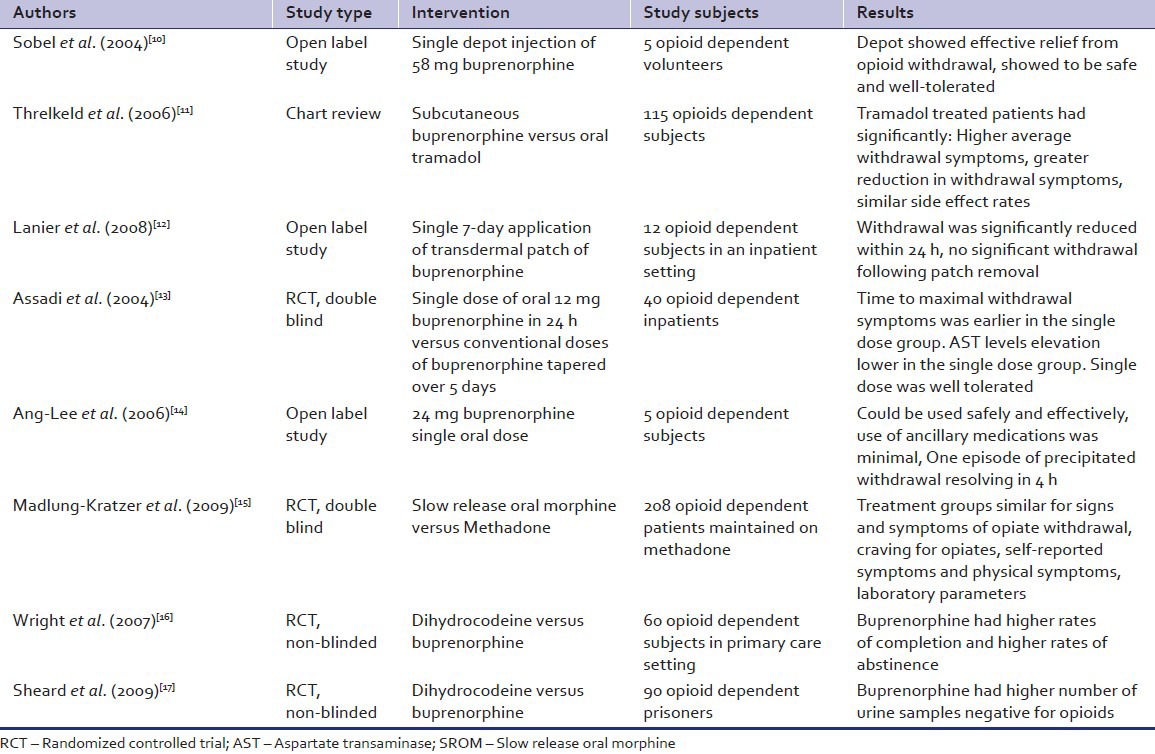
Buprenorphine, a partial μ-agonist and ê-antagonist has been widely used for detoxification. The newer formulations include a depot formulation,[10,11] as a transdermal patches[12] and in a high single oral dose.[13,14] The depot containing 58 mg of buprenorphine in microcapsules was injected subcutaneously. The transdermal patches come in varying strengths and release buprenorphine at a fixed rate. The patch used for detoxification released buprenorphine at 20 μg/h over a period of 7 days.[12] These formulations have been tried mainly in open label studies with small sample sizes. The results are encouraging and demonstrate efficacy of buprenorphine formulations in managing withdrawal symptoms.
Slow release oral morphine delivers a known quantity and purity of morphine, a natural opioid derived from opium poppy, in a controlled manner. It has been shown to be useful in opioid detoxification. A comparatively large double blind randomized controlled trial (RCT) has shown it to be efficacious.[15] It fared equivalent to methadone in attenuating withdrawal symptoms and reducing craving.
Dihydrocodeine, a semi-synthetic opioid analgesic used mainly as an anti-tussive, has also been utilized for opioid detoxification. It was found to be inferior to buprenorphine in reducing the withdrawal symptoms in two studies.[16,17]
Tramadol, is a synthetic opioid, which acts as a weak μ-opioid agonist, releases serotonin, and norepinephrine reuptake inhibitor. It has also been found to be less effective as compared to sub-cutaneous buprenorphine in a chart review study.[11]
Overall, in opioid detoxification the opioid agonists provide the advantages of effective control of withdrawal symptoms and reduction of craving. However, the potential of diversion and misuse remains with these agents.
Newer non-agonist regimen of opioid detoxification
Many non-opioids have been tried for opioid detoxification [Table 2]. Naltrexone, an opioid antagonist was used in a very low-dose as adjunct to methadone tapering in a community based blinded RCT. Its use was associated with attenuated withdrawal symptoms and craving.[18] Buspirone, is a serotonin 5-HT1A receptor partial agonist acting through the serotonin, dopamine, and noradrenergic systems. It was compared to methadone in a placebo controlled comparator RCT.[19] At doses of 30 mg and 45 mg/day, it was as effective as methadone in controlling the subjective and objective opiate withdrawal symptoms.
Table 2.
Alternative non-agonist regimen/formulation for detoxification
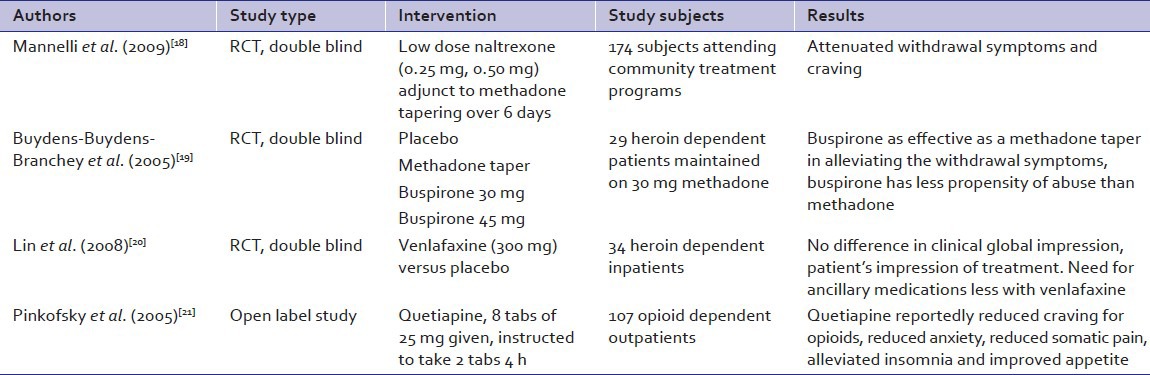
Venlafaxine, an antidepressant serotonin-norepinephrine reuptake inhibitor class, was used at doses of 300 mg/day in opioid dependent subjects in a placebo controlled RCT by Lin et al.[20] Compared to placebo the withdrawal symptom profile was similar, yet lesser amounts of ancillary medications were required with venlafaxine.
Quetiapine, an atypical antipsychotic acting through the dopaminergic and serotoninergic pathways, was tried for opioid detoxification in an open label study.[21] With 4 hourly use of 2 tablets 25 mg each there was reduced anxiety, pain and craving for the opioids.
Lofexidine, a newer α2 adrenergic drug, has been used in titrating the dose to a maximum of 1.6-3.2 mg/day in divided doses, given for 5-18 day-time.[22] It was shown to have lesser propensity to cause hypotension as compared to clonidine. However, withdrawal symptoms of insomnia and aching were not alleviated effectively.
Thus, the non-opioid methods offer advantage of lesser chances of misuse, as also better control of features of withdrawals like irritability. However, the overall reduction of withdrawal symptoms was not complete when compared with opioids.
Other measures
Apart from the pharmacological methods enumerated above, other non-pharmacological or alternative medical treatments have also been evaluated and shown to be beneficial in patients undergoing opioid detoxification. These methods that could possibly be used along with the regular pharmacological measures, have included Chinese herbal preparations,[23] qi-gong[24] and transcutaneous electric acupoint stimulation (TEAS).[25]
A meta-analysis of Chinese herbal preparations tried for opioid detoxification has been conducted.[23] Chinese herbal medicine was found to be superior to α2-adrenergic agonists in relieving withdrawal symptoms during 4th to 10th day, while both worked in an equivalent manner in the first 3 days. Compared with opioid agonists, Chinese herbal medicine was inferior during the first 3 days, but the difference was non-significant during 4th to 9th days. The herbal medicines were also reported to have lesser side-effects.
Qi-gong is a system of exercise that requires maintenance of certain postures for periods of time. Pan – gu qi-gong, a variety of qi-gong was tried in a RCT with medication and no-medication controls.[24] Reduction of withdrawal symptoms in the qigong group occurred more rapidly than in the other groups. As compared to medication group, the qi-gong group had significantly lesser anxiety, and less positive urine samples.
Meade et al.[25] tested the effectiveness of TEAS as an adjunctive treatment for in-patients receiving opioid detoxification with buprenorphine-naloxone. TEAS given in three 30-min treatments daily for 3-4 days was associated with better outcomes, greater improvements in pain and physical health.
It must be acknowledged that in patients being detoxified, a supportive environment, avoidance of cues that elicit craving and good nursing care would greatly reduce the distress and dropouts. The rapidity of taper should be individualized and gradual, as faster tapering is associated with use of substances.[26] Adjunct psychotherapy has been found to be linked to higher retention rates and lesser opioid use in patients undergoing detoxification.[27] The presence of other medical, psychiatric, and substance use comorbidities need to be addressed.[28]
CONCLUSION
The last decade has seen many new medications and techniques being used for opioid detoxification. None of these however, has been an ideal one. Hence the search for an agent or method which is safe and effective for opioid detoxification, has minimal abuse potential, requires minimal monitoring and is cost-effective still continues.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bailey CP, Connor M. Opioids: Cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–8. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Christie MJ. Cellular neuroadaptations to chronic opioids: Tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–96. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray R, Mondal A, Gupta K, Chatterjee A, Bajaj P. UNODC-ROSA-Publications-The Extent, Patterns and Trends of Drug Abuse in India-National Survey. Ministry of Social Justice and Empowerment and United Nations Office on Drugs and Crime. 2004. [Last accessed on 2011 Nov 20]. Available from: http://www.unodc.org/india/national_Survey.html .
- 4.McAdam-Marx C, Roland CL, Cleveland J, Oderda GM. Costs of opioid abuse and misuse determined from a Medicaid database. J Pain Palliat Care Pharmacother. 2010;24:5–18. doi: 10.3109/15360280903544877. [DOI] [PubMed] [Google Scholar]
- 5.Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–6. doi: 10.1016/S0140-6736(98)05356-2. [DOI] [PubMed] [Google Scholar]
- 6.Gowing LR, Ali RL. The place of detoxification in treatment of opioid dependence. Curr Opin Psychiatry. 2006;19:266–70. doi: 10.1097/01.yco.0000218596.54054.a1. [DOI] [PubMed] [Google Scholar]
- 7.Veilleux JC, Colvin PJ, Anderson J, York C, Heinz AJ. A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clin Psychol Rev. 2010;30:155–66. doi: 10.1016/j.cpr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Mee-Lee D, Shulman GD, Fishman M. ASAM patient placement criteria for the treatment of substance-related disorders. American Society of Addiction Medicine. 2001 [Google Scholar]
- 9.Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction – A clinical perspective. Eur J Clin Pharmacol. 2010;66:537–45. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- 10.Sobel BF, Sigmon SC, Walsh SL, Johnson RE, Liebson IA, Nuwayser ES, et al. Open-label trial of an injection depot formulation of buprenorphine in opioid detoxification. Drug Alcohol Depend. 2004;73:11–22. doi: 10.1016/j.drugalcdep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Threlkeld M, Parran TV, Adelman CA, Grey SF, Yu J. Tramadol versus buprenorphine for the management of acute heroin withdrawal: A retrospective matched cohort controlled study. Am J Addict. 2006;15:186–91. doi: 10.1080/10550490500528712. [DOI] [PubMed] [Google Scholar]
- 12.Lanier RK, Umbricht A, Harrison JA, Nuwayser ES, Bigelow GE. Opioid detoxification via single 7-day application of a buprenorphine transdermal patch: An open-label evaluation. Psychopharmacology (Berl) 2008;198:149–58. doi: 10.1007/s00213-008-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assadi SM, Hafezi M, Mokri A, Razzaghi EM, Ghaeli P. Opioid detoxification using high doses of buprenorphine in 24 hours: A randomized, double blind, controlled clinical trial. J Subst Abuse Treat. 2004;27:75–82. doi: 10.1016/j.jsat.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Ang-Lee K, Oreskovich MR, Saxon AJ, Jaffe C, Meredith C, Ellis ML, et al. Single dose of 24 milligrams of buprenorphine for heroin detoxification: An open-label study of five inpatients. J Psychoactive Drugs. 2006;38:505–12. doi: 10.1080/02791072.2006.10400589. [DOI] [PubMed] [Google Scholar]
- 15.Madlung-Kratzer E, Spitzer B, Brosch R, Dunkel D, Haring C. A double-blind, randomized, parallel group study to compare the efficacy, safety and tolerability of slow-release oral morphine versus methadone in opioid-dependent in-patients willing to undergo detoxification. Addiction. 2009;104:1549–57. doi: 10.1111/j.1360-0443.2009.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright NM, Sheard L, Tompkins CN, Adams CE, Allgar VL, Oldham NS. Buprenorphine versus dihydrocodeine for opiate detoxification in primary care: A randomised controlled trial. BMC Fam Pract. 2007;8:3. doi: 10.1186/1471-2296-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheard L, Wright NM, El-Sayeh HG, Adams CE, Li R, Tompkins CN. The Leeds Evaluation of Efficacy of Detoxification Study (LEEDS) prisons project: A randomised controlled trial comparing dihydrocodeine and buprenorphine for opiate detoxification. Subst Abuse Treat Prev Policy. 2009;4:1. doi: 10.1186/1747-597X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: A randomized, controlled trial. Addict Biol. 2009;14:204–13. doi: 10.1111/j.1369-1600.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buydens-Branchey L, Branchey M, Reel-Brander C. Efficacy of buspirone in the treatment of opioid withdrawal. J Clin Psychopharmacol. 2005;25:230–6. doi: 10.1097/01.jcp.0000162804.38829.97. [DOI] [PubMed] [Google Scholar]
- 20.Lin SK, Chen CH, Pan CH. Venlafaxine for acute heroin detoxification: A double-blind, randomized, control trial. J Clin Psychopharmacol. 2008;28:189–94. doi: 10.1097/JCP.0b013e31816727e2. [DOI] [PubMed] [Google Scholar]
- 21.Pinkofsky HB, Hahn AM, Campbell FA, Rueda J, Daley DC, Douaihy AB. Reduction of opioid-withdrawal symptoms with quetiapine. J Clin Psychiatry. 2005;66:1285–8. doi: 10.4088/jcp.v66n1011. [DOI] [PubMed] [Google Scholar]
- 22.Gish EC, Miller JL, Honey BL, Johnson PN. Lofexidine, an (alpha) 2-receptor agonist for opioid detoxification. Ann Pharmacother. 2010;44:343–51. doi: 10.1345/aph.1M347. [DOI] [PubMed] [Google Scholar]
- 23.Liu TT, Shi J, Epstein DH, Bao YP, Lu L. A meta-analysis of Chinese herbal medicine in treatment of managed withdrawal from heroin. Cell Mol Neurobiol. 2009;29:17–25. doi: 10.1007/s10571-008-9290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Chen K, Mo Z. Use of qigong therapy in the detoxification of heroin addicts. (56-9).Altern Ther Health Med. 2002;8:50–4. [PubMed] [Google Scholar]
- 25.Meade CS, Lukas SE, McDonald LJ, Fitzmaurice GM, Eldridge JA, Merrill N, et al. A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. J Subst Abuse Treat. 2010;38:12–21. doi: 10.1016/j.jsat.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: A review. Drug Alcohol Depend. 2011;119:1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005031. CD005031. [DOI] [PubMed] [Google Scholar]
- 28.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]


