Abstract
Background and Objective
Dupuytren’s contractures affecting proximal interphalangeal (PIP) joints are challenging to treat. We explored the effects of collagenase Clostridium histolyticum (CCH) on PIP joint contractures after injection of an affected metacarpophalangeal (MP) joint in the same finger and after injection of an isolated PIP joint contracture.
Methods
Two patient subsets were evaluated: those with MP/PIP joints contractures in the same finger, but only the MP joint contractures were treated (Group A); and those with isolated PIP joint contractures that were treated (Group B). Endpoints included correction and improvement in contracture. Fixed-flexion contracture (FFC) and range of motion (ROM) were also assessed; adverse events (AEs) were monitored.
Results
In Group A, 28 and 43 % of PIP contractures spontaneously corrected after the first and last injection of CCH, respectively, for MP contractures; 40 and 63 %, respectively, improved. In Group B, 31 and 39 % of PIP joint contractures corrected after the first and last injection of CCH, respectively, 56 and 66 %, respectively, improved. In Groups A and B, FFC improvements were largest after the last injection; ROM improvements were largest after the last injection in Group A and third injection in Group B. For 46 and 44 % of patients in Groups A and B, respectively, the first injection was the last injection. In Group B, the median (minimum, maximum) injections/joint was 1.0 (1.0, 4.0). Nearly all patients (98 %) experienced ≥1 AE; most were injection-site reactions.
Conclusions
The efficacy of CCH for improving PIP joint contracture was similar whether treated in isolation or after treatment of an MP joint contracture.
Introduction
In Dupuytren’s disease, the metacarpophalangeal (MP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joints can develop fixed-flexion deformities due to the development and subsequent contraction of diseased cords in the affected tissue. Although PIP joints are not affected as often as are MP joints [1], they can be more disabling for patients, as many routine, daily activities are impaired when these joints are contracted [1, 2]. Frequently, PIP joint contracture is accompanied by MP joint contracture [3]. Research has shown that improvements in PIP joint contractures correlate positively with improved hand function [2, 4]. The PIP joint contractures are also more challenging to treat using corrective surgery [5, 6] or minimally invasive procedures such as percutaneous needle fasciotomy (PNF) [2] and collagenase Clostridium histolyticum (CCH) injections [7]. The CCH injection is the first non-surgical, pharmacologic treatment for Dupuytren’s contracture (DC) with a palpable cord approved for use in the USA and Europe. Clinical trials [7–9] and post-marketing studies [10, 11] have demonstrated the efficacy and safety of CCH for correcting DC.
In this secondary analysis of data from four large clinical trials, we explored the efficacy of CCH on (1) PIP joint contractures when only the adjacent MP joint was treated; and (2) isolated PIP joint contractures treated with CCH.
Methods
Overview of Collagenase Clostridium histolyticum Studies
CORD (Collagenase Option for Reduction of Dupuytren’s) I [8] and II [7] were 90-day, phase III trials conducted at 16 sites in the USA and five sites in Australia, respectively. CORD I also had a 9-month, open-label extension. JOINT I and II were 9-month, open-label studies conducted at 14 sites in the USA and 20 sites in Europe and Australia, respectively [9]. For all four studies, eligible patients (aged ≥18 years) were required to have a fixed-flexion deformity in ≥1 finger (other than the thumb) that was ≥20° and ≤100° in an MP joint or ≥20° and ≤80° in a PIP joint caused by a palpable cord that had not been previously treated with CCH.
Before treatment in all four studies, investigators selected the hand to be treated and prioritized all palpable cords as primary, secondary, and tertiary. The primary cord could affect an MP or PIP joint if the contracture occurred solely in these respective joints. If there were contractures in both the MP and PIP joints of the same finger, the cord causing the MP contracture was deemed the primary cord. After the primary joint was successfully treated, either an MP or PIP joint contracture could be selected. Subsequent joints were selected on the basis of providing the patient with full functionality of the treated hand. Patients could receive a maximum of three injections during the study period. The primary endpoint was clinical success, defined as a reduction in contracture of the primary joint to ≤5° of full extension 30 days after injection. Secondary endpoints included clinical improvement, defined as a ≥50 % reduction in contracture of a treated joint, and a spontaneous effect of treatment, defined as a ≥20° reduction in contracture of any other joint not directly treated with CCH. Changes in fixed-flexion contractures (FFC) and range of motion (ROM) were also assessed.
Inclusion Criteria
For this secondary analysis, patients were included if they had ≥1 PIP joint contracture at study entry and received ≥1 CCH injection during the study. To evaluate the indirect and direct effects of CCH on PIP joint contractures, data for two patient subgroups were analyzed. In Group A, patients had MP and PIP joint contractures in the same finger, but only the cord affecting the MP joint was treated with CCH. In Group B, patients had an isolated PIP joint contracture (or PIP joint contracture combined with an MP joint contracture <20°), and only the cord affecting the PIP joint was treated with CCH. For brevity throughout the report, when we refer to a CCH-treated joint, the cord contracting the joint received the CCH injection(s).
Assessment of Efficacy and Tolerability
In keeping with the definitions used in the phase III studies, in Group A, the indirect effects of CCH on PIP joints after the injection of MP joints were evaluated for correction of contracture, defined as a reduction in FFC to ≤5° 30 days after injection, and as a spontaneous treatment effect (i.e., improvement), defined as a ≥20° reduction in FFC 30 days after injection. In Group B, the direct effects of CCH on PIP joints were evaluated for correction of contracture, as defined previously. Improvement in contracture was defined as a ≥50 % reduction in FFC from baseline 30 days after injection. The results for joints that corrected were included in the results for joints that showed improvement. In both groups, the percentage change in FFC and mean change in ROM were also assessed. In all four trials, the adverse events (AEs) were monitored and recorded for the duration of the studies.
Statistical Analysis
Although the CORD and JOINT studies differed by design (i.e., double-blind vs. open-label), all four protocols used the same inclusion/exclusion criteria to enroll patients, and the treatment paradigms were virtually identical. Moreover, the respective patient populations were relatively homogeneous for baseline demographic (e.g., age, sex) and clinical characteristics (e.g., contracture severity, digits/joints affected). Thus, it was considered appropriate to pool all of the relevant data for this analysis. Inferential statistics were not performed on the data; only descriptive attributes are reported, including means and standard deviation (SD) or medians and ranges (minimum, maximum) when appropriate. The data are reported at the joint level unless otherwise specified (i.e., patient level, finger level).
Results
In total, 616 patients were included in the analysis. Baseline demographic and clinical characteristics are summarized in Table 1. Mean ± SD age was 63 ± 10 years; nearly 70 % of patients were aged 44–74 years. Eighty-three percent of patients were male, and 100 % were white. More than 75 % of patients had ≤2 PIP joints affected; 61 % of patients had ≤3 joints affected. Isolated PIP joints in the fifth finger accounted for the largest percentage (61 %) of affected joints treated with CCH, followed by combined MP/PIP joint contractures on the fifth finger (25 %). The distribution of affected MP and PIP joints by finger is shown in Fig. 1. Nearly half (46 %) of patients with MP and PIP joint contractures on the same finger received CCH injections for the MP joint only; 18 % received CCH injections for the PIP joint contracture, and 36 % received both MP and PIP joint injections. For PIP joint contractures that received direct CCH injections (Group B), the median (minimum, maximum) number of injections per joint overall was 1.0 (1.0, 4.0) [mean ± SD, 1.6 ± 0.8]. The median number of injections per joint for total correction was 1.0 (1.0, 3.0) [mean ± SD, 1.3 ± 0.6].
Table 1.
Baseline demographic and clinical characteristics
| Variable | Value |
|---|---|
| Patients (N = 616) | |
| Age (years) [mean ± SD] | 63 ± 10 |
| Male sex [n (%)] | 508 (83) |
| Joints affected [mean ± SD] | 3.3 ± 2.2 |
| PIP joints affected [mean ± SD] | 1.9 ± 1.2 |
| Same finger MP/PIP contractures/patient [n (%)] | |
| 1 | 268 (44) |
| 2 | 60 (10) |
| 3 | 8 (1) |
| 4 | 1 (0.2) |
| Fingers with MP/PIP contractures (N = 416) [n (%)] | |
| Only MP treated | 191 (46) |
| Both MP and PIP treated | 150 (36) |
| Only PIP treated | 75 (18) |
| Joints treated with CCH (N = 577) [n (%)] | |
| MP | 201 (35) |
| PIP | 376 (65) |
CCH collagenase Clostridium histolyticum, MP metacarpophalangeal, PIP proximal interphalangeal, SD standard deviation
Fig. 1.
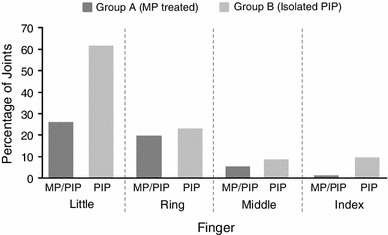
Distribution of affected metacarpophalangeal and proximal interphalangeal joints by finger. MP metacarpophalangeal, PIP proximal interphalangeal
In Group A, 28 % of PIP joint contractures spontaneously corrected after the first CCH injection for the MP joint deformity; 43 % of PIP joint contractures were corrected after the last MP joint injection. In Group B, 31 % of PIP joint contractures were corrected after the first CCH injection; 39 % were corrected after the last injection (Fig. 2a). In Group A, 40 % of PIP joints showed improvement in contracture after the first MP joint injection; 63 % showed improvement after the last MP joint injection, which for 46 % (93/201) of cases was also the first injection. In Group B, 56 % of PIP joints showed improvements in contracture after the first direct CCH injection; 66 % showed improvement after the last injection, which for 44 % (209/376) of cases was also the first injection (Fig. 2b).
Fig. 2.
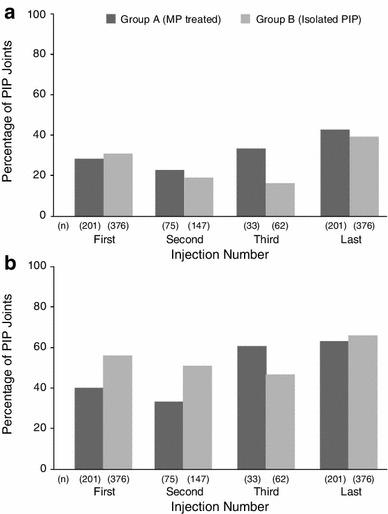
Results for fixed-flexion contracture correction (a) and improvement (b) after collagenase Clostridium histolyticum by group. Correction = reduction in contracture to ≤5° 30 days after injection for both groups; improvement = ≥20° reduction in contracture (Group A) or ≥50 % reduction in contracture (Group B) 30 days after injection. FFC fixed-flexion contracture, MP metacarpophalangeal, PIP proximal interphalangeal
For Group A, the mean change in FFC was 66 % after the first CCH injection and 77 % after the last injection. For Group B, the mean change in FFC was 55 % after the first CCH injection and 62 % after the last injection (Fig. 3a). In both groups, baseline FFC values were higher (i.e., more severe contractures) among joints that received a second or third injection (Table 2). Day 30 values were also higher in both groups. As indicated by the relative changes, FFC values were lowest after the last injection in both groups. Data from Table 2 are plotted graphically in Fig. 4a to show the linear relationship between baseline PIP joint contracture severity and FFC measures 30 days after each injection in both groups. More severe contractures at baseline received two or three CCH injections, and although the change in FFC from baseline was dramatic, day 30 FFC measures remained higher than did those for PIP joints that received only one CCH injection.
Fig. 3.
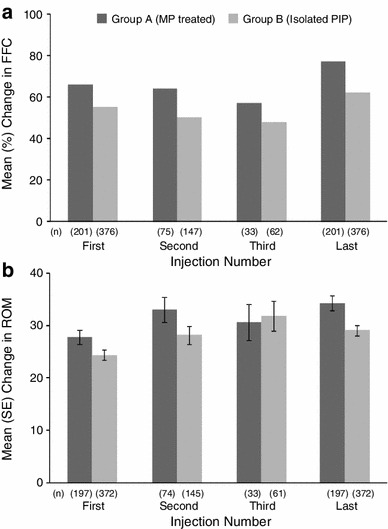
Changes in fixed-flexion contracture (a) and range of motion (b) of proximal interphalangeal joint contractures by group and collagenase Clostridium histolyticum injection. FFC fixed-flexion contracture, MP metacarpophalangeal, PIP proximal interphalangeal, ROM range of motion
Table 2.
Goniometry results for fixed-flexion contracture and range of motion at baseline and day 30 by collagenase Clostridium histolyticum injection number and group
| Injection | Group Aa | Group Bb | ||||
|---|---|---|---|---|---|---|
| Joints (n) | Baseline | Day 30 | Joints (n) | Baseline | Day 30 | |
| FFC | ||||||
| First | 201 | 48.2 ± 20.2 | 19.2 ± 22.4 | 376 | 49.7 ± 18.5 | 24.4 ± 21.4 |
| Second | 75 | 55.6 ± 19.4 | 21.6 ± 20.9 | 147 | 57.5 ± 16.9 | 28.5 ± 20.0 |
| Third | 33 | 59.7 ± 18.9 | 27.4 ± 24.4 | 62 | 60.5 ± 14.5 | 30.2 ± 19.6 |
| Last | 201 | 48.2 ± 20.2 | 13.3 ± 19.4 | 376 | 49.7 ± 18.5 | 20.1 ± 20.4 |
| ROM | ||||||
| First | 197c | 43.1 ± 18.9 | 71.0 ± 22.2 | 372d | 49.9 ± 19.6 | 74.0 ± 23.0 |
| Second | 74c | 37.2 ± 17.8 | 70.6 ± 20.8 | 145 | 41.3 ± 17.2 | 69.5 ± 22.3 |
| Third | 33 | 33.5 ± 16.8 | 64.1 ± 22.9 | 61d | 37.5 ± 16.1 | 69.3 ± 20.3 |
| Last | 197c | 43.1 ± 18.9 | 77.5 ± 19.4 | 372d | 49.9 ± 19.6 | 78.8 ± 22.0 |
Results are mean ± SD (°)
CCH collagenase Clostridium histolyticum, FFC fixed-flexion contracture, ROM range of motion
aGroup A: patients with MP and PIP joint contractures in the same finger, but only the cord affecting the MP joint was treated with CCH
bGroup B: patients had isolated PIP joint contracture (or PIP contracture combined with an MP joint contracture <20°), and only the cord affecting the PIP joint was treated with CCH
cDay 30, n = 199 (first), 75 (second), 199 (last)
dDay 30, n = 374 (first), 62 (third), 373 (last)
Fig. 4.
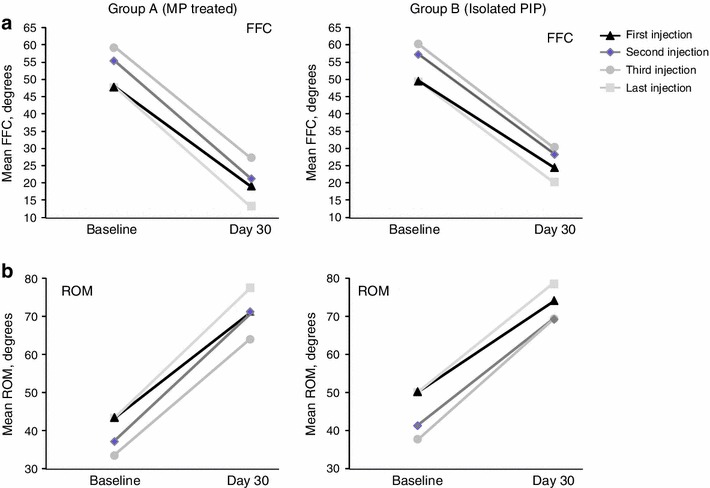
Fixed-flexion contracture (a) and range of motion (b) at baseline and day 30 by group. FFC fixed-flexion contracture, MP metacarpophalangeal, PIP proximal interphalangeal, ROM range of motion
For ROM, the mean ± SD change in Group A was 27.8 ± 19.7° after the first CCH injection and 34.3 ± 19.8° after the last injection; changes in Group B were 24.4 ± 18.1° after the first CCH injection and 29.1 ± 20.0° after the last injection (Fig. 3b). In both groups, baseline ROM values were lower among joints that received a second or third injection (Table 2). The ROM values were highest after the last injection in Group A and after the third injection in Group B. Figure 4b shows the linear relationship between ROM measures at baseline and 30 days after each injection in both groups. Again, despite notable increases in ROM for all PIP contractures, joints with more restricted ROM received two or three CCH injections and the day 30 values were lower than in PIP joint contractures that received only one CCH injection.
Safety and Tolerability
Nearly all patients (98 %) in the PIP joint contracture subgroup experienced ≥1 AE during the study in which they were enrolled. The majority of AEs were mild, transient, and localized to the injection site, including peripheral edema, pain, hemorrhage, tenderness, and swelling. The AEs occurring in ≥10 % of patients in the PIP joint contracture subgroup and compared with all patients from the four studies are summarized in Table 3. With one exception (injection-site pain), slightly larger percentages of patients in the PIP subgroups experienced AEs compared with all patients from the four clinical studies.
Table 3.
Adverse events occurring in ≥10 % of patients
| Adverse event | Groups Aa/Bb (N = 616) | All patients (N = 961) |
|---|---|---|
| Patients with ≥1 adverse event | 604 (98) | 934 (97) |
| General and injection-site conditions | ||
| Edema peripheral | 500 (81) | 736 (77) |
| Injection-site pain | 239 (39) | 381 (40) |
| Injection-site hemorrhage | 231 (38) | 359 (37) |
| Tenderness | 181 (29) | 250 (26) |
| Injection-site swelling | 170 (28) | 255 (27) |
| Contusion | 402 (65) | 574 (60) |
| Pain in extremity | 263 (43) | 383 (40) |
| Pruritus | 94 (15) | 122 (13) |
| Ecchymosis | 87 (14) | 125 (13) |
| Skin laceration | 79 (13) | 106 (11) |
| Blood blister | 70 (11) | 79 (8) |
| Lymphadenopathy | 67 (11) | 86 (9) |
Data are given as n (%)
CCH collagenase Clostridium histolyticum, MP metacarpophalangeal, PIP proximal interphalangeal
aGroup A: patients with MP and PIP joint contractures in the same finger, but only the cord affecting the MP joint was treated with CCH
bGroup B: patients had isolated PIP joint contracture (or PIP contracture combined with an MP joint contracture <20°), and only the cord affecting the PIP joint was treated with CCH
Discussion
In this secondary analysis of data from four large clinical trials of CCH for DC, we explored treatment effects on PIP joint contractures from two perspectives: (1) spontaneous correction and/or improvement after CCH injection for an MP joint contracture affecting the same finger; and (2) correction and/or improvement after direct injection into a cord contracting an isolated PIP joint. More than 600 patients from the CORD I [8] and II [7] trials and the JOINT I and II studies [9] were included; 201 MP/PIP combination contractures and 376 PIP contractures were evaluated. Isolated PIP joints in the fifth finger accounted for the largest percentage (61 %) of affected joints treated with CCH.
Overall, similar percentages of PIP joint contractures showed spontaneous correction after CCH injection for a contracted MP joint in the same finger or after direct CCH injection of an affected PIP joint (43 and 39 %, respectively). Although improvement in contracture was defined differently for Group A (i.e., ≥20° reduction in contracture 30 days after injection) and Group B (i.e., ≥50 % reduction in contracture 30 days after injection), comparable percentages of PIP joint contractures met this endpoint after CCH injection for a contracted MP joint in the same finger or after direct CCH injection of an affected PIP joint (63 and 66 %, respectively).
Interestingly, in both groups, there was a small decrease in the percentage of PIP joint contractures that were corrected or showed improvement after the second CCH injection. This could be due—at least in part—to some residual AEs at the injection site, including edema and stiffness. Overall, 46 % of MP/PIP joint contractures (Group A) and 44 % of isolated PIP contractures (Group B) received only one CCH injection.
In both Groups A and B, the relative (percentage) changes in FFC decreased slightly between the first and third CCH injections. Pre-injection FFC measures were quite similar between Groups A and B, and baseline values increased slightly before the second and then the third injections in both groups. Baseline FFC was lowest before the last injection in both groups. Overall, reductions in FFC were slightly larger among PIP joints in Group A versus Group B at each timepoint, as shown in Fig. 3 and by the slopes of the lines in Fig. 4a. Similarly, changes in ROM were slightly larger in Group A versus Group B. A potential explanation may be related to the mechanics of the entire digit. By releasing two joints, the overall benefit for movement could be larger than the sole improvement in the measure of FFC. Although the treated joint may remain stiff or swollen, the released joint could be moving freely. It is feasible that releasing a proximal cord may reduce some of the tension affecting more distal joints, allowing for improvement along the entire digit. In Group B, the largest change in ROM was observed after the third injection. Overall, regardless of the type or number of CCH injections received, all of the changes in ROM can be considered clinically meaningful. In all but one instance, the change in ROM was twofold larger than the previously demonstrated clinically important difference (CID) of 13.5° (95 % CI 11.9–15.1) [12]. The CID is calculated statistically, but it can help interpret the clinical relevance of changes in objective measures from the patient’s perspective [13, 14].
The vast majority of patients experienced ≥1 AE during the studies; most events were injection-site reactions, including edema, pain, hemorrhage, and swelling. Most AEs were mild and transient in nature. Although slightly larger percentages of patients in the PIP joint contracture subgroups experienced AEs compared with all patients, these differences are not likely to be clinically relevant. As reported for the CORD [7, 8] and JOINT [9] studies, the types, frequencies, and severity of AEs were comparable with those in other published research on the safety and tolerability of CCH.
To our knowledge, this is the first report of spontaneous correction and improvement in PIP joint contractures after treatment of an MP joint contracture in the same finger. Thus, it is not possible to discuss the findings as they relate to the extant literature. However, the body of evidence describing the overall results of surgical or non-surgical interventions for DC is large. In a systematic review of fasciotomy and fasciectomy among European patients, more MP than PIP joint contractures met the pre-specified outcome, with mean improvements of 80 and 49 % after fasciotomy and 94 and 66 % after fasciectomy, respectively [15]. Salhi et al. [16] reported similar results in a systematic review of PNF. In a more recently published study comparing outcomes of PNF versus limited fasciectomy, van Rijssen et al. [17] showed that 55 % of MP and 26 % of PIP joints corrected to ≤5° at 6 weeks after PNF; 94 % of MP and 47 % of PIP joints corrected after limited fasciectomy. In another recent study, Shin and Jones [18] showed that >90 % of MP and 82 % of PIP joints were fully corrected at least 2 weeks after segmental fasciectomies.
Thus, the relative efficacy of different treatment options for correcting MP versus PIP joint contractures is well-established: PIP joints are less responsive to intervention, become even more so over time, and are more susceptible to recurrence [1, 3]. Fundamental anatomical differences [3, 19] between the MP and PIP joints play a large part [20]; other factors not related to Dupuytren’s disease are also involved, including secondary contraction of the volar plate and/or collateral ligaments, arthritic changes and stiffness, and attenuation of the extensor mechanism. Any one or more of these processes may hold the PIP joint in a flexed position even after correction of the MP joint contracture or partial correction of the PIP joint contracture. The irony is that, despite the problematic nature of the contracted PIP joint and its relative resilience to corrective intervention, in some cases—as demonstrated here—the PIP joint contracture resolves spontaneously after treating an MP joint contracture in the same finger. A plausible explanation is that some of the CCH spreads across multiple cords. Alternatively, spontaneous correction of PIP joint contractures may be facilitated by the finger-extension procedure. Perhaps it is a combination of both and other unforeseen factors.
A careful clinical examination is essential for identifying the source and arrangement of the cord or cords causing the PIP joint deformity. These and other patient clinical characteristics, including the extent to which hand function is compromised, should be considered when deciding whether or not to treat the deformity. If affirmative, these factors are again considered in deciding on the approach for corrective intervention. In cases in which the affected MP and PIP joints are in the same finger, the likelihood of achieving full correction for both joint contractures is high after CCH if there is one central, pretendinous cord. If there is a separate cord affecting just the PIP joint, spontaneous correction after treating the MP joint contracture is less likely. Isolated PIP joint contractures are most prevalent in the fifth finger [21, 22]. Although this is the smallest of the fingers, it contains one of the largest digital branches of the ulnar nerve in the hand, which may make the approach to treatment even more challenging.
The abductor digiti minimi cord is commonly observed in the fifth finger. As this type of cord is confined to the finger, PNF is not recommended, although CCH would be a viable alternative. Moreover, although there is no robust clinical evidence to suggest that PNF has an increased risk of iatrogenic nerve, artery or tendon injury, it is reasonable to imagine that a blind procedure with multiple passes of a sharp needle would place such structures at risk. Thus, many surgeons only perform PNF in the palm on well-defined cords. On occasion during open surgery, after the pathological tissue has been removed, the contracted PIP joint can be manipulated straight by the surgeon. In such cases, the accessory collateral and volar plate are manually ruptured with controlled pressure.
This exploratory analysis is not without limitations, and the findings may be most useful for hypothesis generation and as a resource for the design of future clinical studies. For example, the clinical trial data were not analyzed by finger, and details regarding the nature of the cords contracting the MP and/or PIP joints were not available. That said, most surgical studies also do not report on the detailed structure of pathological cords—only that they were divided or excised. Moreover, multiple cords affecting multiple joints or digits may be excised during a single surgical session. By contrast, the product label for CCH stipulates a 30-day interval between injections. Of note, a phase III study evaluating the safety and efficacy of two concurrent injections into the same hand of patients with multiple contractures is in progress (ClinicalTrials.gov identifier no. NCT01407068). Additional studies that investigate different treatment paradigms may afford clinicians a better understanding of these issues. Such designs might include multiple, simultaneous CCH injections to treat MP and PIP joint contractures of the same finger or close, sequential injections to treat residual PIP joint contractures. Finally, it is important that future studies provide for the collection of details regarding the pathological anatomy of affected joints and take these configurations into account when analyzing the results.
Conclusions
The results of this post hoc analysis suggest that the efficacy of CCH for PIP contractures, as reflected by measures of clinical success and clinical improvement, was comparable after treatment of an MP joint contracture in the same finger and injection of an isolated PIP joint contracture. Likewise, improvements in FFC and ROM were quite similar across the two groups. In both Groups A and B, the changes in ROM exceeded the CID, which represents a 1-point change on a 4-point scale for patient-reported improvement. Thus, regardless of the specific pathological palmar anatomy, and consistent with previous research using surgical and non-surgical techniques, some PIP joints not directly treated with CCH will show meaningful spontaneous improvements in contracture. Furthermore, these improvements can be achieved without compromising safety.
Acknowledgments
The CORD I, CORD II, JOINT I, and JOINT II studies were sponsored by Auxilium Pharmaceuticals. Support for this post hoc analysis was provided by Pfizer Inc. Medical writing assistance was provided by Linda Goldstein of UBC Scientific Solutions and was funded by Pfizer.
Conflict of interest
M.J. Hayton serves as a consultant for Pfizer. A. Bayat has received an investigator award from Pfizer; he has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. D.S. Chapman and R.A. Gerber own stock in and are employed by Pfizer Inc.; P.P. Szczypa owns stock in and is employed by Pfizer Ltd.
References
- 1.Leclercq C. Clinical aspects. In: Tubiana R, Leclercq C, Hurst L, Badalamente M, Mackin E, editors. Dupuytren’s disease. London: Martin Dunitz Ltd; 2000. p. 86. [Google Scholar]
- 2.Draviaraj KP, Chakrabarti I. Functional outcome after surgery for Dupuytren’s contracture: a prospective study. J Hand Surg Am. 2004;29(5):804–808. doi: 10.1016/j.jhsa.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Crowley B, Tonkin MA. The proximal interphalangeal joint in Dupuytren’s disease. Hand Clin. 1999;15(1):137–47, viii. [PubMed]
- 4.Skoff HD. The surgical treatment of Dupuytren’s contracture: a synthesis of techniques. Plast Reconstr Surg. 2004;113(2):540–544. doi: 10.1097/01.PRS.0000101054.80392.88. [DOI] [PubMed] [Google Scholar]
- 5.Au-Yong IT, Wildin CJ, Dias JJ, Page RE. A review of common practice in Dupuytren surgery. Tech Hand Up Extrem Surg. 2005;9(4):178–187. doi: 10.1097/01.bth.0000186794.90431.a4. [DOI] [PubMed] [Google Scholar]
- 6.Engstrand C, Borén L, Liedberg GM. Evaluation of activity limitation and digital extension in Dupuytren’s contracture three months after fasciectomy and hand therapy interventions. J Hand Ther. 2009;22(1):21–6 (quiz 27). [DOI] [PubMed]
- 7.Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Smith T, et al. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35:2027–2038. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, et al. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 9.Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am. 2013;38(1):2–11. doi: 10.1016/j.jhsa.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Peimer C, McGoldrick C, Fiore G. Nonsurgical treatment of Dupuytren’s contracture: 1-year US post-marketing safety data for collagenase Clostridium histolyticum. Hand (NY) 2012;7:143–146. doi: 10.1007/s11552-012-9407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pess G, Peimer C, Skodny P, Tursi J, Szczypa P, Gerber R, editors. Efficacy and effectiveness of collagenase Clostridium histolyticum for Dupuytren’s contracture: comparison of real-world data with clinical trial results [abstract no. A-0175]. XVII Congress of the Federation of European Societies for Surgery of the Hand (FESSH); 29 May–Jun 1 2012; Antalya, Turkey.
- 12.Witthaut J, Bushmakin A, Gerber R, Cappelleri J, Hellio Le Graverand-Gastineau M-P. Determining clinically important changes in range of motion in patients with Dupuytren’s contracture: secondary analysis of the randomized, double-blind, placebo-controlled CORD I study. Clin Drug Investig. 2011;31(11):791–798. doi: 10.1007/BF03256918. [DOI] [PubMed] [Google Scholar]
- 13.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/S0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 15.Crean SM, Gerber RA, Le Graverand MP, Boyd DM, Cappelleri JC. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur. 2011;36(5):396–407. doi: 10.1177/1753193410397971. [DOI] [PubMed] [Google Scholar]
- 16.Salhi S, Cardin-Langlois E, Luc M. Percutaneous fasciotomy for the treatment of Dupuytren’s disease—a systematic review. Hand (N Y) 2011;6(4):349–355. doi: 10.1007/s11552-011-9355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rijssen AL, Ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129(2):469–477. doi: 10.1097/PRS.0b013e31823aea95. [DOI] [PubMed] [Google Scholar]
- 18.Shin EK, Jones NF. Minimally invasive technique for release of Dupuytren’s contracture: segmental fasciectomy through multiple transverse incisions. Hand (NY) 2011;6(3):256–259. doi: 10.1007/s11552-011-9336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonkin MA, Burke FD, Varian JP. The proximal interphalangeal joint in Dupuytren’s disease. J Hand Surg Br. 1985;10(3):358–364. doi: 10.1016/S0266-7681(85)80062-0. [DOI] [PubMed] [Google Scholar]
- 20.Adam RF, Loynes RD. Prognosis in Dupuytren’s disease. J Hand Surg Am. 1992;17(2):312–317. doi: 10.1016/0363-5023(92)90413-J. [DOI] [PubMed] [Google Scholar]
- 21.Craft RO, Smith AA, Coakley B, Casey WJ, 3rd, Rebecca AM, Duncan SF. Preliminary soft-tissue distraction versus checkrein ligament release after fasciectomy in the treatment of Dupuytren proximal interphalangeal joint contractures. Plast Reconstr Surg. 2011;128(5):1107–1113. doi: 10.1097/PRS.0b013e31822b67c9. [DOI] [PubMed] [Google Scholar]
- 22.Foucher G, Cornil C, Lenoble E. Open palm technique for Dupuytren’s disease. A five-year follow-up. Ann Chir Main Memb Super. 1992;11(5):362–366. doi: 10.1016/S0753-9053(05)80271-6. [DOI] [PubMed] [Google Scholar]


