Abstract
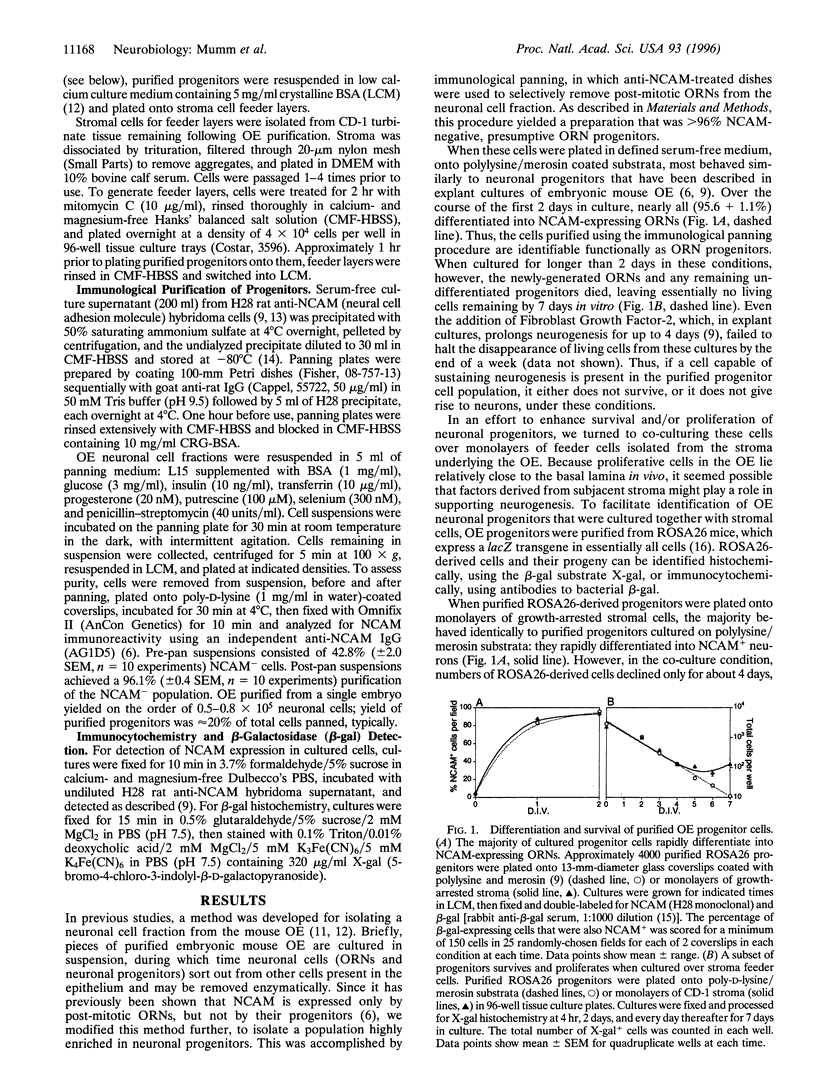
The mammalian olfactory epithelium (OE) supports continual neurogenesis throughout life, suggesting that a neuronal stem cell exists in this system. In tissue culture, however, the capacity of the OE for neurogenesis ceases after a few days. In an attempt to identify conditions that support the survival of neuronal stem cells, a population of neuronal progenitors was isolated from embryonic mouse OE and cultured in defined serum-free medium. The vast majority of cells rapidly gave rise to neurons, which died shortly thereafter. However, when purified progenitors were co-cultured with cells derived from the stroma underlying the OE, a small subpopulation (0.07-0.1%) gave rise to proliferative colonies. A morphologically identifiable subset of these colonies generated new neurons as late as 7 days in vitro. Interestingly, development of these neuronal colonies was specifically inhibited when purified progenitors were plated onto stromal feeder cells in the presence of a large excess of differentiated OE neurons. These results indicate that a rare cell type, with the potential to undergo prolonged neurogenesis, can be isolated from mammalian OE and that stroma-derived factors are important in supporting neurogenesis by this cell. The data further suggest that differentiated neurons provide a signal that feeds back to inhibit production of new neurons by their own progenitors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caggiano M., Kauer J. S., Hunter D. D. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994 Aug;13(2):339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Calof A. L., Chikaraishi D. M. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989 Jul;3(1):115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Calof A. L., Hagiwara N., Holcomb J. D., Mumm J. S., Shou J. Neurogenesis and cell death in olfactory epithelium. J Neurobiol. 1996 May;30(1):67–81. doi: 10.1002/(SICI)1097-4695(199605)30:1<67::AID-NEU7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Calof A. L. Intrinsic and extrinsic factors regulating vertebrate neurogenesis. Curr Opin Neurobiol. 1995 Feb;5(1):19–27. doi: 10.1016/0959-4388(95)80082-4. [DOI] [PubMed] [Google Scholar]
- Calof A. L., Lander A. D. Relationship between neuronal migration and cell-substratum adhesion: laminin and merosin promote olfactory neuronal migration but are anti-adhesive. J Cell Biol. 1991 Nov;115(3):779–794. doi: 10.1083/jcb.115.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah M. I., Au C. Olfactory Schwann cells are derived from precursor cells in the olfactory epithelium. J Neurosci Res. 1991 Jun;29(2):172–180. doi: 10.1002/jnr.490290206. [DOI] [PubMed] [Google Scholar]
- DeHamer M. K., Guevara J. L., Hannon K., Olwin B. B., Calof A. L. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994 Nov;13(5):1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Deryugina E. I., Müller-Sieburg C. E. Stromal cells in long-term cultures: keys to the elucidation of hematopoietic development? Crit Rev Immunol. 1993;13(2):115–150. [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Growth and differentiation in the hemopoietic system. Annu Rev Cell Biol. 1987;3:423–441. doi: 10.1146/annurev.cb.03.110187.002231. [DOI] [PubMed] [Google Scholar]
- Doucette R. Glial progenitor cells of the nerve fiber layer of the olfactory bulb: effect of astrocyte growth media. J Neurosci Res. 1993 Jun 15;35(3):274–287. doi: 10.1002/jnr.490350307. [DOI] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Gordon M. K., Mumm J. S., Davis R. A., Holcomb J. D., Calof A. L. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995 Aug;6(4):363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Gray G. E., Sanes J. R. Migratory paths and phenotypic choices of clonally related cells in the avian optic tectum. Neuron. 1991 Feb;6(2):211–225. doi: 10.1016/0896-6273(91)90357-6. [DOI] [PubMed] [Google Scholar]
- Graziadei G. A., Graziadei P. P. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979 Apr;8(2):197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5(1):113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979 Feb;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Watt F. M. Stem cells: the generation and maintenance of cellular diversity. Development. 1989 Aug;106(4):619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Harding J., Graziadei P. P., Monti Graziadei G. A., Margolis F. L. Denervation in the primary olfactory pathway of mice. IV. Biochemical and morphological evidence for neuronal replacement following nerve section. Brain Res. 1977 Aug 19;132(1):11–28. doi: 10.1016/0006-8993(77)90703-x. [DOI] [PubMed] [Google Scholar]
- Hempstead J. L., Morgan J. I. Monoclonal antibodies to the rat olfactory sustentacular cell. Brain Res. 1983 Dec 12;288(1-2):289–295. doi: 10.1016/0006-8993(83)90105-1. [DOI] [PubMed] [Google Scholar]
- Holcomb J. D., Mumm J. S., Calof A. L. Apoptosis in the neuronal lineage of the mouse olfactory epithelium: regulation in vivo and in vitro. Dev Biol. 1995 Nov;172(1):307–323. doi: 10.1006/dbio.1995.0025. [DOI] [PubMed] [Google Scholar]
- Howlett A. R., Hodges G. M., Rowlatt C. Epithelial-stromal interactions in the adult bladder: urothelial growth, differentiation, and maturation on culture facsimiles of bladder stroma. Dev Biol. 1986 Dec;118(2):403–415. doi: 10.1016/0012-1606(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Jones P. H., Watt F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993 May 21;73(4):713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Kauffman S. L. Lengthening of the generation cycle during embryonic differentiation of the mouse neural tube. Exp Cell Res. 1968 Feb;49(2):420–424. doi: 10.1016/0014-4827(68)90191-2. [DOI] [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Mahanthappa N. K., Schwarting G. A. Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: roles of EGF and TGF-beta s. Neuron. 1993 Feb;10(2):293–305. doi: 10.1016/0896-6273(93)90319-m. [DOI] [PubMed] [Google Scholar]
- Masui T. Differentiation of the yolk-sac endoderm under the influence of the digestive-tract mesenchyme. J Embryol Exp Morphol. 1981 Apr;62:277–289. [PubMed] [Google Scholar]
- Pixley S. K. CNS glial cells support in vitro survival, division, and differentiation of dissociated olfactory neuronal progenitor cells. Neuron. 1992 Jun;8(6):1191–1204. doi: 10.1016/0896-6273(92)90139-5. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Reh T. A. Cell-specific regulation of neuronal production in the larval frog retina. J Neurosci. 1987 Oct;7(10):3317–3324. doi: 10.1523/JNEUROSCI.07-10-03317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh T. A., Tully T. Regulation of tyrosine hydroxylase-containing amacrine cell number in larval frog retina. Dev Biol. 1986 Apr;114(2):463–469. doi: 10.1016/0012-1606(86)90210-1. [DOI] [PubMed] [Google Scholar]
- Schwartz Levey M., Chikaraishi D. M., Kauer J. S. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991 Nov;11(11):3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N. M., Marchionni M. A., Isaacs I., Stroobant P., Anderson D. J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994 May 6;77(3):349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Spooncer E., Lord B. I., Dexter T. M. Defective ability to self-renew in vitro of highly purified primitive haematopoietic cells. Nature. 1985 Jul 4;316(6023):62–64. doi: 10.1038/316062a0. [DOI] [PubMed] [Google Scholar]